This is an old revision of the document!
Table of Contents
Tay Sachs Disease
Tay Sachs is a fatal genetic disorder which occurs due to the deletion of an enzyme called beta-hexosaminidase A (also known as, HEXA), resulting in the accumulation of fatty acid GM2 ganglioside in neurons. This eventually leads to the progressive malfunctioning and degeneration of neurons.
Signs & Symptoms
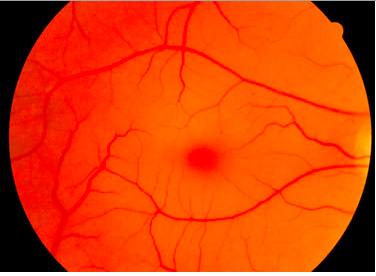
Tay Sachs affects the individual differently based on when their onset is: early (infantile and childhood ages) or late onset (adolescence and onwards). For early onset, the disease is not evident until about 6 months after the baby is born. Around 6 months, the child may show signs of reduced muscle function coming from progressive muscle weakness, twitching and muscle jerks. They will also have a greater sensitivity to sound, for example if they hear an unexpected sound, they will be startled (NORD, 2016). One characteristic sign of Tay Sachs is the development of “cherry red spots” found in the eyes. “Cherry red spots” are apparent in 90% of Tay Sachs disease cases (NORD, 2016). These spots are formed by macular degeneration and causes the choroid of the eye to be exposed. The choroid has blood vessels that supply blood to the retina. The child may also have a difficult time learning new motor movements such as holding eye contact (NORD, 2016). Children between ages 2-10 will experience a reduction in coordination, movement and intellectual abilities. They may also develop a variety of eye disorders such as optic atrophy which results in the loss of nerve function that delivers messages to brain to form images and retinitis pigmentosa which eventually results in the degeneration of the retina (NORD, 2016). Child may also experience a loss of speech and their speech will be slurred.
For late onset symptoms, the individual will experience a reduction of motor coordination resulting from muscle weakness and wasting, involuntary muscle contractions, twitches and tremors. May also experience slurred speech which significantly reduces their ability to interact with others. In addition, you see the sign of mood changes and deterioration in mental health (NORD, 2016). Overall, they’re unable to engage in and complete daily tasks such as driving, walking or interacting with others as one normally would be able to.
<style center>
</style>
Epidemiology
Tay-Sachs Disease is a very rare disease in the general population. Although this disease can occur in any ethnic group, the genetic mutations that are responsible for Tay Sachs are more frequent among the Ashkenazi Jews compared to other Jews (Sephardic and Oriental) and non-Jews (Koeslag 1984). According to a study done in New York City by Kozinn et al. (1957) over 12 years, it was determined that Tay Sachs occurred in 1 per 8300 Jewish people and in 1 per 450, 000 non-Jewish people. The estimated frequency of the heterozygous carrier according to this study was found to by 1 in 50 for Jews and 1 in 300 for non-Jews (Myrianthopoulos 1962). Although less frequent, Tay Sachs is also found in French Canadians, Pennsylvania Amish and Louisiana Cajuns. There are different possible reasons that explain the frequency of Tay Sachs among Ashkenazi Jews and other populations. For one, there may be a conferred adaptive advantage of Tay Sachs. Being a carrier of the gene that is mutated in Tay Sachs provides resistance against tuberculosis (Withrock 2015). Therefore, this gene has a heterozygote advantage (Frisch 2004). As well, there may have been the effect of random genetic drift. Random genetic drift occurs when there is a change in allele frequencies due to chance. Since most of the populations that have Tay Sachs are isolated, genetic drift has a greater effect in changing allele frequencies. Further, the founder effect may have impacted allele frequencies in the population. For example, if the initial population of individuals had the genetic mutation, those individuals would pass on the mutation to their descendants. As the population increases, the number of individuals with the mutation would increase in high frequency as well. It is possible that there may have been a high incidence of the mutation in the founder population. (Chakravarti 1978). Another theory known as reproductive compensation states that parents of infants affected by Tay-Sachs may continue having more children, which maintains the mutated gene in populations in high frequencies (Koeslag 1984).
Genetic Risk Factors
Individuals of the following ethnicities are at a higher risk for being affected by Tay-Sachs or being heterozygous for the mutated allele:
- Ashkenazi (eastern and central European) Jewish
- French-Canadians of Quebec
- Old Order Amish in Pennsylvania
- Cajuns of Louisiana
- Family history of condition
Genetic Inheritance
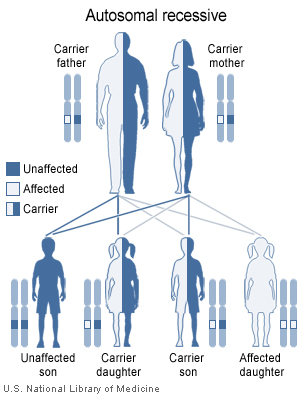
Tay-Sachs disease is caused by mutations in the HEXA gene, which contains information on how to make enzyme beta-hexosaminidase A. This enzyme is important for the central nervous system (brain and spinal cord) and also helps with breaking down GM2 ganglioside. GM2 ganglioside is unable to breakdown properly when there is a mutation in the HEXA gene. This causes a buildup of toxins in the neurons, which results in the observable symptoms of Tay-Sachs disease. This mutation is inherited in an autosomal recessive pattern. For example, if both parents are unaffected carriers of the mutated HEXA gene and they both pass on the mutated gene to their child, the child will inherit both mutated genes to produce a non-functional HEXA gene and will develop Tay-Sachs. If both parents are carriers of the mutated gene, there is a 1 in 4 chance of having a child who is affected with Tay-Sachs, 2 in 4 chance of having a child who is a carrier and 1 in 4 chance of having a child who is an unaffected non-carrier (GHR 2017).
<style center>
</style>
Pathophysiology
Neurobiology
Tay-Sachs disease primarily affects the central nervous system (Genetics Home Reference, 2017). In other words, the disease predominantly affects the brain and spinal cord. This coincides with the disease’s degenerative effects upon motor and intellectual disabilities (Neudorfer et al., 2005).
The pathophysiology of the disease is primarily based upon gangliosides (Yu et al., 2011). Gangliosides are defined as sialic acid containing glycosphingolipids (Sandhoff, 2013). Currently, 188 gangliosides with a varying carbohydrate chain structure have been identified (Sandhoff & Harzer, 2013). In the central nervous system, gangliosides appear on the outer leaflet of neuronal plasma membranes (Sandhoff, 2013). Functionally, gangliosides are involved in cell-cell recognition, adhesion and signal transduction. Naturally, gangliosides are known to have a dynamic level of expression within the brain during development (Sandhoff, 2013).
For the purposes of this disease, the gangliosides of focus are GM2 gangliosides (Walkley, Siegel & Dobrenis, 1995).
HEXA Gene
Tay-Sachs disease involves the HEXA gene (Genetics Home Reference, 2017). The gene is located on chromosome 15 at the location 15q24.1. Through the processes of transcription and translation, the gene is expected to encode the protein, Beta-hexosaminidase A (Genetics Home Reference, 2017).
Beta-Hexosaminidase A
The protein is classified as a lysosomal enzyme prevalent throughout the body (Kaback & Desnick, 1999). In the central nervous system, it is expected to remove excess GM2 ganglioside from the outer membrane of neurons. By doing so, it prevents cellular malfunction and neuronal cell death (Kaback & Desnick, 1999).
Structurally, the protein is classified as an alpha-beta heterodimer (Sandhoff, 2013).
Tay-Sachs disease involves dysfunction of the alpha-subunit of the protein. In effect, this disables the protein as a whole (Sandhoff, 2013)
Cellular Pathway
As previously noted, beta-hexosaminidase is a lysosomal enzyme and works to remove excess GM2 gangliosides from the cell membrane (Kaback & Desnick, 1999).
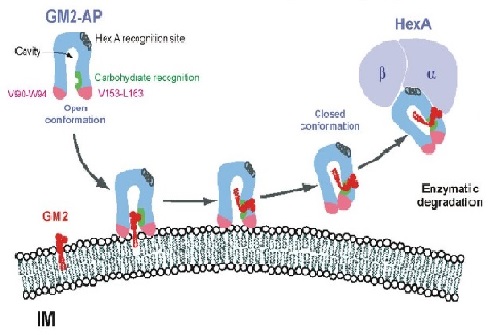
GM2 gangliosides are present throughout the cell membrane (Yu et al., 2004). In order to remove the ganglioside from the membrane a lipid binding protein known as, GM2AP is required (Sandhoff, 2013). GM2AP is required for the overall removal of GM2 gangliosides. GM2AP approaches GM2 ganglioside in an open conformation. Upon attachment to the protein, GM2AP takes on a closed conformation (Sandhoff, 2013).
In this closed conformation, the protein-lipid complex can bind to the alpha subunit of beta-hexosaminidase A (Sandhoff, 2013). Upon binding, the protein exerts catalytic activity on the GM2AP-GM2 ganglioside complex (Sandhoff, 2013).
<style center>
</style>
Mutation
When an individual inherits Tay-Sachs disease, this is primarily due to a single point mutation in the HEXA gene (Myerowitz, 1997). While a wide variety of single point mutations have been identified, the most common is a C (cytosine) to G (guanine) substitution in amino acid 180 (Bergeron, 1997). This changes the codon from UAC to UAG, causing a termination of the polypeptide involved with the alpha subunit of the Beta-Hexosaminidase A protein (Bergeron, 1997). In effect, this will not form the Beta-hexosaminidase A protein (Bergeron, 1997). Which in return, will not allow the removal of excess GM2 gangliosides from the neuronal cell membrane (Yu et al., 2012).
Manifestation of the Mutation
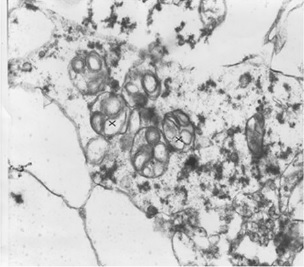
Without the removal of excess GM2 gangliosides, GM2 gangliosidoses will occur. With an excess of lipids within the cell, lipids will be stored within the lysosome (Tuck & Cavalli, 2010). In effect this will cause a swelling of many tissues. In the figure, this is shown in the cortical neuron of a 23 week old fetus (Sandhoff, 2013).
<style center>
</style>
A common symptom of Tay-Sachs disease is a cherry red macular spot (Sandhoff, 2013). Again, this is due to lipid storage in neuronal cells within the eye. Neuronal cells storing lipids lose their ability to cover the fovea centralis. As a result, the fovea (which is naturally yellow) appears red. The red colour is due to the showing of the choroidea behind the retina (Sandhoff, 2013).
Diagnosis
Tay - Sachs disease (TSD) can be diagnosed through the use of tests and clinical evaluation. In a blood test screening, levels of hexosaminidase A are assessed using an enzyme assay (NORDs, n.a). Individuals with Tay-Sachs have reduced levels to almost absent amounts of this enzyme. Another way to diagnose Tay-Sachs is through the use of molecular genetic testing (NORDs, n.a). This method can find mutations in the HEXA gene, which is known to cause the disease. However, this diagnostic test is only offered at certain labs. Tay- Sachs can also be diagnosed prenatally through the use of screening such as chorionic villus sampling (CVS) and amniocentesis. CVS involves removing tissue samples from a part of the placenta and can be conducted around the 11th week of pregnancy (National Human Genome Research Institute, 2011). While in amniocentesis, it is done around the 16th week of pregnancy and involves the testing of a fluid sample surrounding the baby that is removed through the use of a needle (National Human Genome Research Institute, 2011).
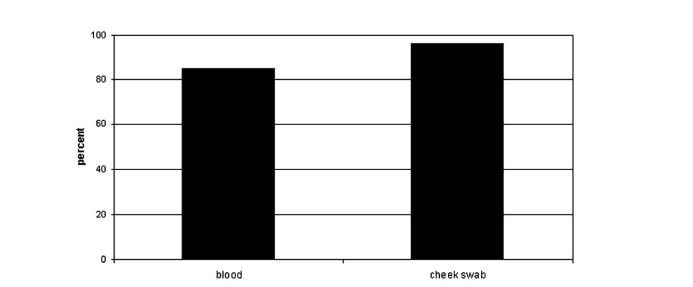
The Tay-Sachs disease carrier screening program was first started in Baltimore Maryland, in 1970 (Lew et al., 2014). Screening programs are important as it has been found that there is about a 90% decrease in the incidence of TSD cases in communities where these programs have been offered (Lew et al., 2014). A study by Gason et al. (2005), examined the efficacy of carrier screening programs in 2 Jewish schools in Melbourne students (ages 14-17) over a two year period. One of the components of the study involved assessing the compliance rate for blood sampling for the enzyme and genetic testing versus using a cheek brush method for solely genetic sampling. Until 2002, Jewish high school students were screened for TSD via an enzyme analysis, along with a mutation detection test. The cheek brush test method was implemented in 2003 and consisted of mutation analysis of the three common mutations found in the Ashkenazi Jewish populations. The study found that was a higher proportion of students who were tested for TSD carrier status via the cheek brush method (96%, N=214) compared to those who took a blood sample (84.9%, N=163). Researchers also saw that receiving a blood test caused more anxiety among the group 8.2% compared to those who received the cheek brush method 20.9%. Student attitudes were considered a powerful motivator for testing as there was a higher level of discomfort linked to blood testing.
In a review article by Lew et al. (2014), there are several barriers to diagnosing TSD that are highlighted. On the clinician’s side, some of these include a lack of clinical education regarding the disease and failure to identify the genetic risk during their medical history. Other barriers include unplanned pregnancies and expenses incurred during testing for diagnosing (Lew et al., 2014). In Sydney Australia, having a test done for TSD costs a patient around $A100 (Lew et al., 2012). However, even when obstacles such as cost and blood testing are removed, there is not always full maximization for carrier screening which makes a diagnosis of TSD more difficult. In the study by Gason et al. (2005), there were students at the high schools who did not want to know if they were carriers at this point in their lives.
Treatment
Unfortunately, there is currently no cure for Tay-Sachs disease. Treatment is directed towards the specific symptoms that are apparent in each individual which may require the coordinated efforts of a team of specialists such as pediatricians, neurologists, speech pathologists and other health care professionals who may need to systematically and comprehensively plan an affected child’s treatment. Genetic counseling may be of benefit for affected individuals and their families. Psychosocial support is recommended for the entire family to help them support the child and cope up with their difficulties.
Current Therapeutic Approach
An anticonvulsant is a medication used to control seizures (convulsions) or stop an ongoing series of seizures and is often used to treat individuals with Tay-Sachs but may not be effective in all people. An anticonvulsant called Miglustat, which is an analogue of D-glucose, is currently in clinical trials and has proven to be successful in tay-sachs induced mouse models (Osher et al., 2011).
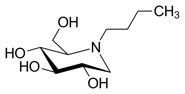
Another strategy is substrate deprivation which utilizes an inhibitor of glycosphingolipid biosynthesis to balance synthesis with the impaired rate of catabolism, thus preventing storage. One such inhibitor is N-butyldeoxynojirimycin, which currently is in clinical trials for the potential treatment of type 1 Gaucher disease, a related disease that involves glycosphingolipid storage in peripheral tissues, but not in the CNS. In a recent study, mouse models with Gaucher disease were treated with the inhibitor N-butyldeoxynojirimycin. The treated mice showed delayed symptom onset, reduced storage in the brain and peripheral tissues, and increased life expectancy. Substrate deprivation therefore offers a potentially general therapy for this family of lysosomal storage diseases, including those with CNS disease (Jeyakumar et al., 1999)
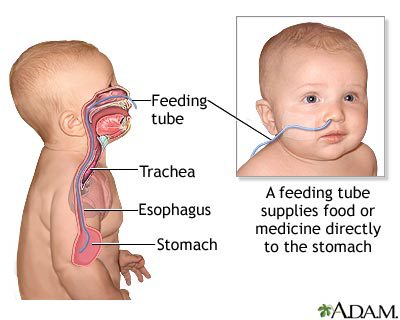
Because of the potential of feeding difficulties, infants should be monitored for nutritional status and proper hydration. In addition to nutritional support and supplementation, a feeding tube may be necessary to help prevent food, liquid or other foreign material from accidentally going into the lungs (Osher et al., 2011).
Investigational Therapies
Further investigation into drug treatments and substrate reduction therapies would be necessary to find a “cure” to this fatal disease. Research is being done to understand and evaluate the effects of different levels of psychosocial support to the affected child and the family. A drug called pyrimethamine has been tried as a treatment for Tay-Sachs disease. Affected individuals who took the medication showed increased activity of hexosaminidase A. However, this increased activity did not lead to any noticeable improvement in neurological or psychiatric symptoms. Therefore, more research is necessary to determine whether pyrimethamine has any significant therapeutic role.
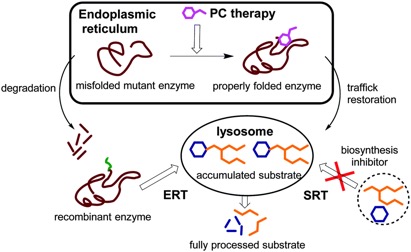
Enzyme Replacement Therapy: Research is ongoing to develop enzyme replacement therapy (ERT) for Tay-Sachs disease which involves replacing a missing enzyme in individuals who are deficient or lack a particular enzyme. Synthetic versions of missing enzymes have been developed and used to treat individuals with other lysosomal storage diseases including Hurler syndrome and Gaucher disease. However, ERT has not proven successful in people with Tay-Sachs disease. One issue is the inability to find a way for the replacement enzyme to cross the blood-brain barrier.
Gene Therapy: Gene therapy is also being studied as another possible approach to therapy where the defective gene present in a patient is replaced with a normal gene to enable the production of active enzyme and prevent the development and progression of the disease. Given the permanent transfer of the normal gene, which can produce active enzyme at all sites of disease, this form of therapy is theoretically most likely to lead to a “cure.”
Chaperone Therapy: Chaperone therapy is also being studied for Tay-Sachs disease which involves very small molecules that attach to newly-created HexA enzymes, before the mutated enzymes are broken down, and guides them to the lysosome, where the enzymes can perform their normal function. Such a molecule can also cross the blood-brain barrier. This therapy is only in initial stages of study, and more research will be necessary to determine its long-term safety and effectiveness.
Conclusion
The National Tay-Sachs & Allied Diseases Association (NTSAD) is an American advocacy group that focuses on providing funding for research, supporting families and individuals, and raising awareness to prevent disease. The organization offers program and services in the areas of education, advocacy, research, and family services.(NTSAD, 2016).
Education stresses the importance of awareness of the disease and plays an important role in diagnoses of Tay-Sachs through programs like carrier screening. Advocacy is not only essential for research funding, but also health insurance coverage as this disease places a financial burden on the individual and their families. As there is no current treatment for Tay-Sachs, research is essential to study current investigational therapies. As Tay-Sachs presents many difficulties for a child and their family, family services such as psychosocial services are recommended to help with support and coping.
Presentation
Family Battles Tay-Sachs Disease
References
1. About NTSAD. (n.d.). Retrieved from https://www.ntsad.org/index.php/about
2. Bergeron, S. (1997). Tay-Sachs. Retrieved from http://www-personal.umd.umich.edu/~jcthomas/JCTHOMAS/1997%20Case%20Studies/S.%20Bergeron.html
3. Cachón-González, M. B., Wang, S. Z., Lynch, A., Ziegler, R., Cheng, S. H., & Cox, T. M. (2006). Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proceedings of the National Academy of Sciences, 103(27), 10373-10378.
4. Chakravarti, A. and Chakraborty R. (1978). Elevated frequency of Tay-Sachs disease among Ashkenazic Jews unlikely by genetic drift alone. Am J Hum Genet, 30(3): 256-261. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1685578/
5. Frisch, A. et. al. (2004). Origin and spread of the 1278insTATC mutation causing Tay-Sachs disease in Ashkenazi Jews: genetic drift as a robust and parsimonious hypothesis. Human Genetics, 114 (4): 366-376.Doi 10.1007/s00439-003-1072-8 http://link.springer.com/article/10.1007%2Fs00439-003-1072-8
6. Gason, A. A., Metcalfe, S. A., Delatycki, M. B., Petrou, V., Sheffield, E., Bankier, A., & Aitken, M. (2005). Tay Sachs disease carrier screening in schools: educational alternatives and cheekbrush sampling. Genetics in Medicine, 7(9), 626-632.
7. Genetics Home Reference. (2017, February 28). Tay-Sachs disease. Retrieved from https://ghr.nlm.nih.gov/condition/tay-sachs-disease
8. Jeyakumar, M., Butters, T. D., Cortina-Borja, M., Hunnam, V., Proia, R. L., Perry, V. H., … & Platt, F. M. (1999). Delayed symptom onset and increased life expectancy in Sandhoff disease mice treated with N-butyldeoxynojirimycin. Proceedings of the National Academy of Sciences, 96(11), 6388-6393.
9. Kaback, M.M., & Desnick, R.J. (1999). GeneReviews: Hexosaminidase A deficiency. Seattle, WA: University of Washington.
10. Koeslag, J. H. and Schach, S. R. (1984), Tay–Sachs disease and the role of reproductive compensation in the maintenance of ethnic variations in the incidence of autosomal recessive disease. Annals of Human Genetics, 48: 275–281. doi:10.1111/j.1469-1809.1984.tb01025.x
11. Lew, R. M., Burnett, L., Proos, A. L., Barlow‐Stewart, K., Delatycki, M. B., Bankier, A., … & Fietz, M. (2015). Ashkenazi Jewish population screening for Tay–Sachs disease: The International and Australian experience. Journal of paediatrics and child health, 51(3), 271-279.
12. Myerowitz, R. (1997). Tay-Sachs disease-causing mutations and neutral polymorphisms in the Hex A gene. Human Mutation, 9(3), 195-208.
13. National Human Genome Research Institute. (2011, March 17). Learning about Tay-Sachs disease. Retrieved from https://www.genome.gov/10001220/
14. Neudorfer, O., Pastores, G.M., Zeng, B.J., Gianutsos, J., Zaroff, C.M., & Kolodny, E.H. (2005). Late-onset Tay-Sachs disease: Phenotypic characterization and genotypic correlations in 21 affected patients. Genetics in Medicine, 7, 119-123.
15. NORD. (2016). Tay Sachs Disease. Retrievedfrom https://rarediseases.org/rare-diseases/tay-sachs-disease/
16. Osher, E., Fattal-Valevski, A., Sagie, L., Urshanski, N., Amir-Levi, Y., Katzburg, S., … & Navon, R. (2011). Pyrimethamine increases β-hexosaminidase A activity in patients with Late Onset Tay Sachs. Molecular genetics and metabolism, 102(3), 356-363.
17. PubMed Health. (n.d.). Tay-Sachs disease. Retrieved from https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0024672/
18. Sandhoff, K. (2013). Metabolic and cellular bases of sphingolipidoses. Biochemical Society Transactions, 41(6), 1562-1568.
19. Sandhoff, K., & Harzer, K. (2013). Gangliosides and gangliosidoses: Principles of molecular and metabolic pathogenesis. Journal of Neuroscience, 33(25), 10195-10208.
20. Tay Sachs Disease. (n.d.). Retrieved from https://rarediseases.org/rare-diseases/tay-sachs-disease/
21. Tay Sachs disease (2017). Genetics Home Reference. Retrieved from https://ghr.nlm.nih.gov/condition/tay-sachs-disease
22. Tuck, E., & Cavalli, V. (2010). Roles of membrane trafficking in nerve repair and regeneration. Communicative & Integrative Biology, 3(3), 209-214.
23. Walkley, S.U., Siegel, D.A., & Dobrenis, K. (1995). GM2 ganglioside and pyramidal neuron dendritogenesis. Neurochemical Research, 20(11), 1287-1299.
24. Withrock, I. C. et. al. (2015). Genetic diseases conferring resistance to infectious diseases. Genes & Diseases, 2(3): 247-254. doi: 10.1016/j.gendis.2015.02.008 http://www.sciencedirect.com/science/article/pii/S2352304215000239
25. Yu, R.K., Bieberich, E., Xia, T., & Zeng, G. (2004). Regulation of ganglioside biosynthesis in the nervous system. Journal of Lipid Research, 45(5), 783-793.
26. Yu, R.K., Tsai, Y., & Ariga, T. (2012). Functional roles of gangliosides in neurodevelopment – An overview of recent advances. Neurochemical Research, 37(6), 1230-1244.
27. Yu, R.K., Tsai, Y., Ariga, T., & Yanagisawa, M. (2011). Structures, biosynthesis, and functions of gangliosides – An overview. Journal of Oleo Science, 60(10), 537-544.