This is an old revision of the document!
Table of Contents
High Protein Diets and Kidney Function
<style justify> Multiple Sclerosis (MS) is an autoimmune disease affecting the central nervous system. The disease specifically affects the myelin and as a result inflammation occurs and the myelin is damaged. Myelin performs the crucial role of nerve impulse through nerve fibers. If myelin is damaged then there will be a decrease in the transmission of nerve impulses. When the damage is significant then scar tissue will replace the myelin. The symptoms of MS vary for each individual, some include fatigue, pain, bladder problems, walking problems, cognitive difficulties and optic neuritis. Canada currently has the highest rate of MS with approximately 100,000 Canadians currently diagnosed (Multiple Sclerosis Society of Canada, 2016). </style>
<br> <br> <br> <br> <br> <br>
Animal studies suggest correlation between high protein intake and kidney damage
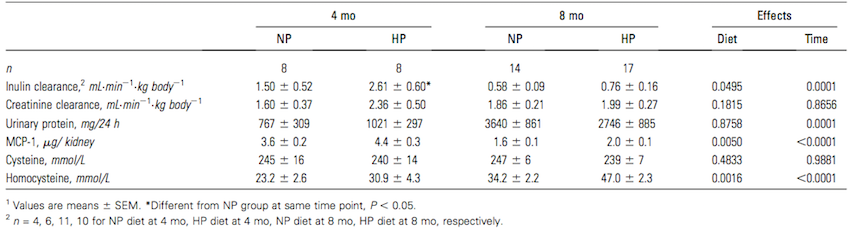
Figure 1: Renal function, MCP-1, and plasma homocysteine in pigs that consumed NP or HP diet for 4 or 8 months (Source: Jia et al, 2010) </style>
<style justify> Research has been conducted on animals, supporting the claim that high protein diets can lead to kidney damage.
<br>
Jia and colleagues (2010) tested the effects of a high protein diet on adult pigs. Pigs have very similar renal anatomy and physiology to that of humans, and thus are an appropriate test animal to use (Jia et al., 2010). Adult pigs were fed a diet consisting of either 15% of total calories from protein or 35% of total calories from protein for eight months. Protein came from a mixture of both plant and animal sources to closely resemble an average human diet. Between both the high and normal protein diets, lipid, fibre and micronutrient compositions were made equal to eliminate the presence of any confounding variables from diet. (Jia et al., 2010) After eight months, adult pigs fed the high protein diet had significantly higher kidney and glomerular volumes, however there was no significant difference in body mass between the high protein and normal protein groups. Additionally, after eight months, pigs fed the high protein diet exhibited more histological renal damage with 55% more fibrosis, and 30% more glomerulosclerosis in the kidney. There were also higher plasma homocysteine levels at 4 and 8 months. However, overall renal function as determined by inulin clearance, creatine clearance, or proteinuria was not affected by a high protein diet, as any increase in values at 4 months did not persist in the long term and differences overall were not statistically significant (Figure 1). Due to the similarity between pig and human renal physiology, this study presents evidence that suggests high protein diets may potentially be harmful to human renal function. (Jia et al., 2010) </style>
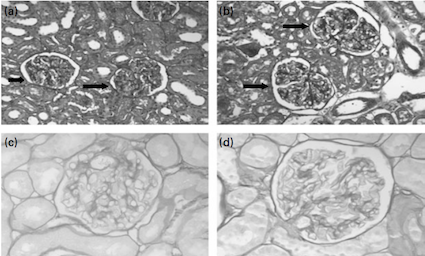
Figure 2: Glomeruli from rats given a) normal-protein or b) high protein diet
for 4 months; Glomeruli from rats given c) NP or d) HP diet for 17 months
(Source: Wakefield et al., 2011) </style>
<br>
<style justify> Wakefield and colleagues (2011) studied the effects of a high protein diet on Sprague-Dawley rats to investigate if the findings of Jia and colleagues’ (2010) pig model were species specific or more generalizable. 70 day old female Sprague-Dawley rats were randomized to either a normal-protein or a high protein diet for 4, 8, 12 and 17 months. The normal-protein diet consisted of 15% of calories from protein and the high protein diet consisted of 35% of calories from protein. The two diets were kept similar with regards to caloric content, lipids, fibre and micronutrient content. (Wakefield et al., 2011) The rats fed with a high protein diet displayed concerning kidney adaptations. Rats fed on a high protein diet had larger kidneys and glomeruli, and exhibited 33% more glomerulosclerosis compared to the rats fed a normal-protein diet (In Figure 2, d) shows a hardened glomerulus, compare to c)). This finding is consistent with the histological damage shown in Jia and colleagues' (2010) study with adult pigs. Rats fed high protein diets exhibited altered renal function with a 27% higher creatinine clearance. Creatinine clearance is a marker of glomerular filtration rate (GFR) and proteinuria, both of which were significantly elevated in the rats fed on the high protein diet. These findings contrast to Jia and colleagues' (2010) experiment where renal function in adult pigs was not affected. The evidence presented in this study suggests that in rats, high protein diets are associated with kidney damage, consistent to Jia and colleagues (2010) study with pigs, however this relationship cannot conclusively be generalized to humans. (Wakefield et al., 2011) </style>
<br> <br> <br> <br>
Short and long-term effects of high protein diets
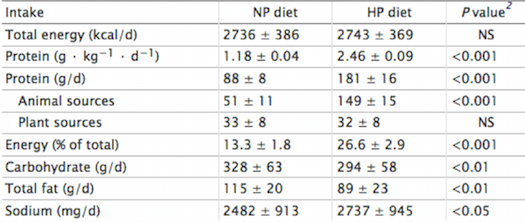
Figure 3: Total energy and macronutrient intakes during a 7-d normal-protein (NP) and high-
protein (HP) diet in 24 healthy normal-weight men </style>
<style justify> Frank and colleagues (2009) Investigated the effects of a short-term high protein diet on healthy kidney functions, such as renal hemodynamics and clinical-chemical factors. Young men were assigned to either a high protein diet containing 2.4 g/ kg•d or a normal protein diet containing 1.2 g/kg•d for a duration of seven days. The filtration fraction was taken from the 24 subjects on day seven. Researchers found that renal-dynamics were altered, with a significant rise in GFR (Glomerular filtration rate) and the filtration fraction. However, adaptive changes in renal function might be caused by sodium intake, which is significantly parallel to the high protein diet. Importantly, sodium is known for its possible effects on GFR through tubular reabsorption. This study suggests that further research should be conducted to analyze the effects of chronic high protein intake on GFR, while controlling for sodium. </style>
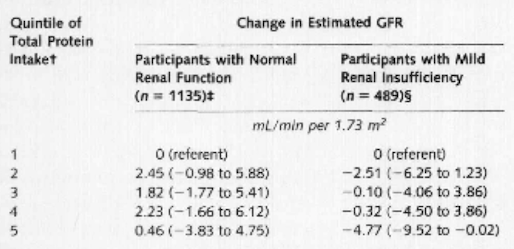
Figure 4: Multivariate linear regression results for change in estimated glomerular filtration
rate according to quintile of total protein intake </style>
<br> <br> <br> <br> <br> <br> <br> <br>
<style justify> Knight and colleagues (2003) A long-term study where protein intake had an impact on the change in rate of renal function in women over an 11 year period was examined. Over 1500 women between the ages of 42 and 68 enrolled in the Nurses’ Health study and gave blood samples, which measured creatinine levels. As protein intake increased, the change in estimated GFR became more pronounced as well as the confidence interval (1.14 mL/min per 1.73 m2 [CI, −3.63 to 5.92 mL/min per 1.73 m2]). Through multivariate linear regression analyses, it was shown that there was no significant relation between high protein intake and change in glomerular filtration rate in women with normal renal function. However, high intake of non-dairy animal protein might be linked with accelerated renal function decline in women with mild renal insufficiency. </style>
<br> <br> <br> <br>
High protein intake and at risk individuals
<style justify> According to the National Kidney Foundation (2015), a healthy GFR is approximately 90mL/min/1.73m2, while glomerular hyperfiltration results when GFR ranges from 125mL/min/1.73m2 to 175mL/min/1.73m2 (Helal, Fick-Brosnahan, Reed-Gitomer and Schrier, 2012). Glomerular hyperfiltration leads certain individuals with diabetes or hypertension to an increased risk of developing kidney disease. This can ultimately which can cause a decrease in the GFR (National Kidney Foundation, 2015). </style>
<br>
<style justify> Jesudason and colleagues (2013) They conducted a study to examine the effects of a high protein diet on weight loss in individuals with type 2 diabetes mellitus and early kidney disease. Approximately, forty-five individuals were selected for the study. These individuals were diagnosed with Type 2 Diabetes and showed symptoms of early Kidney Disease. They were then assigned to either a moderate protein diet (30% of total calories from protein) or a standard protein diet (20% of total calories from protein) for a duration of one year. Both groups demonstrated weight loss, however there was no significant difference in the weight loss between the two groups. The results demonstrated that weight loss was associated with changes to GFR. From Figure 5, it can be concluded that individuals with a normal baseline GFR (< 120mL/min/1.73m2) displayed an increase in GFR, while individuals with hyperfiltration experienced a decline in the GFR. Results were similar between both groups indicating that long-term diets that consisted of high proteins are not necessarily associated with hyperfiltration (Jesudason, Pedersen and Clifton, 2013). </style>
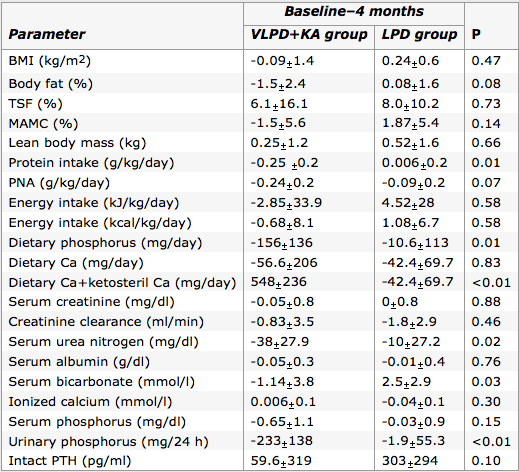
Figure 6: Comparison of the changes through 4 month period in VLPD+KA group with LPD
group on various parameters. (Source: Feiten et al., 2005) </style>
<style justify> At-risk individuals have been recommended to refrain from eating high protein diets (Schwingshackl and Hoffman, 2014), due to the presumed associated increase in GFR and the concept of increased GFR leading to higher risk of developing Kidney Disease in individuals with Diabetes (National Kidney Foundation, 2015). However, the research suggests that high protein diets associated with weight loss may be beneficial to individuals with Type 2 Diabetes and early stage Kidney Disease, since such diets appeared to normalize GFR in some individuals. Controversy exists regarding the association of hyperfiltration and Kidney Disease. It is suggested that an increase of GFR in the absence of renal hypertension is not associated with kidney pathology (Helal, Fick-Brosnahan, Reed-Gitomer and Schrier, 2012). Therefore, in order to demonstrate that high protein diets are associated with kidney damage, research would need to support that such diets result in increased GFR and increased renal blood pressure. </style>
Figure 5: Demonstrates the baseline and end GFR values of both moderate and standard
protein diet groups after 1 year period. (Source: Jesudason, Pedersen and Clifton, 2013 ) </style>
<br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br>
<style justify> Feiten and colleagues (2004) It is important to note when the use of a Very Low Protein Diet would be more beneficial to an individual than a high protein diet. This is usually the case with individuals afflicted with chronic kidney disease (CKD). This study challenged the normal treatment of a Low Protein Diet (0.6g/kg/day) with that of a Very Low Protein Diet (0.3g/kg/day) supplemented with ketoacids. They had two groups of 12 subjects with one group undergoing the Low Protein Diet and the other with a Very Low Protein Diet & ketoacids supplementation for four months. The results highlighted in Figure 6, were able to conclude that the Very Low Protein Diet & ketoacid group had the same nutritional standing as the other group, however had better results with phosphorus and calcium metabolism while also leading to lower serum urea nitrogen levels. These results are indicative of an effective therapy for advanced CKD patients. It is important to recognize when a person requires a Very Low Protein Diet instead of a high protein diet because of the varying amount of short-term stress it causes on the kidney. </style>
<br> <br>
High protein diet and obesity
<style justify> Zhaoping and colleagues (2010) They conducted a research experiment which investigated the adverse effects of a high protein diet on kidney function during weight loss. The experiment integrated 100 obese men and women who were monitored for the duration of one year. During the experiment, high protein consumption was clearly defined at 2.2g/kg versus standard protein ingestion, which was at 1.1g/kg. As for results, researchers had not found a direct or indirect correlation between inhibited renal function and high protein diet. Furthermore, no variation was found in creatinine clearance, which suggests an unmodified GFR rate. Thus, it is evident that this study supports the idea that a high protein diet safely reduces an obese individual’s Body Mass Index with no adverse concerns regarding kidney function. </style>
<style justify> Friedman and colleagues (2012) It was a controlled study that examined whether low-carbohydrate high protein diets led to any harmful renal effects. People that took part in the study were healthy obese individuals who followed the high protein diet for two years, as well as, another group who followed a low fat diet. The primary focus for the researchers was the GFR measured by creatinine clearance and albuminuria. The researchers found that even though the levels of solute excretion such as calcium and sodium were slightly raised in the high protein diet subjects, it was still lower than the low fat diets. In addition, they also measured urinary calcium excretion and found that despite an increase, there was no presence of new kidney stones. Besides that, small reductions in serum creatinine at three months (4.2% relative to low fat diet), as well as serum urea at two years (8.2%) were observed, indicating kidney adaptation. Upon review of all collected data, the researchers concluded that low carbohydrate and high protein diets in healthy obese individuals does lead to weight loss without causing renal damage to the kidney. </style>
Does Protein source Affect Renal Function?
<style justify>
There are many different types of protein sources available to humans today. Perhaps some sources of protein provide better protection for renal function than others. Aukema et al. (2010) compared the effects of soy based protein diets to those of plant based protein diets on renal protection. They used Weanling Han rats who were afflicted with kidney disease, and subjected them to hemp, pea, soy, and casein protein sources. Upon review of the experimental data they found that hemp and soy protein sources resulted in the least amount of kidney inflammation, as well as a more normal serum creatinine level, and smaller cyst sizes. This allowed them to conclude that there was a difference in kidney disease protection when it came to the source of the protein. Despite using rat test subjects instead of humans, the study still provides valuable insight into human chronic kidney disease treatment. There is a strong misconception that vegetarian diets are the healthiest type of diet, and while acquiring the necessary nutrients from a vegetarian diet is possible, it is crucial to include soy based protein for chronic kidney disease patients. Limiting one’s self to a single source of protein would be an opportunity passed to slowing renal disease progression.
Another study done by Moe et al. (2011) looked at animal based protein and plant based protein with respect to phosphorus homeostasis in chronic kidney disease. Increases in parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF23) induce phosphaturia which maintains phosphorus levels in an appropriate range. Moe et al. (2011) performed a crossover trial involving nine patients who had an average GFR at 32 ml/min to allow for a fair comparison of both diets. They found that after a week of implementing the vegetarian diets, there was a decrease in serum phosphorus and FGF23 levels. Progressive deterioration of renal function is usually indicative in an increase in FGF23 levels. This study was able to show the significant effect on phosphorus homeostasis in patients which might be due to decreased bioavailability of phytate-bound phosphorus or the higher availability of protein in meat-based diets, placing strain on the kidney. High FGF23 were more prevalent in the meat based diet, and could have possibly inhibited PTH. These studies show the importance of phosphate and protein source that the phosphate comes from, especially regarding those with chronic kidney disease. Future studies should look at the long term effects of high protein intake on phosphorus levels.
<br>
Maintaining Remission
There are treatments available to modify the disease course by helping to maintain remission and delay relapse in MS. For RRMS, SPMS, disease modifying drugs (DMDs) may be prescribed. There are many DMDs available for MS. Interferon beta and glatiramer acetate are the current front-line therapies for maintaining remission. These make up the ABC-R therapy of MS, which consists of Avonex (interferon b-1a), Betaseron (interferon b-1b), Copaxone (glatiramer acetate), and Rebif (interferon b-1a). (Hillman, 2014)
Interferon Beta
Interferon beta is in the family of cytokines, which possess antiviral and immunoregulatory activities. The mechanisms of interferon beta in MS, however, are not clearly understood. (Hedley, 2012) There are two types of interferon beta (interferon beta-1a and interferon beta-1b) that have no difference in effectiveness. They are only different in the way they are manufactured. Interferon beta use in MS patients reduces the number and severity of relapses, and improves MRI measures. (Hillman, 2014) Studies show that MS patients using interferon beta therapy showed 75% fewer brain lesions on a brain MRI compared with a placebo. (Hedley, 2012) The effect of the treatment on long-term progression is not clear. There are some serious side effects of interferon beta. These include flu-like symptoms such as myalgia, fever, chills, asthenia, headache, and nausea, as well as psychological symptoms such as depression. Interferon beta may also lead to the development of neutralizing antibodies that may lead to a decrease in the effectiveness of therapy. (Hillman, 2014)
Glatiramer Acetate
Glatiramer acetate is thought to alter the immune processes believed to be responsible for the pathogenesis of MS, however its mechanism is not fully known. (Hedley, 2012) Studies have shown that there is delay of progression from CIS to “clinically definite MS” in MS patients for up to three years with use of glatiramer acetate. (Hedley, 2012) This treatment reduces the number and severity of relapses, and the formation of new lesions on a brain MRI, however its effects on long-term progression are not clear. (Hedley, 2012) Adverse effects of glatiramer acetate minor and mainly consist of injection site reactions, seen in 70% of patients. (Hedley, 2012) Other less common side effects include lipoatrophy, flushing, shortness of breath, chest tightness, and palpitations. (Hillman, 2014)
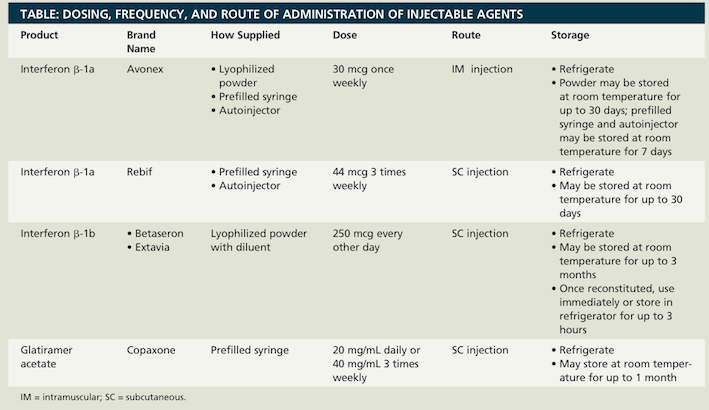
Deciding on which treatment to use (interferon beta or glatiramer acetate) depends on patient preference in type and frequency of injection. (See Figure 7)
</style>
<style float-right>
Figure 7: The dosing, frequency, and route of administration for the ACB-R therapies. (Source: Hillman & Khorassani, 2014)
</style>
<style justify>
There are several other treatments available aside from the front-line ABC-R therapies.
Natalizumab
Natalizumab is a monoclonal antibody that is currently the best treatment for preventing relapses. (Hedley, 2012) 67% of patients using Natalizumab are relapse free (compared to 41% of patients with a placebo) after two years of treatment. (Hedley, 2012) This treatment is recommended for patients with rapidly evolving severe RRMS. There are however, several side effects including infections, urticaria, and headaches to name a few. (Hedley, 2012) One side effect that could possibly escalate to something serious is an increased risk of progressive multifocal leukoencephalopathy (PML). With PML, there is a progressive inflammation of white matter in the brain. Due to the morality of up to 50% with PML, Natalizumab is not a first-line therapy. (Hedley, 2012)
Mitoxantrone
Mitoxantrone is not used as often since the development of natalizumab. It is a DNA-reactive agent of which the mechanism of action is not yet known, but is approved in the US to treat SPMS and RRMS. (Hedley, 2012) It is cytotoxic, thus has serious side effects such as life threatening reactions and cardiac toxicity. Mitoxantrone has a maximum lifetime dose and thus is not used long-term. (Hedley, 2012)
Azathioprine
Azathioprine is an immunosuppressant that is used to try to prevent relapse and to slow disease progression. It is not commonly used as side effects include gastrointestinal disturbances, bone marrow suppression, and hepatic toxicity. (Hedley, 2012)
Fingolimod
Fingolimod is a sphingosine-1-phosphate receptor modulator that retains lymphocytes in lymph nodes. This prevents lymphocytes from reaching the CNS and causing damage. (Hedley, 2012) In RRMS patients where interferon beta is unsuccessful, fingolimod is used. It is administered orally (one capsule daily). (Hedley, 2012) The development of oral agents are exciting as it is a easier method of administration than injections.
Maintaining Relapse
There are also treatments to manage relapse MS. Relapses in MS are caused by inflammation in the CNS, damaging the myelin of nerve fibers. Due to the varying lengths, severity, and nature of relapses, treatment is not always needed. (Hedley, 2012) Treatment is recommended for severe relapses when distress is experienced or when there is increased limitation of activities. Corticosteroids are the treatment of choice when there is demyelination. Methylprednisolone is a synthetic glucocorticoid that is a strong anti-inflammatory. (Hedley, 2012) It is unclear what it does exactly with MS, but it is thought to aid by immunosuppression or to reduce the accumulation of fluid around nerve damage. Studies have shown that steroids are effective in speeding up recovery from relapse, however there are no benefits to the long-term progression of disease. (Hedley, 2012) There are short-term and long-term treatments available, however long-term treatment should be avoided due to the side effects. Side effects of long-term treatment include weight gain, acne, cataracts, osteoporosis, deterioration of the head of the thigh bone, and diabetes. The side effects of short-term treatment are minor. (Hillman, 2014)
Maintaining Symptoms
Treatment for complications that are associated with MS are also available. Common symptoms of MS are fatigue, bladder and bowel problems, weakness, spasticity, and swallowing difficulties.
Fatigue
Up to 95% of MS patients experience fatigue, and more than half describe it as one of the most troubling symptoms of MS. (Amato & Portaccio, 2012) The pathophysiology of fatigue in MS is not well understood. There are both pharmacological and non-pharmacological therapeutic approaches to MS. The most studied pharmaceuticals are amantadine, modafinil, and aminopyridines. There are several non-pharmacological interventions. One is neuro-rehabilitation, which improves compensation, adaptation and reconditioning. Another is exercise, which has very promising results such as reduced fatigue, improve in mood and quality of life. (Amato & Portaccio, 2012) Finally there is behavioural therapy, which strives to educate patients and caregivers about how to make small behavioural changes to reduce fatigue. This may include education on planning rest, relaxation techniques, adapting daily living activities, making everyday tasks more energy efficient, and controlling external temperature. (Amato & Portaccio, 2012)
</style>
<style float-right>
Figure 8: Body weight assisted treadmill training. There is physical assistance for each
leg due to impaired walking ability. (Source: http://agelessphysio.com)
</style>
<style justify>
Bladder
Another symptom of MS is an overactive bladder. To manage an overactive bladder, MS patients can reduce fluid intake and caffeine to reduce symptoms of urgency and frequency. However, a daily intake of 1-2 litres of water is recommended. (Fowler et al., 2009) Pharmacological interventions such as antimuscarinic medication is also available to reduce incontinence, frequency, and urgency in MS. (Fowler et al., 2009) Some physical interventions would include pelvic floor exercises to strengthen the pelvic floor and treat stress incontinence. This is effective in patients whose neural pathways to their pelvic floor muscles are intact. (Fowler et al., 2009) In MS patients with neurogenic bladder dysfunction, clean intermittent self-catheterization is used. (Fowler et al., 2009)
Walking Ability
A common symptom of MS is impaired walking ability. Dalfampridine (Fampyra or Ampyra) can be used to improve walking ability. Dalfampridine is a potassium channel blocker that enhances conduction along demyelinated nerve fibres. (Hillman, 2014) In phase 3 clinical trials, walking speed was increased by about 25% within weeks in MS patients. (Hedley, 2012) There are, however, side effects such as a greater risk of seizures, anxiety, insomnia, dizziness, and tremor. (Hedley, 2012) In terms of physical therapy, exercise through treadmill training has been shown to improve walking endurance and velocity. (Amato & Portaccio, 2012) (See Figure 8)
Depression
Depression can be a psychological symptom of MS or from interferon beta therapy. For MS patients with severe depression, interferon is avoided. (Hillman, 2014)
Complementary Therapies
Finally, there are therapies available that can be used alongside MS treatment that may help with the general sense of wellbeing in MS patients and help them to feel and cope better with the disease and the treatment. Some complementary therapies include reflexology, massage, tai chi, magnetic field therapy, neural therapy, and fish oils. (Hedley, 2012) Also linoleic acid, found in sunflower, corn, soya, and safflower oils, may reduce the progression of MS. (Hedley, 2012)
Current Research
The majority of research being done on MS primarily involves targeting new medications which better combat relapsing-remitting MS, as well as improving symptomatic treatments. However there is current research being done which aims to promote myelin repair, thus acting as a protective agent against MS. A study done by de la Fuente et al. in 2015, looked at vitamin D as an agent to promote remyelination, which is the process which drives specialized cells to fix damaged myelin found in the CNS nerve fibres. This process happen naturally in a healthy individual, however those afflicted with MS experience a decrease in the function over time due to the continuous damage being done to their myelin (de la Fuente et al. 2015). Cells termed oligodendrocyte progenitor cells (OPC) are responsible for creating oligodendrocytes which manufacture the myelin sheath (de la Fuente et al. 2015). Vitamin D is important because it binds to a specific protein, retinoid X receptor-y (RXR-y), which aids in swiftly maturing OPC into oligodendrocytes. Thus increasing the rate at which the myelin repairing oligodendrocytes, this would be greatly beneficial in battling MS. The study looked at a MS like disease in rats, more specifically focusing on the vitamin D receptors. They found that the RXR-y protein interacts with the vitamin D receptor, which stays active during the remyelination stage. This led to improved remyelination. Furthermore when the vitamin D receptors were prevented from binding and functioning correctly, the OPCs did not develop properly into oligodendrocytes, therefore leading to malfunctioning remyelination in the rats. This type of study investigating the remyelination enhancement possibility from vitamin D is the trend in current research.
</style>
<br>
Conclusion
Multiple Sclerosis is an inflammatory, autoimmune disease which disrupts the neurons’ ability to trigger action potentials. As of today, the pathophysiology of this disease is not fully understood, however, considerable advances have been made in understanding the factors behind this disease. It is evident that further research needs to be conducted to figure out the precise mechanism of the demyelination and remyelination process. The findings of this research will lead to more efficient treatment mechanisms and may even lead to a cure for Multiple Sclerosis.
<br>
References
Amato, M. P. & Portaccio, E. (2012). Management options in multiple sclerosis-associated fatigue. Expert Opinion on Pharmacotherapy, 13(2), 207-216
Ascherio A, Munger KL (April 2007). “Environmental risk factors for multiple sclerosis. Part I: the role of infection”. Annals of Neurology. 61 (4): 288–99.
Brosnan, C. F., & Raine, C. S. (1996). Mechanisms of immune injury in multiple sclerosis. Brain Pathology, 6(3), 243-257.
Compston A, Coles A (October 2008). “Multiple sclerosis”. Lancet. 372 (9648): 1502–17
de la Fuente AG et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol. 2015; 211(5):975-85. Multiple Sclerosis Clinical Presentation. (2016). Retrieved September 18, 2016, from http://emedicine.medscape.com/article/1146199-clinical
Dyment DA, Ebers GC, Sadovnick AD (February 2004). “Genetics of multiple sclerosis”.Lancet Neurol. 3 (92): 104–10
Fowler, C. J., Panicker, J. N., Drake, N., Harris, C., Harrison, S. C. W., Kirby, M., Lucas, M., Macleod, N., Mangnall, J., North, A., et al. (2009). A UK consensus on the management of the bladder in multiple sclerosis. Postgraduate Medical Journal. 85, 552-559.
Gilden DH (March 2005). “Infectious causes of multiple sclerosis”. The Lancet Neurology. 4 (3): 195–202
Hedley, L. (2012). Multiple sclerosis treatment options. The Pharmaceutical Journal. 288, 247-250.
Hillman, A. & Khorassani, F. (2014). Multiple sclerosis management. Pharmacy Times. Retrieved from http://www.pharmacytimes.com/publications/health-system-edition/2014/march2014/multiple-sclerosis-management
Löscher, W., & Potschka, H. (2005). Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx, 2(1), 86-98.
Multiple Sclerosis Clinical Presentation. (2016). Retrieved September 18, 2016, from http://emedicine.medscape.com/article/1146199-clinical
Multiple Sclerosis Society of Canada. (2016). Retrieved September 18, 2016, from https://mssociety.ca/about-ms/what-is-ms
Rolak, L. A. (2003). Multiple Sclerosis: It's Not The Disease You Thought It Was.Clinical Medicine & Research, 1(1), 57-60. doi:10.3121/cmr.1.1.57
Smith, K. J., & McDonald, W. I. (1999). The pathophysiology of multiple sclerosis⋮ the mechanisms underlying the production of symptoms and the natural history of the disease. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 354(1390), 1649-1673.
Tzartos, J. S., Friese, M. A., Craner, M. J., Palace, J., Newcombe, J., Esiri, M. M., & Fugger, L. (2008). Interleukin-17 Production in Central Nervous System-Infiltrating T Cells and Glial Cells Is Associated with Active Disease in Multiple Sclerosis. The American Journal of Pathology, 172(1), 146–155.
Wingerchuk, D. M., Lucchinetti, C. F., & Noseworthy, J. H. (2001). Multiple sclerosis: current pathophysiological concepts. Laboratory investigation, 81(3), 263-281.
Wu, G. F., & Alvarez, E. (2011). The immuno-pathophysiology of multiple sclerosis. Neurologic Clinics, 29(2), 257–278.
Wucherpfennig, K. W., & Strominger, J. L. (1995). Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell, 80(5), 695-705.