Table of Contents
Progeria
Overview
Introduction
Hutchinson- Gilford Progeria syndrome, also known as Progeria, is a rare fatal genetic disease affecting 1 in 4 million babies globally. As of 2014, there were 118 confirmed cases of progeria globally. It is responsible for accelerated aging throughout their childhood, commencing at age 1, resulting in scleroderma, reduced joint mobility, hair loss and more (Progeria101/FAQ, 2018). These symptoms will appear at age 1, The disease progresses atherosclerosis which raises the probability of a heart attack or stroke (Genetics Home Reference, 2018).
History
Progeria is an extremely rare genetic disease of childhood characterized by dramatic, premature aging with death occurring on average at the age of 13, usually from heart attack or stroke (Kashyap et al., 2014). Hutchinson-Gilford progeria syndrome (HGPS) is the most severe form of the disease and the classic type. The disease was named after the doctors who first described it in England; in 1886 by Dr. Jonathan Hutchinson and in 1897 by Dr. Hastings Gilford. The term progeria is derived from the Greek work geras, meaning old age (DeBusk, 1972).
In 1886, the syndrome was first reported by Hutchinson of a 6-year-old boy whose overall appearance was that of an old man. Hutchinson described the case as “congenital absence of hair and its appendages” (DeBusk, 1972). It was a year later that Gilford described a second patient with similar clinical findings. To date, there are only 100 patients with HGPS that have been described in literature (Kashyap et al., 2014). These two boys were further described in 1897 and 1904 by Gildford, who was the one to proposal the term “progeria” and described the post-mortem characteristics (DeBusk, 1971). Little research was done on the disease until the 1990’s due to the rarity of the disease, causing it to be frequently diagnosed erroneously in patients with some of the features such as alopecia and skin of aged appearance (DeBusk, 1972). However, there are three features present in early life; mid-facial cyanosis, skin resembling scleroderma, and glyphic nasal tip, which all facilitate an early diagnosis of HGPS (DeBusk, 1972).
Epidemiology
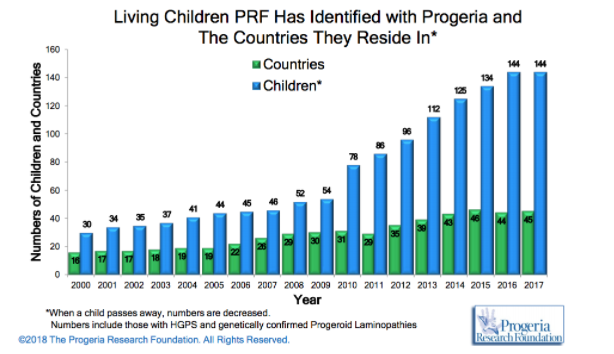
HPGS is an extremely rare genetic disorder affecting about 1 in 4 million live births, if unreported or misdiagnosed cases are taken into account. The reported prevalence rate of the disease is 1 in 8 million births, based on the number of cases (Coppedè, 2013). According to the Progeria Research Foundation database, there are an estimated 350-400 children living with progeria worldwide at any one time. As of January 2018, there are a recorded 114 children living with progeria worldwide with numbers steadily increasing as the years go by (http://www.progeriaresearch.org/prf-by-the-numbersprf.html).
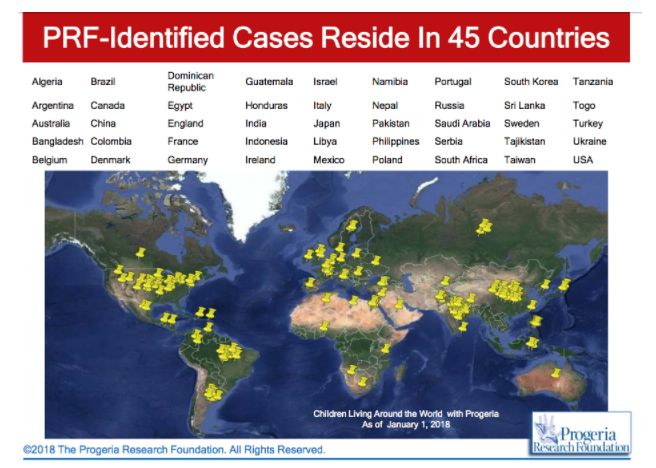
HPGS affects all races; cases of progeria have been discovered in 45 different countries. However, 97% of affected patients are white. Males are affected 1 ½ times more often than females. The disease was thought to be autosomal recessive in the past, however observations made an autosomal recessive inheritance very unlikely and favour a sporadic, dominant mutation. The mutation results in life spans for progeria syndrome to be in the second/third decades of life, with the majority of patients dying of cardiovascular or cerebrovascular disease between 7 and 27 years of age (Sarkar and Shinton, 2001).
Etiology
The cause of Progeria is known to be mutations of the Lamin A, or LMNA gene. These mutations have been found to be sporadic and new, rather than being inherited by their parents. In the sporadic cases leading to Progeria, an autosomal dominant mutation takes place. However, this does not occur when Progeria is inherited and is known as Werner’s syndrome (Sinha, Raghunath & Ghosh, 2018). What is known is the fact that the LMNA gene is responsible for creating a protein which helps keep a nuclei intact. Unfortunately, in individuals with Progeria, this does not occur as the LMNA is abnormal. Abnormal LMNA proteins, or Progerin, accumulates in the body and thus creates instability in cells. As a result, this leads to the abnormal aging process of children (Mayo Clinic, 2018). Interestingly, the LMNA mutations being the cause of Progeria was discovered in April 2003, and have claimed it has significant implications on treating the disease, aging, and cardiovascular disease as well (Progeria101/FAQ, 2018).
Diagnosis/Symptoms
The only way to diagnose is once the symptoms become visible. There are no early diagnostic techniques.
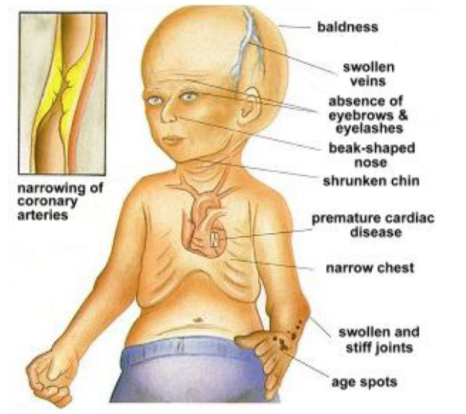
HPGS is a genetic condition that can later be characterized by a rapid appearance of aging that begins in childhood. An affected child will usually look normal at birth and even early infancy but will usually grow more slowly than other children and will not gain weight at the expected rate of a normal child . There is a distinctive appearance that is developed in affected children (De Sandre-Giovannoli et al, 2003). Usually what is seen is baldness, aged-looking skin, a pinched nose, and a small face and jaw in relation to head size. In addition to this they will usually suffer from symptoms that are usually seen in older individuals such as stiffness of joints, hip dislocations and severe cardiovascular disease (De Sandre-Giovannoli et al, 2003). They experience severe hardening of the arteries that begin in early childhood and put individuals at risk of having a heart attack or a stroke at an early age. The condition does not have and affect on intellectual development or the development of motor skills. As of now clinical professional look for these physical symptoms in early childhood in order to make a proper diagnosis (De Sandre-Giovannoli et al, 2003).
A mutation in the LMNA gene is the cause of HPGS. The LMNA gene makes a protein called Lamin A. This protein determines the shape of the nucleus within cells. It is a supporting component of the nuclear envelope (De Sandre-Giovannoli et al, 2003). A mutation in this gene causes an abnormal version of the lamin A protein. That being said there is still continuing research going on to understand how this impact on the nucleus leads to the characteristics that are seen in HPGS (De Sandre-Giovannoli et al, 2003).
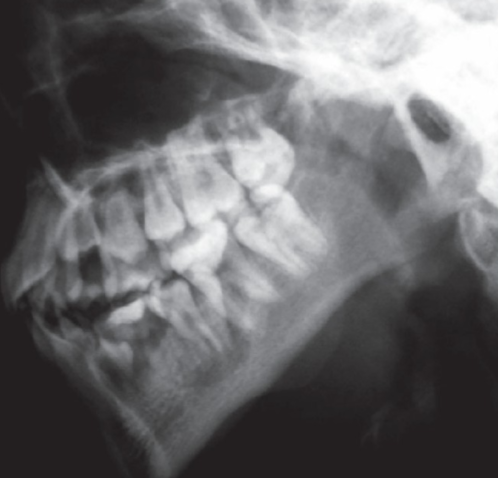
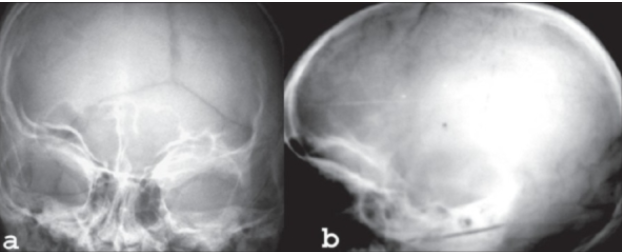
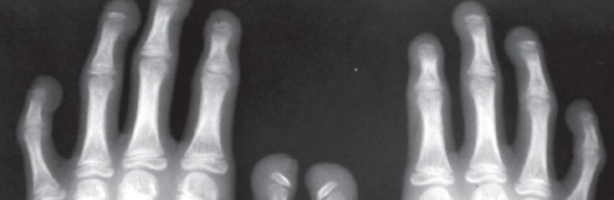
CASE (X-Ray) of individual with HPGS.
Pathophysiology
Background
The nuclear lamina is a filamentous protein layer that provides mechanical support to the inner nuclear membrane (Gonzalez, 2011). The functions of the nuclear lamina include nuclear positioning, chromatin organization, nuclear pore complex organization, nuclear envelope breakdown and reassembly during mitosis, DNA replication, DNA damage response and cell cycle progression, transcriptional control and apoptosis (Gonzalez, 2011). The main components of the nuclear lamina are type V intermediate filaments known as lamins that contain a central α- helical rod surrounded by globular N and C terminal domains; the C terminal region contains the nuclear localization sequences (Gonzalez, 2011). As proteins they form coiled- coil dimers that can associate head to tail. These protofilaments then create the final lamin filaments. Lamins can be classified into two types: A- type and B- type. A -type lamins are basic and type B is acidic. Type A are encoded by the LMNA gene with its two isoforms being Lamin A and C. B type lamins are therefore encoded by the LMNB1 and LMNB2 genes (Gonzalez, 2011). Lamin A is affected in Progeria so an understanding of the normal transcriptional and translational mechanisms of this protein is essential (Gonzalez, 2011). In cells containing the normal LMNA gene, prelamin A undergoes post- translational modifications before it is found in its mature Lamin A form. Firstly, the cysteine in the C – terminal CaaX motif is farnesylated by farnesyltransferase. Rce1, an endoprotease then cleaves the three terminal amino acids. Then the newly- available cysteine is then methylated by carboxyl methyltransferase, ICMT. Lastly to create mature Lamin A, 15 C- terminal residues that include the farnesylated and carbosymethylated C- terminal cysteine are cleaved by another endoprotease, Zmpste24/ FACE-1 (Gonzalez, 2011).
Genetics
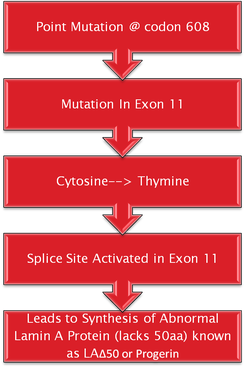
Hutchinson- Gilford Progeria syndrome is commonly caused by a single de novo silent mutation in codon 608 of the LMNA gene (Gonzalez, 2011). The mutation in HGPS is a nucleotide substitution from cytosine to thymine at position 1824 (Goldman et al., 2004). This substitution causes for the partial activation of a cryptic splice site (Goldman et al., 2004). It causes deletion of 150 nucleotides in exon 11 (Goldman et al., 2004). This causes for a 50 amino acid deletion near the C- terminus of the protein, which includes the Zmpste24 cleavage site (Pollex & Hegele 2004). This causes for a mutant prelamin A to remain farnesylated throughout the lifespan of the protein (Pollex & Hegele, 2004). The mutant protein that results from this is known as progerin (Pollex & Hegele, 2004). Around 80% of HGPS cases contain this mutation that is known as the LAΔ50 mutation. Evidence has suggested that transfection of progerin or a non-cleavable form of prelamin A causes for nuclear abnormalities (Pollex & Hegele, 2004). During mitosis, the abnormal association of progerin causes a delay in the onset and progression of cytokinesis and impairs the targeting of lamina components to the nucleus of daughter cells (Gonzalez, 2011). In addition, it can change the entry of the cell into S- phase mediated by hyperphosphorylation of the retinoblastoma gene product (pRB) by cyclin D1/cdk4 (Gonzalez, 2011). Progerin accumulation also causes abnormal chromosome segregation and binucleation (Gonzalez, 2011). It also promotes DNA- damage, changes in DNA repair, causes for genomic instability as well as interfering with nuclear architecture. This leads to premature cell death (Gonzalez, 2011).
Additional heterozygous mutations for atypical Progeria patients have also been revealed. R644C affects the C- terminus, E578V also in the C- terminus and T10I within the N- terminal globular domain seen in Seip syndrome (Pollex & Hegele, 2004). The most common hypothesis for the inheritance of this disorder is sporadic autosomal dominant (Pollex & Hegele, 2004).
Genomic Instability
Hutchinson- Gilford Progeria syndrome is commonly caused by a single de novo silent mutation in codon 608 of the LMNA gene (Gonzalez, 2011). The mutation in HGPS is a nucleotide substitution from cytosine to thymine at position 1824 (Goldman et al., 2004). This substitution causes for the partial activation of a cryptic splice site (Goldman et al., 2004). It causes deletion of 150 nucleotides in exon 11 (Goldman et al., 2004). This causes for a 50 amino acid deletion near the C- terminus of the protein, which includes the Zmpste24 cleavage site (Pollex & Hegele 2004). This causes for a mutant prelamin A to remain farnesylated throughout the lifespan of the protein (Pollex & Hegele, 2004). The mutant protein that results from this is known as progerin (Pollex & Hegele, 2004). Around 80% of HGPS cases contain this mutation that is known as the LAΔ50 mutation. Evidence has suggested that transfection of progerin or a non-cleavable form of prelamin A causes for nuclear abnormalities (Pollex & Hegele, 2004). During mitosis, the abnormal association of progerin causes a delay in the onset and progression of cytokinesis and impairs the targeting of lamina components to the nucleus of daughter cells (Gonzalez, 2011). In addition, it can change the entry of the cell into S- phase mediated by hyperphosphorylation of the retinoblastoma gene product (pRB) by cyclin D1/cdk4 (Gonzalez, 2011). Progerin accumulation also causes abnormal chromosome segregation and binucleation (Gonzalez, 2011). It also promotes DNA- damage, changes in DNA repair, causes for genomic instability as well as interfering with nuclear architecture. This leads to premature cell death (Gonzalez, 2011).
Additional heterozygous mutations for atypical Progeria patients have also been revealed. R644C affects the C- terminus, E578V also in the C- terminus and T10I within the N- terminal globular domain seen in Seip syndrome (Pollex & Hegele, 2004). The most common hypothesis for the inheritance of this disorder is sporadic autosomal dominant (Pollex & Hegele, 2004).
Current Treatments
Unfortunately today there are no treatments for Progeria. Physicians and healthcare providers’ main goals right now are to delay or reduce symptoms. This can be achieved by: small doses of aspirin, preventative medications, physical and occupational therapy, nutrition and dental care. Common with Progeria, small doses of Aspirin attempt to reduce the risk of heart attacks and strokes. Preventative medications that can help lower cholesterol, blood pressure, and reduce the chance of getting a blood clot can all be prescribed if the individual seems to be at risk. Physical and occupational therapy can help an individual maintain healthy movement capability and nutrition and dental care for being overall healthy (Mayo Clinic, 2018). Progeria is commonly screened phenotypically or through medical history at the physician’s office. A genetic test for the LMNA mutations can be ordered if the physician deems this appropriate (Sinha, Raghunath & Ghosh, 2018).
For potential future treatments, there are many different angles of approach. Genetics currently is a big area of research for this disease. Anything from early detection capability, to actual cures, genetics is a significant field to target. Additionally, there is also a lot of interest in reducing the severity of symptoms, common with individuals with Progeria. First, heart and blood vessel disease is a target of interest. Farnesyltransferase inhibitors (FTIs), which are drugs for treating cancer, are being investigated to whether they can help vasodilate blood vessels and reduce weight gain (Mayo Clinic, 2018). In 2012, 25 children with Progeria underwent a clinical trial that showed these results (Gordon et al., 2012). FTIs also have shown in mouse models to improve nuclear shape and reduce the negative effects of built up prelamin A. Lonafarnib, an FTI, certainly gives confidence for developing a potential cure to Progeria (Sinha, Raghunath & Ghosh, 2018). (a is Progerin cell, d is a healthy cell - treated with FTI - Capell reference)
Conclusion
With the description of the history, diagnosis, symptoms, risks, pathophysiology, etiology, epidemiology, and treatments there are clear outlines of progeria however further research is required. The future implications of progeria details a focus on increasing the lifespan of diagnosed individuals. Although there are not specific treatments available, the future developments are promising. With the use of models and genetics, the advancements to create a clinical trial are near (Swahari and Nakamura, 2016).
References
Capell, B., Erdos, M., Madigan, J., Fiordalisi, J., Varga, R., & Conneely, K. et al. (2018). Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. PNAS. Retrieved 24 February 2018, from http://www.pnas.org/content/102/36/12879
Coppedè, F. (2013). The epidemiology of premature aging and associated comorbidities. Clinical interventions in aging, 8, 1023. DeBusk, F. L. (1972). The Hutchinson-Gilford progeria syndrome: report of 4 cases and review of the literature. The Journal of pediatrics, 80(4), 697-724.
De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., … & Lévy, N. (2003). Lamin a truncation in Hutchinson-Gilford progeria. Science, 300(5628), 2055-2055.
Goldman, R., Shumaker, D., Erdos, M., Eriksson, M., Goldman, A., & Gordon, L. et al. (2004). Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proceedings Of The National Academy Of Sciences, 101(24), 8963-8968.
Gonzalez, J. (2011). A-type lamins and Hutchinson-Gilford progeria syndrome: pathogenesis and therapy. Frontiers In Bioscience, S3(1), 1133.
Gonzalo, S., & Kreienkamp, R. (2015). DNA repair defects and genome instability in Hutchinson–Gilford Progeria Syndrome. Current Opinion In Cell Biology, 34, 75-83.
Gordon, L., Kleinman, M., Miller, D., Neuberg, D., Giobbie-Hurder, A., & Gerhard-Herman, M. et al. (2012). Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proceedings Of The National Academy Of Sciences, 109(41), 16666-16671. http://dx.doi.org/10.1073/pnas.1202529109
Kashyap, S., Shanker, V., & Sharma, N. (2014). Hutchinson–Gilford progeria syndrome: A rare case report. Indian dermatology online journal, 5(4), 478. Pollex, R., & Hegele, R. (2004). Hutchinson-Gilford progeria syndrome. Clinical Genetics, 66(5), 375-381.
Progeria - Symptoms and causes. (2018). Mayo Clinic. Retrieved 14 February 2018, from https://www.mayoclinic.org/diseases-conditions/progeria/symptoms-causes/syc-20356038
Progeria 101/FAQ. (2018). The Progeria Research Foundation. Retrieved 14 February 2018, from https://www.progeriaresearch.org/progeria-101faq/
Rastogi, R., & Mohan, S. C. (2008). Progeria syndrome: a case report. Indian journal of orthopaedics, 42(1), 97.
Sangita Devi, A., Thokchom, S., & Mamata Devi, A. (2017). Children Living with Progeria. Nursing & Care Open Access Journal, 3(4). http://dx.doi.org/10.15406/ncoaj.2017.03.00077
Sarkar, P. K., & Shinton, R. A. (2001). Hutchinson-Guilford progeria syndrome. Postgraduate medical journal, 77(907), 312-317.
Sinha, J., Raghunath, M., & Ghosh, S. (2018). Progeria: A rare genetic premature ageing disorder. PubMed Central (PMC). Retrieved 14 February 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4140030/
Swahari, V., & Nakamura, A. (2016). Speeding up the clock: The past, present and future of progeria. Development, growth & differentiation, 58(1), 116-130.