This is an old revision of the document!
Table of Contents
TYPE 1 DIABETES
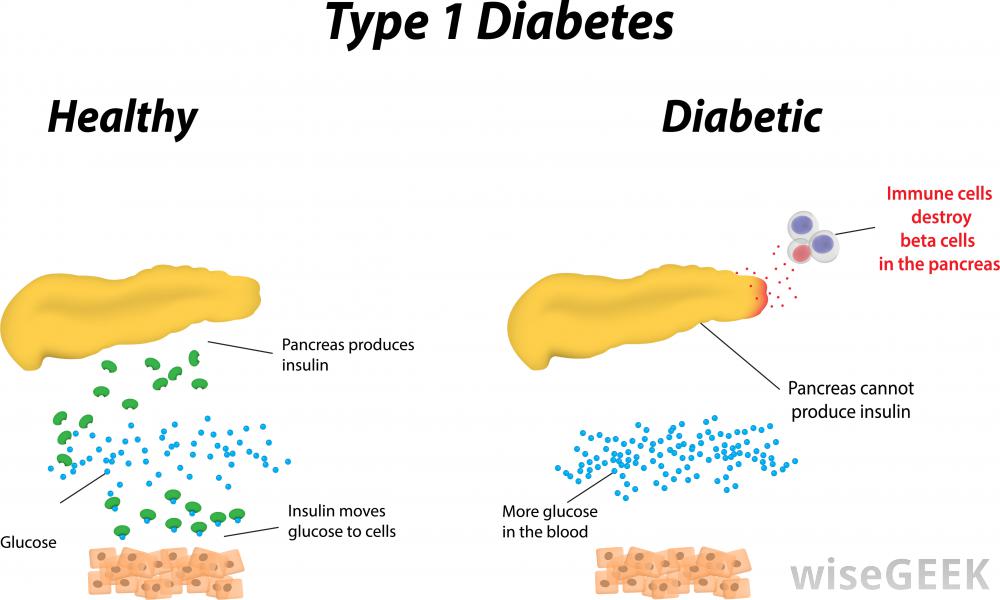
Diabetes mellitus is a disease in which blood sugar levels rise above the normal for a prolonged period of time. There are two forms of diabetes: Type 1 and Type 2. According to the International Diabetes federation, it is estimated that 1 out of 12 people have diabetes. 50% of which are undiagnosed and therefore unaware of their condition, and of these 5-10% have Type 1 (Daneman, 2006). In Type 1 diabetes, the pancreas does not produce enough insulin. Insulin is a hormone in the body that promotes the absorption of blood sugar known as glucose, to be stored in the muscles and in the liver. This can then be broken down and use for energy later when needed (Daneman, 2006).
Decreased insulin production causes an increase in blood glucose levels. For this reason, fat and muscle cells are deprived of glucose for energy. In a healthy person, the insulin is produced by special cells known as beta cells. They allow for these sugars to be moved by other cells. But in a Type 1 diabetic individual, the body’s defense cells attack these beta cells and destroy them (Alberti, G., & Zimmet, 1998).
Signs and Symptoms
Most patients will have symptoms of high blood sugar levels or hyperglycaemia such as: excessive hunger, excessive thirst, feeling tired, frequent urination, unexplained weight loss, blurred vision,and fatigue.
The specific clinical symptoms associated with the classic onset of hyperglycaemia and T1DM includes: polyuria, polydipsia, and weight loss.Polyuria is when the serum glucose concentration rises significantly above 180 mg/dL (10 mmol/L), exceeding the renal threshold for glucose, which leads to increased urinary glucose excretion. Polyuria may present as nocturia, bedwetting, or daytime incontinence. Polydipsia is enhanced thirst because of the increased serum osmolality from hyperglycaemia and hypovolemia. Weight loss is a result of hypovolemia (decreased volume of circulating blood in the body) and increased catabolism. Insulin deficiency in diabetic children impairs glucose utilization in skeletal muscle and increases fat and muscle breakdown. Initially, appetite is increased, but over time, children are more thirsty than hungry, and ketosis leads to nausea and anorexia, contributing to weight loss.
If hyperglycaemia goes untreated, your body resorts to using its fats and protein stores as an alternate source of energy. As fat breakdown continues, certain byproducts, known as ketone bodies, accumulate in the blood, resulting in ketosis. When ketone build up to dangerously high levels, a life threatening condition called diabetic ketoacidosis results. If one’s blood glucose level remains high over time, long term health problems can occur, such as atherosclerosis, nerve damage, blindness, and kidney disease.
Diagnosis
The complete diagnosis of T1DM is based on your symptoms and blood tests. Blood tests are a measure of blood sugar levels. Higher blood sugar levels coupled with the aforementioned symptoms is an indication of DM.
T1DM is characterized primarily by insulin deficiency, whereas T2DM is characterized primarily by insulin resistance with relative insulin deficiency. As the incidence of T2DM increases in children and adolescents, it becomes increasingly important to distinguish type 1 from type 2 disease, because long-term management differs.
Causes
T1DM is a chronic medical condition that occurs when the pancreas produces little to no insulin. Insulin is a hormone that helps the body absorb and use glucose and other nutrients from food, store fat, and build up protein. Without insulin, blood glucose levels become elevated. T1DM normally starts in childhood or young adulthood, but can develop at any age.
T1DM usually develops when the immune system destroys the insulin-producing cells, called the beta cells, in the pancreas. Hence, the pancreatic beta cells loose their ability to produce insulin. In T1DM, your immune system, specifically your WBCs, mistake your pancreatic beta cells for foreign invaders. In an autoimmune response, your WBC secrete autoantibodies that destroy your own beta cells. Thus the pancreas produce little to no insulin. This is an autoimmune response.
Pathobiology
Management of Type 1 Diabetes
Monitoring of Blood Glucose Levels
Blood glucose levels must be tested a minimum of 4 times/day when the patient is in a fasted state and before bed. The blood glucose meter is the instrument used to monitor blood glucose levels and only requires a small sample of blood of around 0.3-1 microliter that is taken by a fingerstick.
Frequent monitoring of blood glucose levels is essential for those suffering from T1DM and has shown to optimize glycemic control and decrease the severity of hypoglycaemia.T1DM Leads to increased production of ketoacids. These small molecules have the effect of reducing the pH of whatever solution they reside in leading to an acidic environment. In order to prevent diabetic ketoacidosis, it is essential for the patient to check urine and blood ketones when blood glucose levels reach optimal levels of more than or equal to 250 mg/dL. Periods of acute episodes such as increases in stress may also lead to ketoacidosis. When hypoglycemic levels and blood ketones increase in a patient, it is essential for the patient to be treated with additional insulin and strict monitoring precautions must be put in place of their ketone concentrations and blood glucose levels.
Devices known as “continuous glucose monitoring (CGM) systems” use glucose sensors that continuously measure interstitial fluid glucose levels. There are several different types of CGM devices: The first device is the CGM without real-time feedback, in which a patient needs to go to a physician in order to obtain the blood glucose data. The second device is the CGM with real-time feedback, in which a patient can receive instant information about his/her blood glucose levels within each time interval. Lastly, sensor-augmented insulin pump devices are used in combination with an insulin pump. The insulin dosage is adjusted based on the blood glucose data that the CGM shows on the screen.
Insulin Injections & Insulin Pump
Insulin can be taken through multiple daily injections (as a shot several times a day), or infused through a small needle known as a catheter, which is attached to an insulin pump. Insulin pumps consist of rapid or short acting insulin delivered in small increments every few minutes. Therefore, a patient can easily increase the dosage after a meal. The instructions for the dosage are entered into the pump’s small computer and the appropriate amount of insulin is then injected into the body. Insulin is delivered by inserting a catheter into the abdominal fat and is replaced every 2 to 3 days.
Treatments and drugs
Islet Transplantation
Islet cell transportation is a procedure that is performed to transplant the islets taken from a donated pancreas usually from a deceased donor, into a diabetic patient’s liver. Inorder to isolate the islets of Langerhans from the deceased donor’s pancreas, a thin tube is introduced into the pancreatic duct which runs throughout the whole pancreas carrying with it a mixture of highly purified enzymes known as collagenases. The delivery of the collagenase solution into the pancreatic duct leads to the enlargement and distention of the organ, due to the internal pressure. The swollen pancreas is chopped into small pieces and further transferred into a machine called Ricordi's chamber. Inside, the Ricordi’s chamber, digestion takes place in order to isolate the islets and prepare for their removal from the solution. They then go through a process of purification in order to separate the isolated islets from the exocrine tissue and debris. Transplantation starts with placing a small catheter through a tiny incision into the upper abdomen and into the portal vein of the liver. The patient receives a local anesthetic, and then the islets are infused through the catheter allowing them to enter the diabetic patient’s liver. Finding the proper placement of the catheter is very risky, and requires the use of an ultrasound and radiography techniques. After the islets are successfully transplanted in the liver, insulin starts to be produced, resulting in the immediate release of beta cells. Immunosuppressant drugs are prescribed to patients after the transplant in order to ensure that their immune system doesn’t attach the transplanted islets.
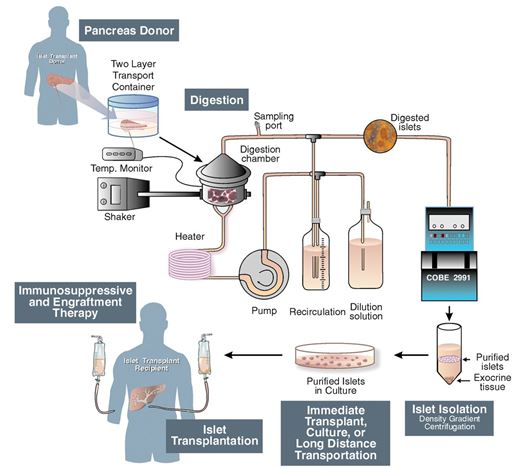 Figure 1: Schematic of Islet Transplantation (Shapiro, 2006)
Figure 1: Schematic of Islet Transplantation (Shapiro, 2006)
Bi-hormonal Bionic Pancreas
A bionic pancreas that would automatically control blood glucose levels for type 1 diabetes patients is proposed to be brought to the market by 2017. The Bionic Pancreas is worn externally and the insulin is infused inside the skin. This device functions similarly to the biologic pancreas (physiological function) with a push/pull mechanism combining insulin and glucagon. The device consists of a Dexcom continuous glucose monitor (CGM), two infusion pumps and mathematical algorithms which runs on a smartphone. The Dexcom continuous glucose monitor (CGM) device checks glucose levels every 5 min, in order to make decisions whether or not to release insulin or glucagon. The dual pump (insulin pump and glucagon pump) is commanded by the smartphone’s app to deliver either insulin if glucose levels rise or glucagon if glucose levels drop. There are several advantages to using this device. This device has the ability in removing human error and lack of precision out of diabetes management. This treatment option will not only ease the burden of therapy for the insulin-dependent patients, but will also improve insulin therapy until glycemic control becomes normal.
The first human study of a Bi-Hormonal Bionic endocrine Pancreas was performed by Russel et al in 2008. The researchers tested a closed-loop system that took venous plasma glucose (PG) measurements every 5 minutes. These measurements went into a computer algorithm that ran on a laptop. Nurses would take the insulin and glucagon doses delivered by the algorithm, and using dual pumps, they would manually enter the doses every 5 minutes over a course of 24 hours. These insulin pumps delivered the insulin and glucagon through subcutaneous infusion sets (Cleo® 90, Smiths Medical) inserted into the abdomen. The study only included T1DM subjects with undetectable C-peptides. Undetectable C-peptides allowed the researchers to ensure that the device is completely controlling their glucose levels, and that the subjects were not making any of their own insulin. A closed-loop control was performed for 24 hours, including three high carbohydrate meals. Blood samples were taken every 10 minutes to measure insulin and glucagon levels. This gave researchers insight in how these drugs were managed in real life. It was found that glucose control without hypoglycemia is feasible with a bi-hormonal bionic pancreas. Moreover, the micro dose glucagon was effective and well tolerated. However, the system still required an accurate continuous glucose monitor (CGM) that was able to make blood glucose measurements every 5 minutes.
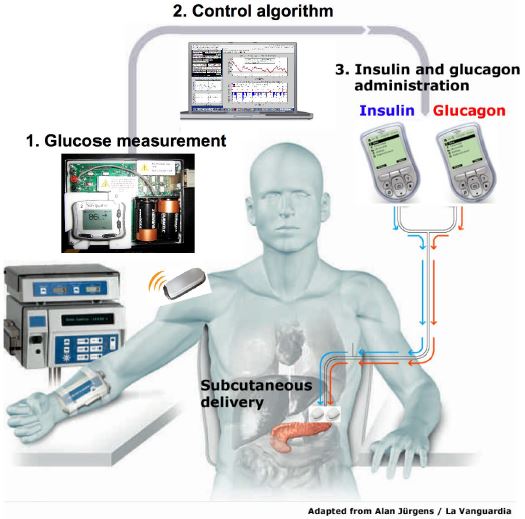 Figure 2: the bihormonal bionic endocrine pancreas used in the clinical trial. (Russell, 2012)
Figure 2: the bihormonal bionic endocrine pancreas used in the clinical trial. (Russell, 2012)
The second part of the study tested the accuracy and reliability of three different continuous glucose monitors (CGMs). During the study the subjects wore three CGMs across their abdomen and they compared the results from each of the CGM’s blood glucose values to measure the accuracy of the devices. The three CGM devices included the Dexcom Seven (2nd generation), Abbott Navigator and the Minimed Gaurdian RT. It was found that there was a huge difference in their accuracy. The mean absolute relative deviation (MARD) measured the average error in each of the devices. It was found that the average error for the Abbott Navigator device was 90%, the Dexcom Seven device was 56% and the Minimed Gaurdian RT was 69%. The researchers decided to move forward with a 48 hour CGM driven closed-loop control. The researchers used the Abbott Navigator CGM device that ran automatically every 5 minutes. A portable system was developed. Since, there was a delay/lag in picking up blood glucose levels with the CGM; the researchers announced the meals to the controlled, which would give a very small priming bolus of insulin at the exact same time the subjects started to eat. The bolus of insulin was intended to be half of the insulin required to manage the meal. The closed loop control lasted for 48 hours, and subjects had 6 meals with the incorporation of exercise. It was found that the mean blood glucose bolus was 152 mg/dl with most of the blood glucose values between the 70–180 mg/dl range (refer to figure 2). These results indicate that a hypoglycemic range was rare, and the subjects were able to manage the disease. It was also found that the incorporation of exercise allowed the subjects to uptake glucose into the muscle, independent of insulin. This study showed that a CGM driven, bihormonal blood glucose control is effective for 2 continuous days in the face of exercise and large meals. However, optimizing meal priming boluses may have improved control.
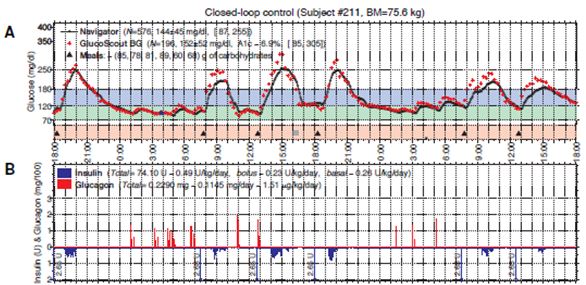 Figure 3: Typical 48-hour closed-loop experiment using a meal priming bolus of 0.035 U/kg. Figure 2A shows the blood glucose values (high carb meals) over the course of 48 hours. Figure 2B shows the insulin levels in blue and glucagon level is in red. (Russell, 2012)
Figure 3: Typical 48-hour closed-loop experiment using a meal priming bolus of 0.035 U/kg. Figure 2A shows the blood glucose values (high carb meals) over the course of 48 hours. Figure 2B shows the insulin levels in blue and glucagon level is in red. (Russell, 2012)
Since, the bionic pancreas was still in its experimental stages, the study used a laptop driven system, instead of a phone driven system. Nowadays, the algorithm is controlled using a smartphone through a custom app. Bluetooth technology allows the pumps and the smartphone to communicate and calculate the required doses of insulin and glucagon needed. This allows the smartphone to control the pumps wirelessly, instructing them to release either insulin or glucagon.
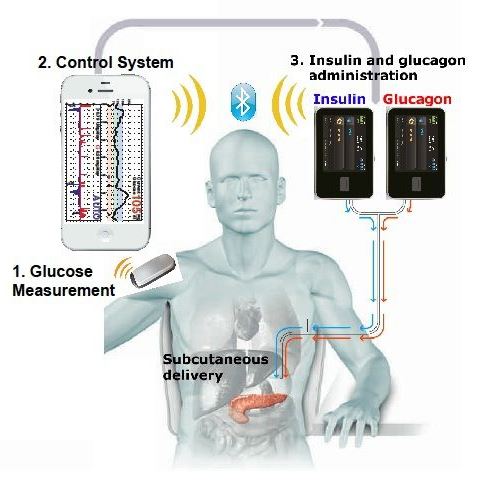 Figure 4: the bihormonal bionic endocrine pancreas, with the incorporation of a smartphone. (Philip Elmer-DeWitt, 2014)
Figure 4: the bihormonal bionic endocrine pancreas, with the incorporation of a smartphone. (Philip Elmer-DeWitt, 2014)
References
Alberti, K. G. M. M., & Zimmet, P. F. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation.Diabetic medicine, 15(7), 539-553.
Atkinson, M. A., Eisenbarth, G. S., & Michels, A. W. (2014). Type 1 diabetes.The Lancet, 383(9911), 69-82.
Atkinson, M. A., & Eisenbarth, G. S. (2001). Type 1 diabetes: new perspectives on disease pathogenesis and treatment. The Lancet,358(9277), 221-229.
Daneman, D. (2006). Type 1 diabetes. The Lancet, 367(9513), 847-858.
Karvonen, M., Viik-Kajander, M., Moltchanova, E., Libman, I., LaPorte, R. O. N. A. L. D., & Tuomilehto, J. (2000). Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes care,23(10), 1516-1526.
Levitsky, L., Misra, M. (2015). Management of type 1 diabetes mellitus in children and adolescents.UptoDate.
Russell, S. et al. (2012). Blood Glucose Control in Type 1 Diabetes with a Bihormonal Bionic Endocrine Pancreas. Diabetes Care, 35(11), 2148-55.
Shapiro, J., Merani, S. (2006). Current Status of Pancreatic Islet Transplantation. Clinical Science, 110(6), 611-625.
Robertson R (2004). Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med 350 (7): 694–70
Enriquez, J. (2015). Could A Bionic Pancreas Be Ready By 2017?. Yale News. Retrieved January 20, 2016, from http://www.meddeviceonline.com/doc/could-a-bionic-pancreas-be-ready-by-0001
Elmer-DeWitt, P. (2014). Apple-powered bionic pancreas one step closer. Fortune. Retrieved January 20, 2016, from http://fortune.com/2014/06/16/apple-powered-bionic-pancreas-one-step-closer/