Table of Contents
Powerpoint: group_5_chronic_myeloid_leukemia.pptx
CHRONIC MYELOID LEUKEMIA
Introduction
Leukemia is the eleventh-most prevalent cancer worldwide, with over 350,000 patients diagnosed each year worldwide (Siegel, Miller, & Jemal, 2015). Leukemia can generally be defined as a non-tumorous cancer of the blood, developing exclusively in the bone marrow of an individual (Apperley, 2015). It is at this stage where leukemia is further classified into varying types. The first distinction made for diagnosed patients is whether the leukemia is myelogenous or lymphocytic (Apperley, 2015). While lymphocytic leukemia occurs specific to blood stem cells predetermined to differentiate into lymphocytes, myelogenous leukemia targets blood stem cells destined to produce red blood cells (RBCs) and platelets (Vardiman et al., 2009). Both lymphocytic and myelogenous leukemia can also be sub-classified into acute and chronic leukemia. Acute leukemia patients display a rapid increase in immature myeloid or lymphocytic cells while chronic leukemia patients display increased numbers of more relatively mature cells (Vardiman et al., 2009). Based on the unique characteristics displayed by both acute and chronic leukemia, acute patients require a more immediate response to the disease.
The most notable characteristic of CML patients is the increased growth of myeloid cells which are unregulated can be observed in the bone marrow (Pui et al., 1989). This highly-proliferated stem cell fails to differentiate into its intended functional cell, thus failing to produce red blood cells and platelets required by the body (Deininger, Goldman, & Melo, 2000). The abundancy of myeloid cells in leukemic patients result in an accumulation within the individual’s bloodstream.
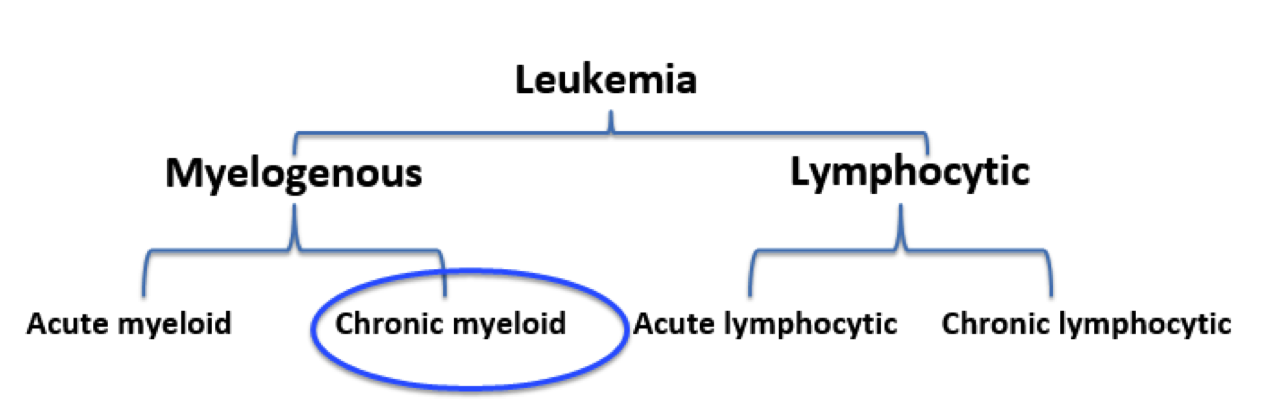
Figure 1: Leukemia and its respective classifications. Chronic Myeloid Leukemia is both myelogenous and long-term.
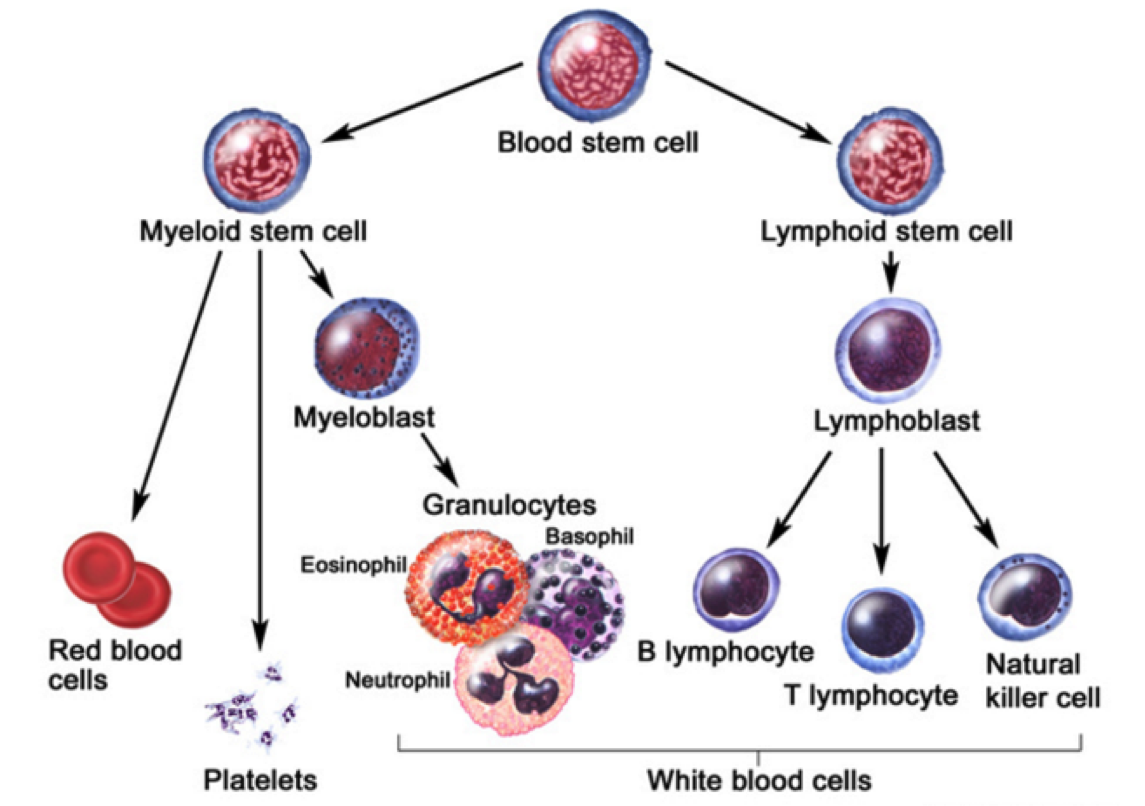
Figure 2: Stem cell differentiation of a blood stem cell. A blood stem cell can differentiate via a myeloid or lymphoid stem cell pathway
Risk Factors, Diagnosis, Signs and Symptoms
Notable risk factors associated with CML include age, gender, and exposure to radiation. The average recorded age of a CML patient is 65 years, implying that individuals are more prone to CML at an older age (Siegel, Miller, & Jemal, 2015). Recent studies also suggest men having a higher genetic predisposition to CML as opposed to their female counterparts (Siegel, Miller, & Jemal, 2015). In addition, individuals that have been exposed to high dosages of radiation throughout their lifetime have also been contingent with the disease.
Clinical diagnosis of CML include various methods of approach. The most prevalent method of diagnosis is a complete blood count (CBC) of an individual. An increased level of granulocytes revealed during a CBC is indicative of CML, thus requiring further testing to verify the cause (Deininger, Goldman, & Melo, 2000). A more invasive study requires a bone marrow biopsy, which helps identify CML by gathering physical data at the locus of the disease. A less common, but more in-depth approach includes a genetic screening of the patient in order to display the source of the disease on a chromosomal level (Deininger, Goldman, & Melo, 2000).
In contrast to acute leukemia, which can be observed within days or weeks, the onset of symptoms cannot be observed in the early stages of the disease (Pui et al., 1989). The development of disease is very gradual in nature, thus making the diagnosis of CML occur in its later stages. CML is usually diagnosed during routine blood tests which later reveal an elevated white blood cell count. The physiological impact of CML primarily results in the lack of both RBCs and platelet count. Symptoms contingent to a lack of RBCs include both fatigue and anemia whereas a low platelet count is associated with widespread bleeding or bruising, frequent infections, bone pain, and weight loss due to loss of appetite (Baccarani et al., 2009).
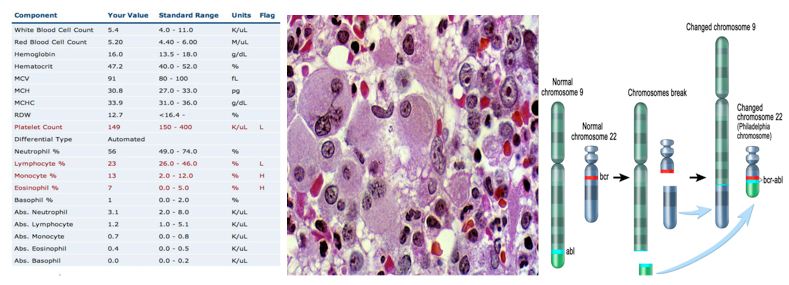
Figure 3: An example of data obtained from a complete blood count. Both a low platelet count and a high granulocyte level are indicative of chronic myeloid leukemia
Pathogenesis
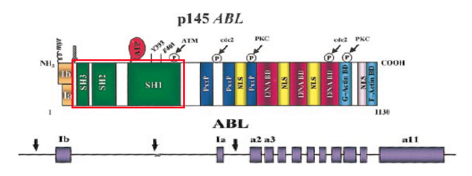
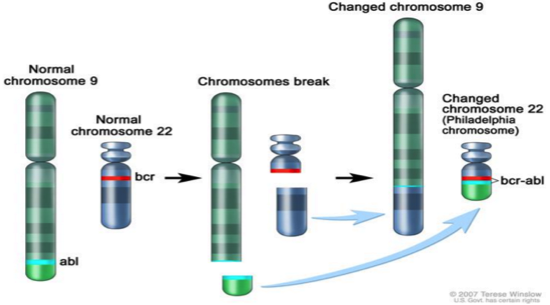
Chronic Myeloid Leukemia (CML) is a result of the reciprocal translocation between chromosome 9 and chromosome 22 (Apperley, 2015). Abnormal chromosome 22 is also referred to as the Philadelphia chromosome (Ph), since it was in Philadelphia, USA where scientists first discovered it (Apperley, 2015). The Abl gene is a non-receptor tyrosine kinase and is located on chromosome 9 (Corey & Cortes, 2010). Abl stands for Abelson murine Leukemia and it is the v-abl oncogene (viral gene) human homologue (Deniniger et al., 2000). SH1, SH2 and SH3 are the 3 SRC homology domains found on Abl (Deniniger et al., 2000). SH1 domain is important in carrying out tyrosine kinase function, whereas SH2 & SH3 allow Abl to interact with other proteins (Deniniger et al., 2000).
Figure 4: Abl gene with its respective exons
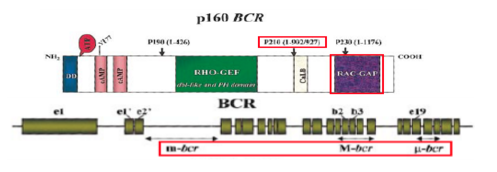
The Bcr gene is located on chromosome 22, and it is a serine/threonine kinase that is involved in the activation of RAc and CDc42 GTPases (Corey & Cortes, 2010). Bcr stands for breakpoint cluster region, due to the different breakpoints that are found at the site for transcription (Corey & Cortes, 2010). Chromosome 9 has a constant breakpoint, whereas, chromosome 22 can have breakpoints in up to 3 different areas: m-bcr, M-bcr, and µ-bcr (Hurtado et al., 2007). These three breakpoints produce proteins that contain different molecular weights: p190, p210, or p230 (Hurtado et al., 2007). The protein with molecular weight p210 is the one that is seen in 95% of CML patients (Hurtado et al., 2007).
Figure 5: Bcr gene with its respective exons
When the partial gene, Abl fuses with another partial gene, Bcr, this results in a Bcr-Abl mRNA transcript that gets further translated into Bcr-Abl protein (Corey & Cortes, 2010). Due to the many different breakpoints located on Bcr , it results in two types of Bcr-Abl mRNA, named b2a2 and b3a2 (Hurtado et al., 2007). The Bcr-Abl protein leads to upregulated tyrosine kinase activity, causing an excessive proliferation of leukemia cells, and the inhibition of apoptosis of HSC or progenitors (Corey & Cortes, 2010). There are several ways to deregulate the Abl tyrosine kinase. The SH3 domain is an essential domain on the Abl gene, and is involved in negatively regulating activity (Deniniger et al., 2000). It has been found that the deletion or the change in position of the SH3 domain can activate the kinase and turn Abl into an oncogene (Deniniger et al., 2000). Since, the serine/kinase part that tells Bcr to turn off is replaced by Abl’s tyrosine kinase, and the SH3 domain that tells Abl to turn off is deleted, the protein never stops working. Moreover, oxidative stress such as ionizing radiation has been found to oxidize a protein called Pag/Msp23 (Deniniger et al., 2000). This oxidation leads to the dissociation of this protein from Abl, leading to the activation of the kinase (Deniniger et al., 2000).
β1 integrins are important transmembrane receptors, that allow progenitor cells to attach and interact with the stroma, resulting in the inhibition of proliferation (Deniniger et al., 2000). In CML, an adhesion-inhibitory variant of β1 integrin is present, which causes a decrease in the adhesion of these progenitor cells to bone marrow, resulting in the over proliferation of these cells(Deniniger et al., 2000).
Figure 6: The reciprocal translocation between chromosome 9 (location of Abl) and chromosome 22 (location of Bcr)
Pathophysiology
When Abl is in its proto-oncogene form, it is weakly active and functions like a normal gene (Corey & Cortes, 2010). Abl serves a complex role in the human body, when it is in its proto-oncogene form. As a normal gene, it serves in regulating the cell cycle, responding to genotoxic stress, and using integrin signals to influence decisions regarding cell cycle and apoptosis (Corey & Cortes, 2010). However, when it fuses to Bcr, Abl acts as a constitutively active tyrosine kinase protein (constantly active) (Corey & Cortes, 2010). This transformation of the Abl proto-oncogene into an Abl oncogene, is a leading cause of the elevation in tyrosine kinase activity (Corey & Cortes, 2010).
There are several substrates that have the potential in binding to Bcr-Abl and becoming tyrosine phosphorylated (Deniniger et al., 2000). The choice of substrate is dependent upon the type of cell they function and reside in (Deniniger et al., 2000). Under physiologic conditions, tyrosine phosphatases function to regulate tyrosine kinases (Deniniger et al., 2000). Syp and PTP1B are two tyrosine phosphatases that have the ability to dephosphorylate Brc-Abl (Deniniger et al., 2000). More research needs to be conducted on these tyrosine phosphatases, and their role in managing this disease.
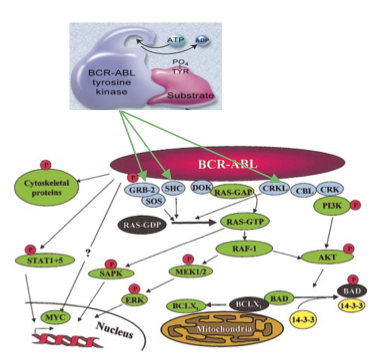
There are numerous substrates and signaling pathways found to be influenced by Bcr-Abl. The Ras and the MAP kinase pathways play an important role in the activation of mitogenic signaling (Deniniger et al., 2000). An adaptor molecule known as Grb-2 acts as a substrate and is tyrosine phosphorylated by Brc-Abl (Deniniger et al., 2000). It binds to the Sos protein, which allows for the activation of Ras from its GDP bound form to its GTP bound form (Deniniger et al., 2000). Two other adaptor molecule known as Shc and Crkl act as substrates of Brc-Abl and are also capable of activating the Ras GTP bound form (Deniniger et al., 2000). The SH2 domain on Shc and the SH3 domain on Crkl allows for the binding to Brc-Abl (Deniniger et al., 2000). Unlike tumours, Ras activation does not involve active mutations, it causes the pathway to stay consistently active (Deniniger et al., 2000). When IL-3 cytokine receptors are stimulated, this leads to the activation of Ras (Deniniger et al., 2000). After this occurs, a serine/threonine kinase known as Raf is recruited to the cell membrane (Deniniger et al., 2000). Mek1/Mek2 and Erk are serine/threonine kinases involved in the gene transcription (Deniniger et al., 2000). Ras signaling may be relayed through Rac (the GTP-GDP exchange factor), then to germinal center kinase (Gckr) and then to Sapk (Deniniger et al., 2000). Another part of the MAP pathway involves Rac relaying information to p38, which is activated in the same way by the Bcr-Abl complex (Deniniger et al., 2000).
Figure 7: MAP and RAS kinase pathways
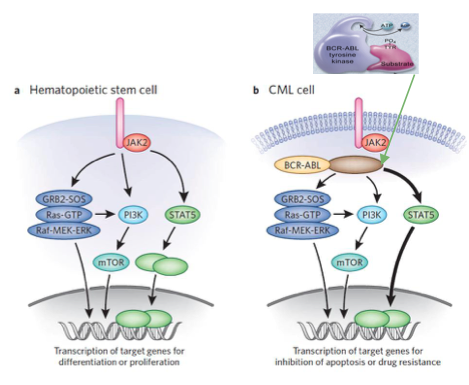
Jak-Stat pathways have also been found to play a role in mitogenic signaling (Deniniger et al., 2000). Bcr-Abl constitutively phosphorylates and activates target proteins involved in cell growth and proliferation of Jak2, such as the STAT5 transcription factor (Fabbro, 2012). When STAT5 proteins get phosphorylated, they dimerize, enter the nucleus, and allow for transcription of apoptotic genes, which is a driver of proliferation of leukemic cells.
Figure 8: JAK-STAT Pathway in a Hematopoietic Stem Cell and CML cell
Therapeutics
Options:
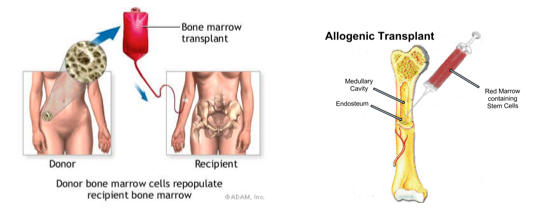
The only truly curative treatment is bone marrow transplant or allogeneic stem cell transplant. However, these treatments can be invasive and hence other treatment options include tyrosine kinase inhibitors.
Figure 9: Curative treatment options for CML
Inhibiting Bcr-Abl as a potential drug target:
CML is caused by the reciprocal translocation between chromosome 9 (location of Abl1 gene) and 22 (location of BCR gene) which creates the Philadelphia chromosome. This molecular pathogenesis generates the Bcr-Abl fusion protein which has elevated tyrosine kinase activity. The Bcr-Abl fusion protein is found predominately in CML cells and thus provides the desired specificity (minimized systemic toxicity) for an inhibitor or therapeutic to interfere with this fusion protein’s function (Deininger, Goldman, Melo 2000). It is easy to target the ATP pocket of this fusion protein (something common to kinase proteins) than other protein-protein interactions as ATP pockets are well defined and mimicking ATP is an easy starting point for drug development (Deininger, Goldman, Melo 2000).
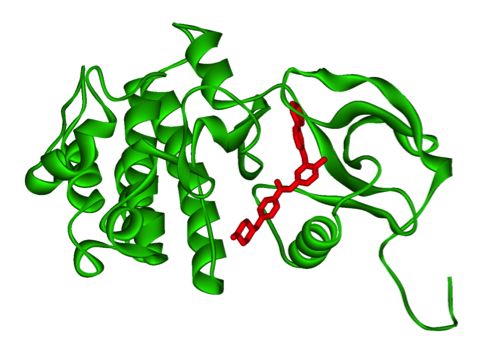
There are two conformation of Bcr-Abl that can be inhibited, the active and inactive conformation (Reddy and Aggarwal 2014). The open or active conformer is an active kinase in which ATP can bind. The kinase can use ATP to activate downstream proteins through phosphorylation. A possible inhibitor, and hence therapeutic for CML, would be an ATP mimetic of the open/active conformation. The second conformation is the closed/inactive conformation which is an inactive kinase in which ATP cannot bind and hence no downstream phosphorylation occurs (Reddy and Aggarwal 2014). It is harder to design an inhibitor/therapeutic for the closed conformer as the ATP pocket is not exposed.
Figure 10: Bcr-Abl kinase being inhibited by a compound, imatinib, in red
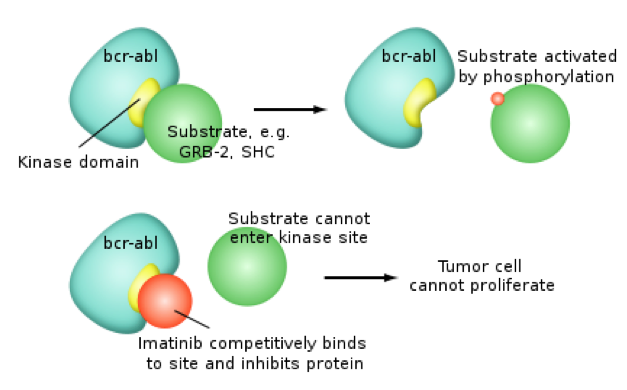
Gleevec: a tyrosine kinase inhibitor
Gleevec (imatinib mesylate) is a therapeutic that was FDA approved for CML treatment in 2001. It is now used to treat gastrointestinal stroll tumours (Pray, 2008). Though at first the rationale was that Gleevec would compete with ATP in the active Bcr-Abl conformer, however that wasn't the case. Gleevec doesn’t compete with ATP, instead it just keeps the kinase in a closed state preventing ATP from binding to the conformer. There has been vast improvement seen for CML patient survival over other therapeutics. However, there is acquired drug resistance seen too. Drug resistance is common for chemotherapies due to prolonged exposure to drugs. In particular, some CML cell populations survive due to a genetic mutation in Bcr-Abl, giving rise to a new population of Bcr-Abl CML cells that are resistant to Gleevec. This mutation is at Thr315Ile location and it pushed Gleevec out of its pocket (Pray, 2008).
Figure 11: Pharmacodynamics of imatinib, Gleevec
Results from tyrosine kinase inhibitors:
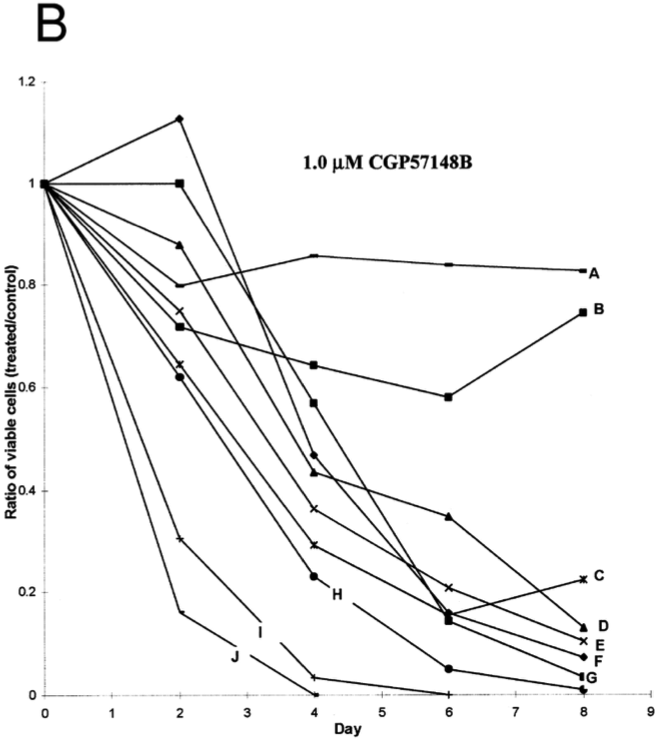
In the study by Deininger, Goldman, Lydon and Melo (1997), Bcr-Abl positive CML cells were exposed to tyrosine kinase inhibitor CGP57148B. Of all the cell lines examined, most showed a drastic reduction showing that tyrosine kinase inhibitors were effective in treating CML cells. Two cells that were resistant to the initial 1.0uM were treated with 10uM of the inhibitor.
Figure 12: Results from study showing effectiveness of tyrosine kinase inhibitors on Bcr-Abl positive CML cells
Implications of treatment:
One strategy to overcome resistance is to develop new inhibitors of Bcr-Abl. For example, Dasatinib inhibits most Bcr-Abl mutants, except for the Thr351Ile mutant. Also Ponatinib is able to inhibit the T35I mutant (Shah, Tran, Lee, Chen, Norris, Sawyers 2004).
Numerous substrates and signaling pathways found to be influenced by Bcr-Abl. The result of this includes increased proliferation or decreased apoptosis of HSCs or progenitor cells. For example, Bcr-Abl constitutively phosphorylates STAT5, leading to transcription of target genes for proliferation/inhibition of apoptosis. As a result, this maintains CML. Finding inhibitors of the the STAT5 pathway is another possible alternative target for CML treatment (Cumaraswamy et al. 2014).
Conclusion
In summary, Chronic Myeloid Leukemia is non-tumorous and develops in the bone marrow of the individual. Specifically, it is the rapid growth of the myeloid cells which characterized Chronic Myeloid Leukemia, resulting in an extreme number of White Blood cells in the body.
It is the totipotent stem cell which targets the translocation of Chromosome 9 and Chromosome 22. This juxtapositioning of the chromosome creates the Philadelphia chromosome (Ph) which creates the fusion gene known as the BCR-ABL gene, observed in all CML patients. This leads to the upregulated activity in tyrosine kinase, causing excessive proliferation and clonal expansion of the leukemic cells. Additionally, the nucleus cascade involves specific pathways triggering the inhibition of cell death (otherwise known as apoptosis) of the hematopoietic stem cells.
One treatment that is showing significant promise is the Gleevec (otherwise called the Wonder drug) that has results where the cell growth is significantly decreasing with exposure to the drug in controlled settings. More research needs to be done in this field to determine if the drug can be used over varying patients in different stages of the disease.
References
Apperley, J. (2015). Chronic myeloid leukaemia. The Lancet. 385: 9976, 1447–1459
Baccarani, M., Cortes, J., Pane, F., Niederwieser, D., Saglio, G., Apperley, J., & Hochhaus, A. (2009). Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet.Journal of clinical oncology, 27(35), 6041-6051.
CML Society of Canada. (2010, March 18). CML Animation. Retrieved from https://www.youtube.com/watch?v=3tu3CWKbb4M
Corey, S., Cortes, J. (2010). Chronic Myeloid Leukemia: Pathophysiology and Therapeutics.
Deininger, M., Goldman, J., Lyon, N., & Melo, J. (1997). The Tyrosine Kinase Inhibitor CGP57148B Selectively Inhibits the Growth of BCR-ABL–Positive Cells. Blood Journal, 90(9), 3691-3698. Retrieved from http://www.bloodjournal.org/content/90/9/3691?sso-checked=true#F4
Deniniger, M., Goldman, J., Melo, J. (2000). The Molecular Biology of Chronic Myeloid Leukemia. Blood, 96(10), 3343 - 3356.
Druker, B. (2008). Translation of the Philadelphia chromosome into therapy for CML. Blood, 12(13), 4808 - 4817.
Druker, B. J., Talpaz, M., Resta, D. J., Peng, B., Buchdunger, E., Ford, J. M., … & Sawyers, C. L. (2001). Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. New England Journal of Medicine, 344(14), 1031-1037.
Fabbro, D. (2012). BCR-ABL signaling: A new STATus in CML. Nature Chemical Biology, 8, 228-229.
Goldman, J. M., & Melo, J. V. (2003). Chronic myeloid leukemia—advances in biology and new approaches to treatment. New England Journal of Medicine, 349(15), 1451-1464.
Hurtado et al. (2007). Chronic Myeloid Leukemia Current Concepts in Physiopathology and Treatment. Cancerología, 2, 137-147.
Pray, L. A. (2008). Gleevec: The Breakthrough in Cancer Treatment. Nature Education, 1(1), 37th ser. Retrieved from http://www.nature.com/scitable/topicpage/gleevec-the-breakthrough-in-cancer-treatment-565
Pui, C. H., Behm, F. G., Raimondi, S. C., Dodge, R. K., George, S. L., Rivera, G. K., & Crist, W. M. (1989). Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. New England Journal of Medicine, 321(3), 136-142.
Reddy, E. P., & Aggarwal, A. K. (2012). The Ins and Outs of Bcr-Abl Inhibition. Genes & Cancer, 3(5-6), 447-454. Retrieved from http://www.bloodjournal.org/content/96/10/3343.long?sso-checked=true
Sawyers, C. L. (1999). Chronic myeloid leukemia. New England Journal of Medicine, 340(17), 1330-1340.
Shah, N. P. (2004). Overriding Imatinib Resistance with a Novel ABL Kinase Inhibitor. Science, 305(5682), 399-401. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15256671
Siegel, R. L., Miller, K. D., & Jemal, A. (2015). Cancer statistics, 2016. CA: A cancer journal for clinicians.
Vardiman, J. W., Thiele, J., Arber, D. A., Brunning, R. D., Borowitz, M. J., Porwit, A., & Bloomfield, C. D. (2009). The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood, 114(5), 937-951.