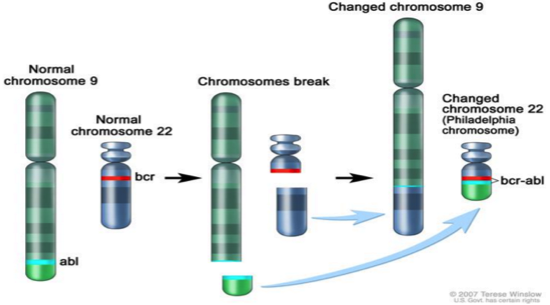
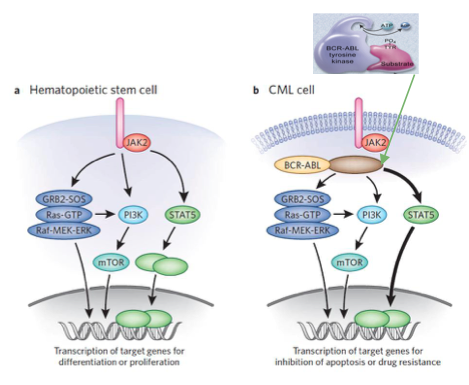
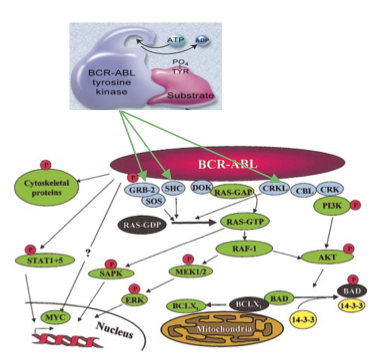
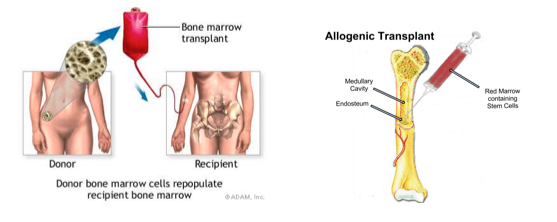
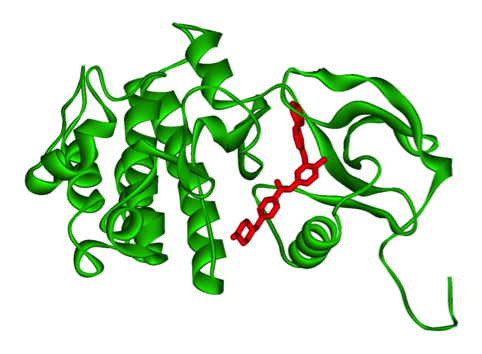
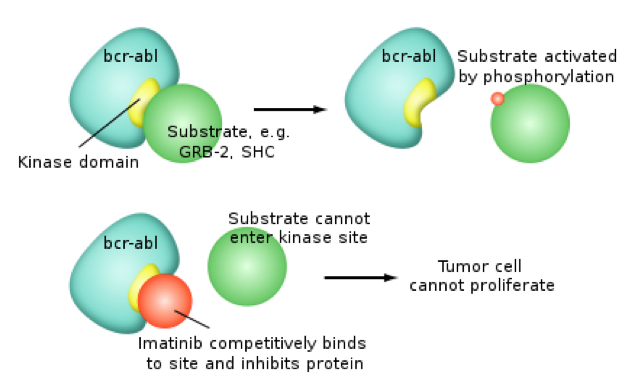
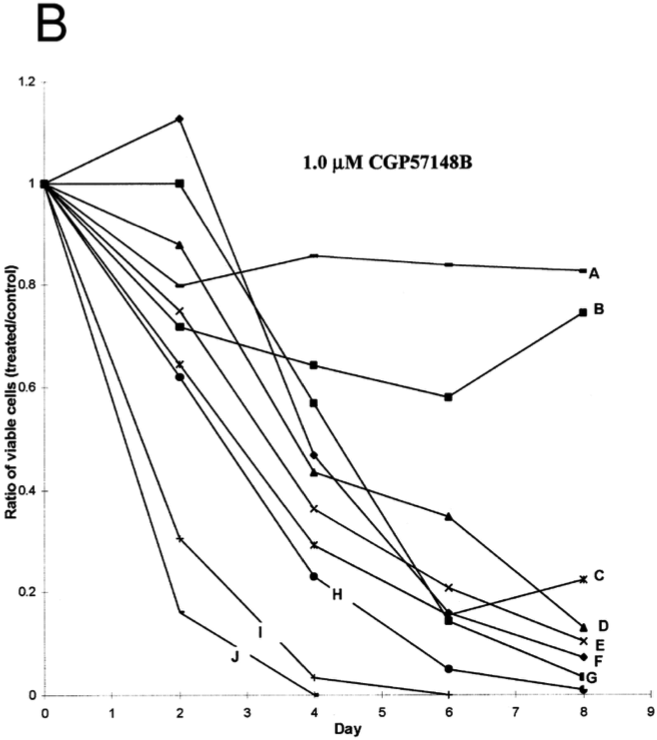
This is an old revision of the document!
Table of Contents
CHRONIC MYELOID LEUKEMIA
Introduction
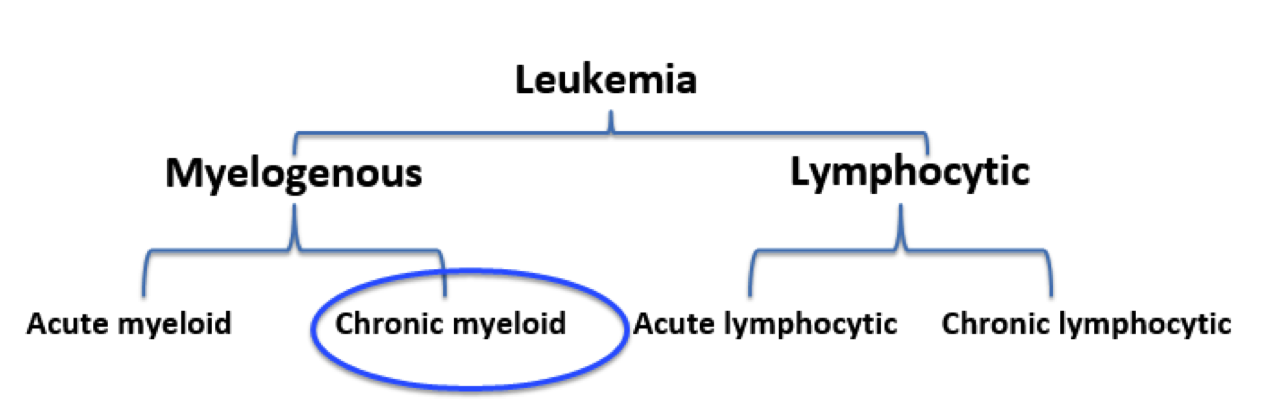
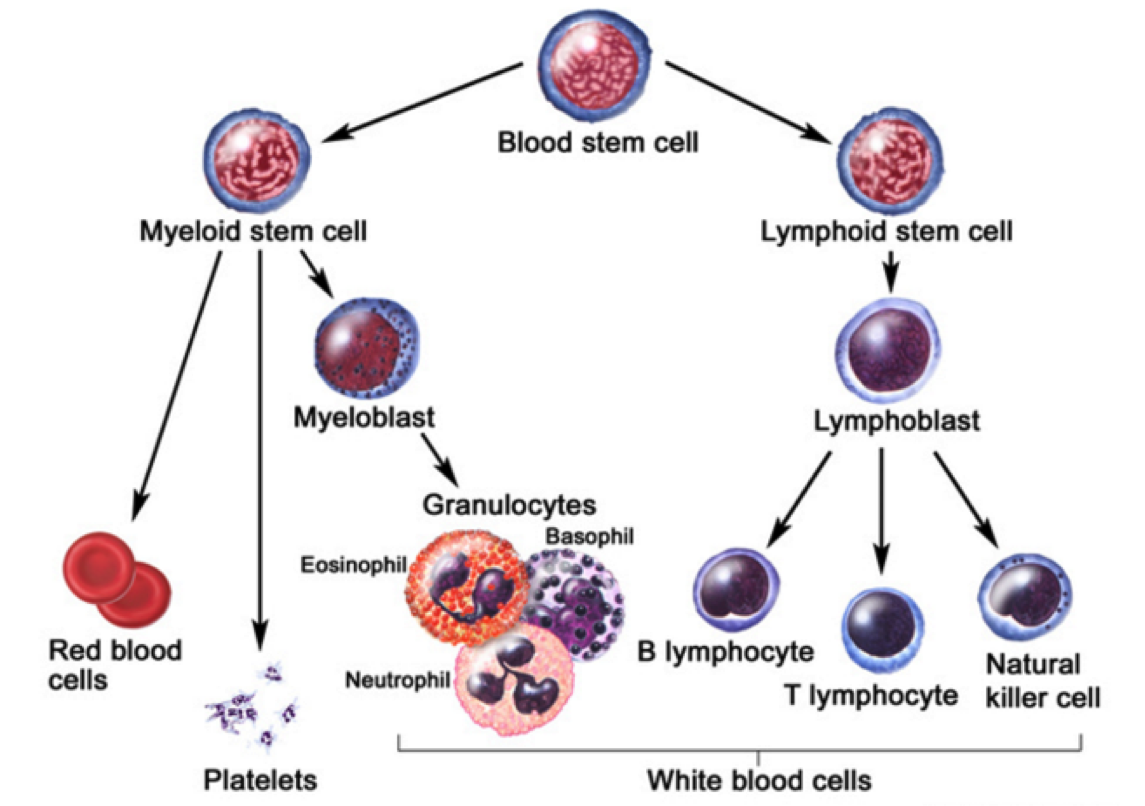
Leukemia is the eleventh-most prevalent cancer worldwide, with over 350,000 patients diagnosed each year worldwide (Siegel, Miller, & Jemal, 2015). Leukemia can generally be defined as a non-tumorous cancer of the blood, developing exclusively in the bone marrow of an individual (Apperley, 2015). It is at this stage where leukemia is further classified into varying types. The first distinction made for diagnosed patients is whether the leukemia is myelogenous or lymphocytic (Apperley, 2015). While lymphocytic leukemia occurs specific to blood stem cells predetermined to differentiate into lymphocytes, myelogenous leukemia targets blood stem cells destined to produce red blood cells (RBCs) and platelets (Vardiman et al., 2009). Both lymphocytic and myelogenous leukemia can also be sub-classified into acute and chronic leukemia. Acute leukemia patients display a rapid increase in immature myeloid or lymphocytic cells while chronic leukemia patients display increased numbers of more relatively mature cells (Vardiman et al., 2009). Based on the unique characteristics displayed by both acute and chronic leukemia, acute patients require a more immediate response to the disease.
The most notable characteristic of CML patients is the increased growth of myeloid cells which are unregulated can be observed in the bone marrow (Pui et al., 1989). This highly-proliferated stem cell fails to differentiate into its intended functional cell, thus failing to produce red blood cells and platelets required by the body (Deininger, Goldman, & Melo, 2000). The abundancy of myeloid cells in leukemic patients result in an accumulation within the individual’s bloodstream.
Risk Factors, Diagnosis, Signs and Symptoms
Notable risk factors associated with CML include age, gender, and exposure to radiation. The average recorded age of a CML patient is 65 years, implying that individuals are more prone to CML at an older age (Siegel, Miller, & Jemal, 2015). Recent studies also suggest men having a higher genetic predisposition to CML as opposed to their female counterparts (Siegel, Miller, & Jemal, 2015). In addition, individuals that have been exposed to high dosages of radiation throughout their lifetime have also been contingent with the disease.
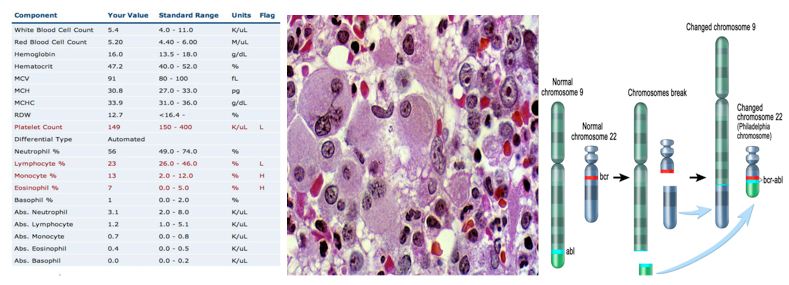
Clinical diagnosis of CML include various methods of approach. The most prevalent method of diagnosis is a complete blood count (CBC) of an individual. An increased level of granulocytes revealed during a CBC is indicative of CML, thus requiring further testing to verify the cause (Deininger, Goldman, & Melo, 2000). A more invasive study requires a bone marrow biopsy, which helps identify CML by gathering physical data at the locus of the disease. A less common, but more in-depth approach includes a genetic screening of the patient in order to display the source of the disease on a chromosomal level (Deininger, Goldman, & Melo, 2000).
In contrast to acute leukemia, which can be observed within days or weeks, the onset of symptoms cannot be observed in the early stages of the disease (Pui et al., 1989). The development of disease is very gradual in nature, thus making the diagnosis of CML occur in its later stages. CML is usually diagnosed during routine blood tests which later reveal an elevated white blood cell count. The physiological impact of CML primarily results in the lack of both RBCs and platelet count. Symptoms contingent to a lack of RBCs include both fatigue and anemia whereas a low platelet count is associated with widespread bleeding or bruising, frequent infections, bone pain, and weight loss due to loss of appetite (Baccarani et al., 2009).
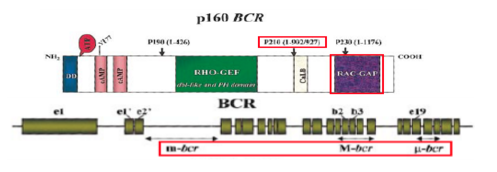
Pathogenesis
Pathophysiology
Therapeutics
Conclusion
References
Apperley, J. (2015). Chronic myeloid leukaemia. The Lancet. 385: 9976, 1447–1459
Baccarani, M., Cortes, J., Pane, F., Niederwieser, D., Saglio, G., Apperley, J., & Hochhaus, A. (2009). Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet.Journal of clinical oncology, 27(35), 6041-6051.
CML Society of Canada. (2010, March 18). CML Animation. Retrieved from https://www.youtube.com/watch?v=3tu3CWKbb4M
Corey, S., Cortes, J. (2010). Chronic Myeloid Leukemia: Pathophysiology and Therapeutics.
Deniniger, M., Goldman, J., Melo, J. (2000). The Molecular Biology of Chronic Myeloid Leukemia. Blood, 96(10), 3343 - 3356.
Druker, B. (2008). Translation of the Philadelphia chromosome into therapy for CML. Blood, 12(13), 4808 - 4817.
Druker, B. J., Talpaz, M., Resta, D. J., Peng, B., Buchdunger, E., Ford, J. M., … & Sawyers, C. L. (2001). Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. New England Journal of Medicine, 344(14), 1031-1037.
Fabbro, D. (2012). BCR-ABL signaling: A new STATus in CML. Nature Chemical Biology, 8, 228-229.
Goldman, J. M., & Melo, J. V. (2003). Chronic myeloid leukemia—advances in biology and new approaches to treatment. New England Journal of Medicine, 349(15), 1451-1464.
Hurtado et al. (2007). Chronic Myeloid Leukemia Current Concepts in Physiopathology and Treatment. Cancerología, 2, 137-147.
Pui, C. H., Behm, F. G., Raimondi, S. C., Dodge, R. K., George, S. L., Rivera, G. K., & Crist, W. M. (1989). Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. New England Journal of Medicine, 321(3), 136-142.
Sawyers, C. L. (1999). Chronic myeloid leukemia. New England Journal of Medicine, 340(17), 1330-1340.
Siegel, R. L., Miller, K. D., & Jemal, A. (2015). Cancer statistics, 2016. CA: A cancer journal for clinicians.
Vardiman, J. W., Thiele, J., Arber, D. A., Brunning, R. D., Borowitz, M. J., Porwit, A., & Bloomfield, C. D. (2009). The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood, 114(5), 937-951.