This is an old revision of the document!
Table of Contents
Progeria
 Retrieved from: http://fcscortland.org/schizophrenia
Retrieved from: http://fcscortland.org/schizophrenia
Overview
Introduction
Hutchinson- Gilford Progeria syndrome, also known as Progeria, is a rare fatal genetic disease affecting 1 in 4 million babies globally. As of 2014, there were 118 confirmed cases of progeria globally. It is responsible for accelerated aging throughout their childhood, commencing at age 1, resulting in scleroderma, reduced joint mobility, hair loss and more (Progeria101/FAQ, 2018). These symptoms will appear at age 1, The disease progresses atherosclerosis which raises the probability of a heart attack or stroke (Genetics Home Reference, 2018).
History
Progeria is an extremely rare genetic disease of childhood characterized by dramatic, premature aging with death occurring on average at the age of 13, usually from heart attack or stroke (Kashyap et al., 2014). Hutchinson-Gilford progeria syndrome (HGPS) is the most severe form of the disease and the classic type. The disease was named after the doctors who first described it in England; in 1886 by Dr. Jonathan Hutchinson and in 1897 by Dr. Hastings Gilford. The term progeria is derived from the Greek work geras, meaning old age (DeBusk, 1972).
In 1886, the syndrome was first reported by Hutchinson of a 6-year-old boy whose overall appearance was that of an old man. Hutchinson described the case as “congenital absence of hair and its appendages” (DeBusk, 1972). It was a year later that Gilford described a second patient with similar clinical findings. To date, there are only 100 patients with HGPS that have been described in literature (Kashyap et al., 2014). These two boys were further described in 1897 and 1904 by Gildford, who was the one to proposal the term “progeria” and described the post-mortem characteristics (DeBusk, 1971). Little research was done on the disease until the 1990’s due to the rarity of the disease, causing it to be frequently diagnosed erroneously in patients with some of the features such as alopecia and skin of aged appearance (DeBusk, 1972). However, there are three features present in early life; mid-facial cyanosis, skin resembling scleroderma, and glyphic nasal tip, which all facilitate an early diagnosis of HGPS (DeBusk, 1972).
Epidemiology
HPGS is an extremely rare genetic disorder affecting about 1 in 4 million live births, if unreported or misdiagnosed cases are taken into account. The reported prevalence rate of the disease is 1 in 8 million births, based on the number of cases (Coppedè, 2013). According to the Progeria Research Foundation database, there are an estimated 350-400 children living with progeria worldwide at any one time. As of January 2018, there are a recorded 114 children living with progeria worldwide with numbers steadily increasing as the years go by (http://www.progeriaresearch.org/prf-by-the-numbersprf.html).
HPGS affects all races; cases of progeria have been discovered in 45 different countries. However, 97% of affected patients are white. Males are affected 1 ½ times more often than females. The disease was thought to be autosomal recessive in the past, however observations made an autosomal recessive inheritance very unlikely and favour a sporadic, dominant mutation. The mutation results in life spans for progeria syndrome to be in the second/third decades of life, with the majority of patients dying of cardiovascular or cerebrovascular disease between 7 and 27 years of age (Sarkar and Shinton, 2001).
Etiology
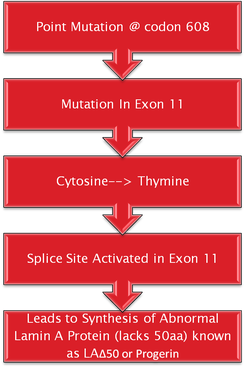
The cause of Progeria is known to be mutations of the Lamin A, or LMNA gene. These mutations have been found to be sporadic and new, rather than being inherited by their parents. In the sporadic cases leading to Progeria, an autosomal dominant mutation takes place. However, this does not occur when Progeria is inherited and is known as Werner’s syndrome (Sinha, Raghunath & Ghosh, 2018). What is known is the fact that the LMNA gene is responsible for creating a protein which helps keep a nuclei intact. Unfortunately, in individuals with Progeria, this does not occur as the LMNA is abnormal. Abnormal LMNA proteins, or Progerin, accumulates in the body and thus creates instability in cells. As a result, this leads to the abnormal aging process of children (Mayo Clinic, 2018). Interestingly, the LMNA mutations being the cause of Progeria was discovered in April 2003, and have claimed it has significant implications on treating the disease, aging, and cardiovascular disease as well (Progeria101/FAQ, 2018).
Diagnosis
The only way to diagnose is once the symptoms become visible. There are no early diagnostic techniques.
HPGS is a genetic condition that can later be characterized by a rapid appearance of aging that begins in childhood. An affected child will usually look normal at birth and even early infancy but will usually grow more slowly than other children and will not gain weight at the expected rate of a normal child . There is a distinctive appearance that is developed in affected children (De Sandre-Giovannoli et al, 2003). Usually what is seen is baldness, aged-looking skin, a pinched nose, and a small face and jaw in relation to head size. In addition to this they will usually suffer from symptoms that are usually seen in older individuals such as stiffness of joints, hip dislocations and severe cardiovascular disease (De Sandre-Giovannoli et al, 2003). They experience severe hardening of the arteries that begin in early childhood and put individuals at risk of having a heart attack or a stroke at an early age. The condition does not have and affect on intellectual development or the development of motor skills. As of now clinical professional look for these physical symptoms in early childhood in order to make a proper diagnosis (De Sandre-Giovannoli et al, 2003).
A mutation in the LMNA gene is the cause of HPGS. The LMNA gene makes a protein called Lamin A. This protein determines the shape of the nucleus within cells. It is a supporting component of the nuclear envelope (De Sandre-Giovannoli et al, 2003). A mutation in this gene causes an abnormal version of the lamin A protein. That being said there is still continuing research going on to understand how this impact on the nucleus leads to the characteristics that are seen in HPGS (De Sandre-Giovannoli et al, 2003).
CASE (X-Ray) of individual with HPGS.
Symptoms
Pathophysiology
Background
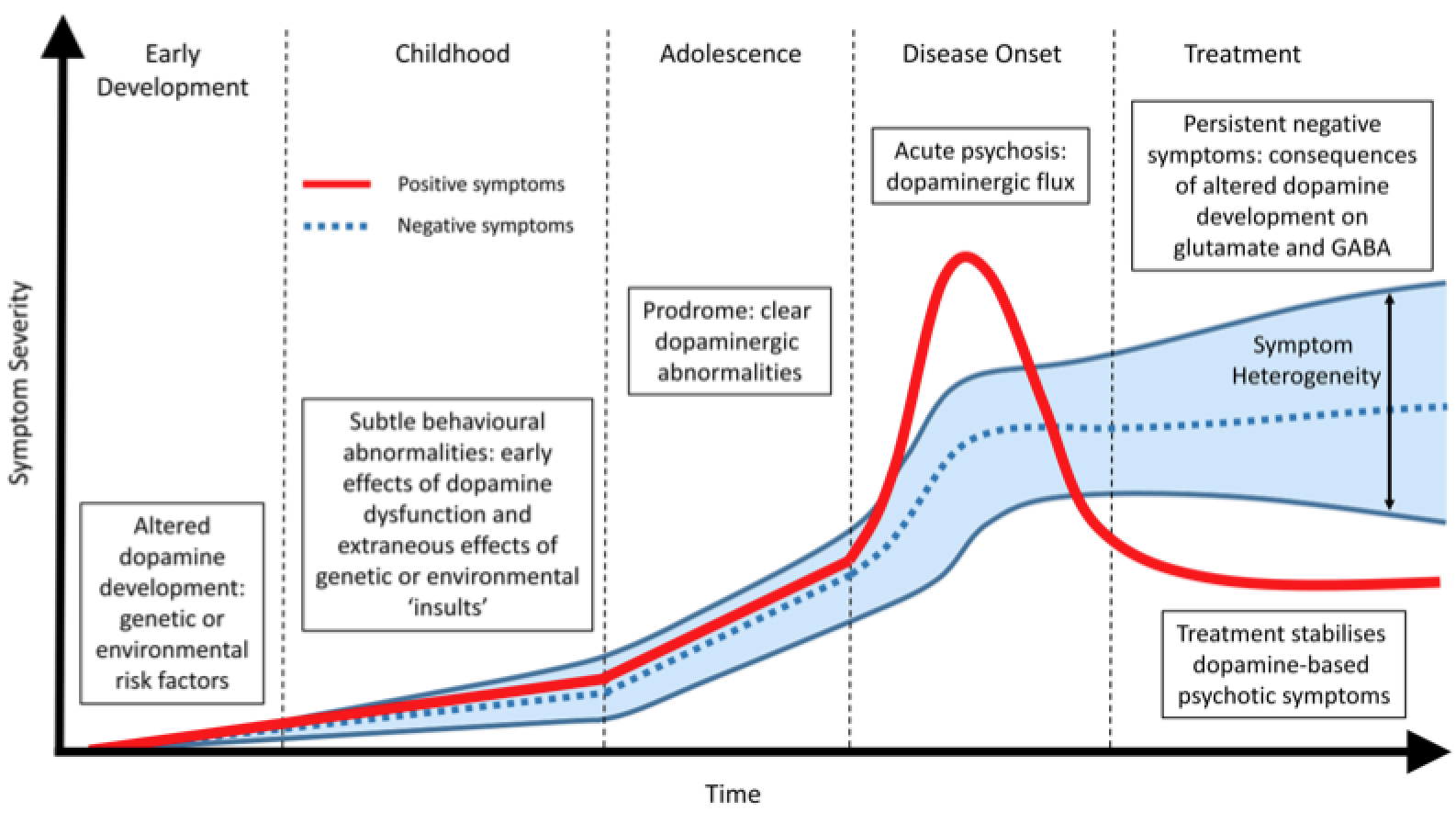
Both structural and functional abnormalities are common in patients with schizophrenia. The changes occur in several key brain systems, including prefrontal and medial temporal lobe regions, as well as in the cortex and in the connections between different cortical regions (Karlsgodt et al. 2010). Individuals with schizophrenia have smaller volume in the hippocampus, thalamus, amygdala, nucleus accumbens and intracranial space, and larger pallidum and ventricle volumes (McIntosh et al. 2011). At illness onset, prefrontal hypoactivity and hippocampal and subcortical hyperactivity represent a core illness pathophysiology (Gong et al. 2015). Over the course of the illness, changes in the brain progress and differ from the brain deficits at the onset of the illness, which has correlations with clinical symptoms (Gong et al. 2015). It is commonly seen in drug-free patients with schizophrenia that the functions and anatomic brain abnormalities overlapped in the default mode (DMN) and auditory networks (AN) however, the pattern of changes differed between the two (Gao et al. 2017). Decreased grey matter was associated with decreased activation within the DMN, whereas it was associated with increased activation within the AN (Gao et al. 2017). Changes in grey matter have been most pronounced in the thalamo-cortical networks, whereas altered brain activity has been seen in fronto-parietal and default-mode networks (Gong et al. 2015). White and grey matter volumes are found to be 5-6% smaller in individuals with schizophrenia (Sigmundsson et al. 2001). Similarly, to the grey matter changes, white matter changes are present by the time of the first episode and are an indicator that the subject is at risk for the disorder (Karlsgodt et al. 2010).
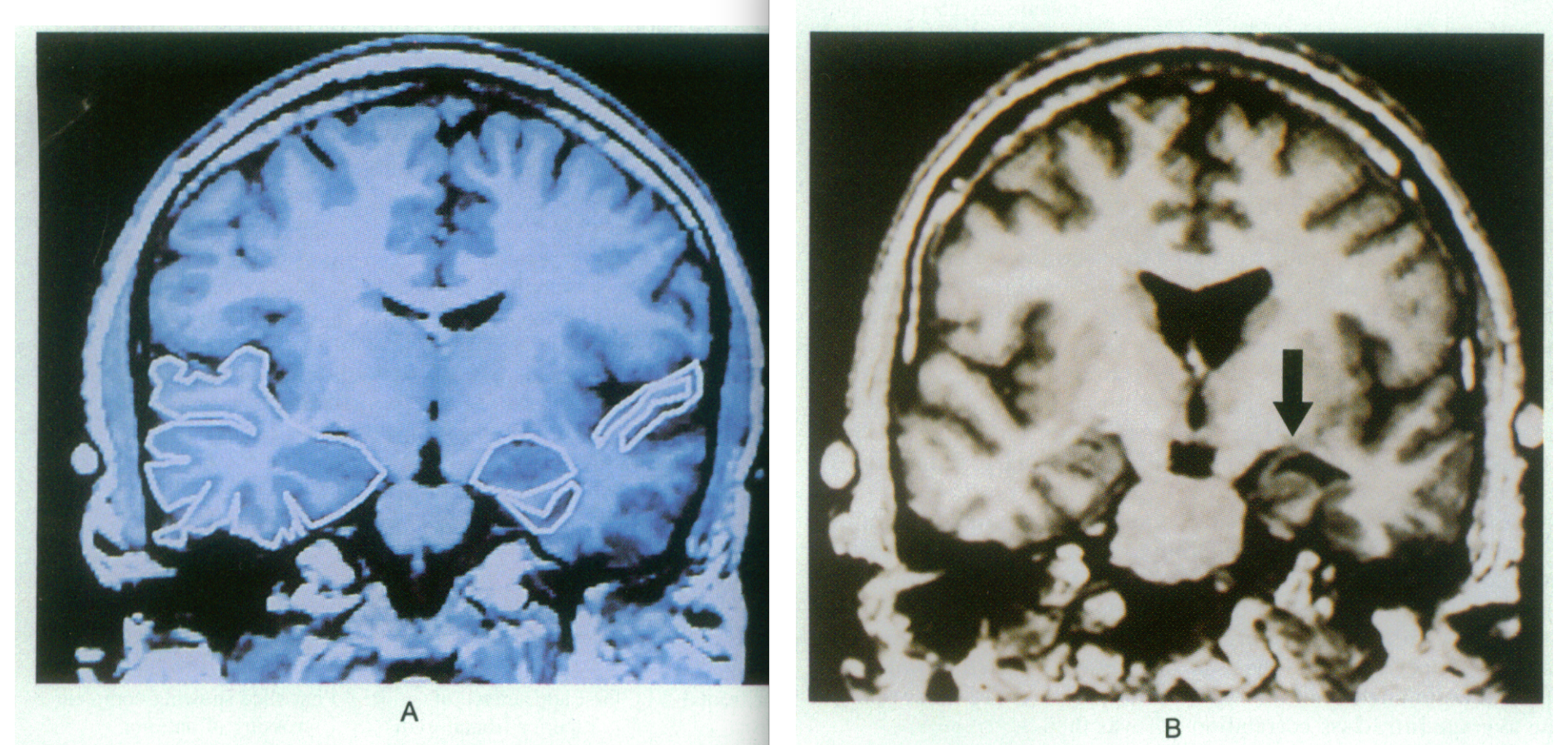
Figure 4: Coronal slide of the temporal lobe in control (A) and one of a patient with schizophrenia (B). Regions of interest in A include the grey matter of the superior temporal gyrus (right) as well as the amygdala- hippocampal complex (medial) which is almond- shaped. In Image B, the cerebrospinal fluid (CSF) surrounding the left superior temporal gyrus has increased compared to the control. Arrow points to tissue loss in the parahippocampal gyrus and increase in the temporal horn surrounding the amygdala- hippocampal complex. Retrieved from : http://www.nejm.org/doi/pdf/10.1056/NEJM199208273270905 Abnormalities of the left temporal lobe and thought disorder in schizophrenia
Neurotransmitter System Abnormalities
Currently, it is a common belief that both positive and negative symptoms are associated with neurotransmitter imbalances in patients’ brain (Frankenburg, 2018). Neurotransmitters are chemicals in the brain that are responsible for communication within the brain (Snyder, 2018). The neurotransmitters that may be imbalanced include: dopamine, serotonin, GABA and glutamate. In schizophrenic patients, dopamine is thought to be in excess in the brain, and as a result antipsychotic drugs are the gold standard medication to lower levels. Breaking down symptoms, positive symptoms are usually attributed to excess dopamine activity in the mesolimbic system (midbrain to the nucleus accumbens). Negative symptoms on the other hand are attributed to low dopamine activity in the mesocortical system (Ventral tegmentum to prefrontal cortex). Lastly, it is thought that lower glutamate levels are also found in schizophrenic patients, which produce psychotic symptoms (Frankenburg, 2018).
Inflammation and Immune Function
Schizophrenia has been found to have many immunological effects. Observations have been made of a diffuse non-specific overactivation of the immunological response system, of a T-helper cell type 1 immune activation and of a T-helper cell type 2 immune activation in subgroups of schizophrenia patients (Strous et al. 2006). Cytokines play an important role in infection and inflammation and are crucial mediators between the brain and the immune system (Ptovin et al. 2008). An imbalance in inflammatory cytokines is common in individuals with schizophrenia leading to a decrease in Th1 and an increase in Th2 cytokine secretion (Ptovin et al. 2008). The cytokines exhibit a number of critical and overlapping regulating functions in the immunological system and interact in a highly complex fashion with a range of neurotransmitters including; increased levels of interleukin (IL) 2 and soluble IL-2 receptor, increased cerebrospinal fluid levels IL-4, increased levels of IL-6 and soluble IL-6 receptor, increased levels of IL-10, increased levels on tumor necrosis factor alpha, as well as decreased mitogen stimulated levels of interferon-y (Strous et al. 2006).
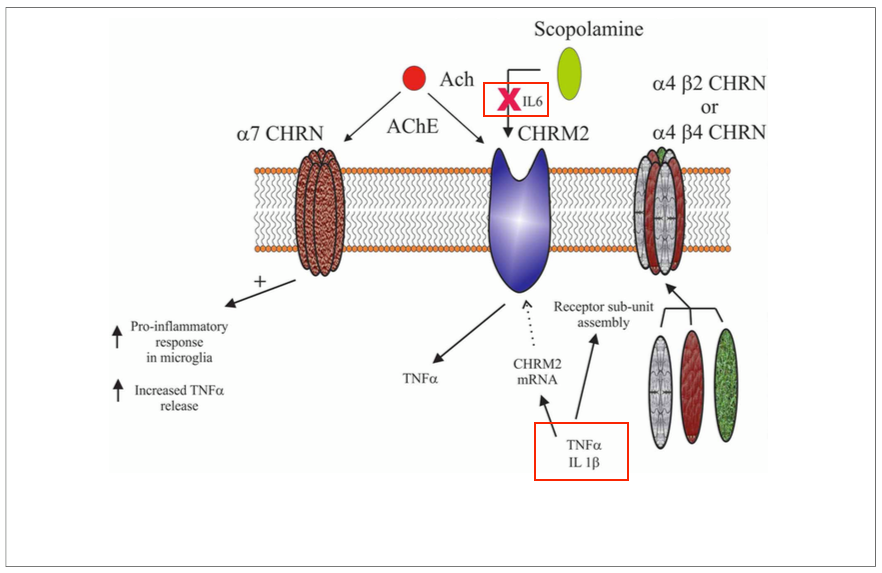
There are three prominent lines in the overactivation of the immune system: 1. Elevated serum levels of the pleiotropic cytokine IL-6, released from astrocytes, lymphocytes, and macrophages; 2. Increased levels of serum IL-2 and soluble IL-2 receptor (sIL-2) levels have been commonly noted in the illness (Gaughran et al. 2998); 3. Decreased mitogen-stimulated IL-2 production by overloaded activated lymphocytes (Muller et al. 1999 & Ginestet et al. 19890 (Strous et al. 2006). The elevated levels of pro-inflammatory cytokines activate the kynurenine pathway, by which tryptophan is metabolized into kynurenic and quinolinic acids; these acids regulate NMDA receptor activity and may be involved in dopamine regulation (Strous et al. 2006). The kynurenine pathway, when dysfunctional, is commonly associated with a number of disorders one being schizophrenia. Dysregulation of molecules in individuals with schizophrenia is associated with inflammation/immune responses and centered around disrupted interactions between the central cholinergic system (Deng et al. 2013).
Treatments
Unfortunately today there are no treatments for Progeria. Physicians and healthcare providers’ main goals right now are to delay or reduce symptoms. This can be achieved by: small doses of aspirin, preventative medications, physical and occupational therapy, nutrition and dental care. Common with Progeria, small doses of Aspirin attempt to reduce the risk of heart attacks and strokes. Preventative medications that can help lower cholesterol, blood pressure, and reduce the chance of getting a blood clot can all be prescribed if the individual seems to be at risk. Physical and occupational therapy can help an individual maintain healthy movement capability and nutrition and dental care for being overall healthy (Mayo Clinic, 2018). Progeria is commonly screened phenotypically or through medical history at the physician’s office. A genetic test for the LMNA mutations can be ordered if the physician deems this appropriate (Sinha, Raghunath & Ghosh, 2018).
For potential future treatments, there are many different angles of approach. Genetics currently is a big area of research for this disease. Anything from early detection capability, to actual cures, genetics is a significant field to target. Additionally, there is also a lot of interest in reducing the severity of symptoms, common with individuals with Progeria. First, heart and blood vessel disease is a target of interest. Farnesyltransferase inhibitors (FTIs), which are drugs for treating cancer, are being investigated to whether they can help vasodilate blood vessels and reduce weight gain (Mayo Clinic, 2018). In 2012, 25 children with Progeria underwent a clinical trial that showed these results (Gordon et al., 2012). FTIs also have shown in mouse models to improve nuclear shape and reduce the negative effects of built up prelamin A. Lonafarnib, an FTI, certainly gives confidence for developing a potential cure to Progeria (Sinha, Raghunath & Ghosh, 2018).
Conclusion
With the description of the history, diagnosis, symptoms, risks, pathophysiology, etiology, epidemiology, and treatments there are clear outlines of progeria however further research is required. The future implications of progeria details a focus on increasing the lifespan of diagnosed individuals. Although there are not specific treatments available, the future developments are promising. With the use of models and genetics, the advancements to create a clinical trial are near (Swahari and Nakamura, 2016).
References
Capell, B., Erdos, M., Madigan, J., Fiordalisi, J., Varga, R., & Conneely, K. et al. (2018). Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. PNAS. Retrieved 24 February 2018, from http://www.pnas.org/content/102/36/12879
Coppedè, F. (2013). The epidemiology of premature aging and associated comorbidities. Clinical interventions in aging, 8, 1023. DeBusk, F. L. (1972). The Hutchinson-Gilford progeria syndrome: report of 4 cases and review of the literature. The Journal of pediatrics, 80(4), 697-724.
De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., … & Lévy, N. (2003). Lamin a truncation in Hutchinson-Gilford progeria. Science, 300(5628), 2055-2055. Goldman, R., Shumaker, D., Erdos, M., Eriksson, M., Goldman, A., & Gordon, L. et al. (2004). Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proceedings Of The National Academy Of Sciences, 101(24), 8963-8968. Gonzalez, J. (2011). A-type lamins and Hutchinson-Gilford progeria syndrome: pathogenesis and therapy. Frontiers In Bioscience, S3(1), 1133.
Gonzalo, S., & Kreienkamp, R. (2015). DNA repair defects and genome instability in Hutchinson–Gilford Progeria Syndrome. Current Opinion In Cell Biology, 34, 75-83.
Gordon, L., Kleinman, M., Miller, D., Neuberg, D., Giobbie-Hurder, A., & Gerhard-Herman, M. et al. (2012). Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proceedings Of The National Academy Of Sciences, 109(41), 16666-16671. http://dx.doi.org/10.1073/pnas.1202529109
Kashyap, S., Shanker, V., & Sharma, N. (2014). Hutchinson–Gilford progeria syndrome: A rare case report. Indian dermatology online journal, 5(4), 478. Pollex, R., & Hegele, R. (2004). Hutchinson-Gilford progeria syndrome. Clinical Genetics, 66(5), 375-381.
Progeria - Symptoms and causes. (2018). Mayo Clinic. Retrieved 14 February 2018, from https://www.mayoclinic.org/diseases-conditions/progeria/symptoms-causes/syc-20356038
Progeria 101/FAQ. (2018). The Progeria Research Foundation. Retrieved 14 February 2018, from https://www.progeriaresearch.org/progeria-101faq/
Rastogi, R., & Mohan, S. C. (2008). Progeria syndrome: a case report. Indian journal of orthopaedics, 42(1), 97. Sangita Devi, A., Thokchom, S., & Mamata Devi, A. (2017). Children Living with Progeria. Nursing & Care Open Access Journal, 3(4). http://dx.doi.org/10.15406/ncoaj.2017.03.00077 Sarkar, P. K., & Shinton, R. A. (2001). Hutchinson-Guilford progeria syndrome. Postgraduate medical journal, 77(907), 312-317.
Sinha, J., Raghunath, M., & Ghosh, S. (2018). Progeria: A rare genetic premature ageing disorder. PubMed Central (PMC). Retrieved 14 February 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4140030/
Swahari, V., & Nakamura, A. (2016). Speeding up the clock: The past, present and future of progeria. Development, growth & differentiation, 58(1), 116-130.