Table of Contents
TYPE 1 DIABETES
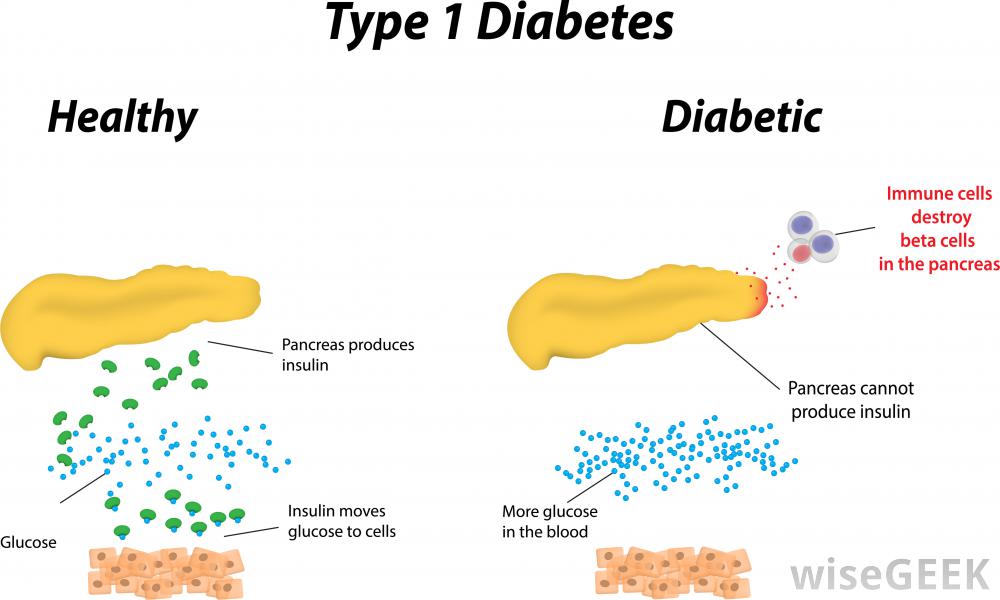
Diabetes mellitus is a disease in which blood sugar levels rise above the normal for a prolonged period of time. There are two forms of diabetes: Type 1 and Type 2. According to the International Diabetes federation, it is estimated that 1 out of 12 people have diabetes. 50% of which are undiagnosed and therefore unaware of their condition, and of these 5-10% have Type 1 (Daneman, 2006). In Type 1 diabetes, the pancreas does not produce enough insulin. Insulin is a hormone in the body that promotes the absorption of blood sugar known as glucose, to be stored in the muscles and in the liver. This can then be broken down and used for energy later when needed (Daneman, 2006).
Decreased insulin production causes an increase in blood glucose levels. For this reason, fat and muscle cells are deprived of glucose for energy. In a healthy person, the insulin is produced by special cells known as beta cells. They allow for these sugars to be moved by other cells. But in a Type 1 diabetic individual, the body’s defense cells attack these beta cells and destroy them (Alberti, G., & Zimmet, 1998). When attacked, the body suffers making it unable for the individual to properly intake glucose, affecting their eating choices. Additionally, without the presence of insulin, the body will allow glucose to build up to unsafe amounts instead of being used for energy intake. It is especially hard for younger children since it is one of the most common illnesses faced (Davendra, Liu, & Eisenbarth, 2004).
Figure 1: Pathway of insulin in healthy vs. Diabetic individuals
Epidemiology
Type 1 Diabetes, being an autoimmune disease, is largely considered to be a disorder with a heavy genetic component, inherited through the HLA complex in individuals (Atkinson, Eisenbarth, & Michels, 2014). Even though previous descriptions of the disease show onset in early adulthood, recent studies by Atkinson et al. (2014) show only 50-60% of individuals affected are around 16-18 years of age. Additionally, patterns of Type 1 Diabetes have shown that incidences of Type 1 Diabetes has increased around 2-5% per year on a global scale. Specifically in demographic populations, China is considered to be the country with the lowest incidence rate, followed by the United Kingdom, and Finland with 100 times more cases per year (Daneman, 2006).
According to a consensus by Daneman (2006), a trend of decreasing age at diagnosis shows a possibility of environmental factors affecting younger populations. Additionally, the increasing weight of younger populations in society has shown to increase incidences of Type 1 Diabetes in children under the age of 5. (Daneman, 2006)
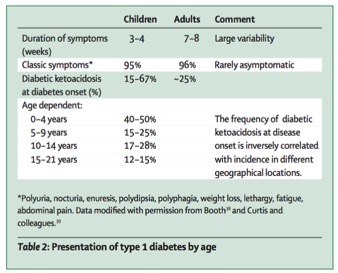 Figure 2: Type 1 diabetes incidence rates in children vs. adults
Figure 2: Type 1 diabetes incidence rates in children vs. adults
Signs and Symptoms
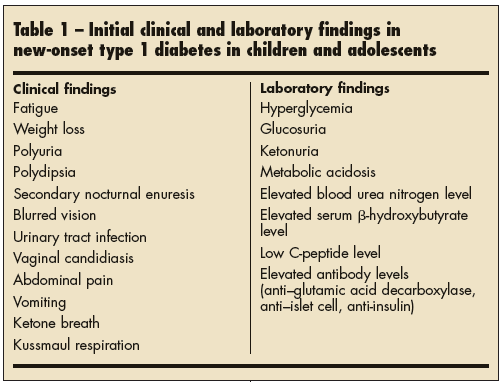
Figure 3: Clinical and laboratory signs and symptoms of Type 1 Diabetes
Most patients will have symptoms of high blood sugar levels or hyperglycaemia such as: excessive hunger, excessive thirst, feeling tired, frequent urination, unexplained weight loss, blurred vision,and fatigue.
The specific clinical symptoms associated with the classic onset of hyperglycaemia and T1DM includes: polyuria, polydipsia, and weight loss.Polyuria is when the serum glucose concentration rises significantly above 180 mg/dL (10 mmol/L), exceeding the renal threshold for glucose, which leads to increased urinary glucose excretion. Polyuria may present as nocturia, bedwetting, or daytime incontinence. Polydipsia is enhanced thirst because of the increased serum osmolality from hyperglycaemia and hypovolemia. Weight loss is a result of hypovolemia (decreased volume of circulating blood in the body) and increased catabolism. Insulin deficiency in diabetic children impairs glucose utilization in skeletal muscle and increases fat and muscle breakdown. Initially, appetite is increased, but over time, children are more thirsty than hungry, and ketosis leads to nausea and anorexia, contributing to weight loss.
Patients with the aforementioned symptoms normally present in the ambulatory setting appearing ill, with general complaints, such as weight loss and lethargy (Quinn, Fleischman, Rosner et al. 2006) In a study, the mean duration of symptoms before presentation was found to be ten days (Roche, Menon, Gill, & Hoey 2005). The classic symptoms of polyuria and polydipsia are present in more than 90 percent of patients, but are not always the initial complaints patients present with, and may become apparent only after obtaining a careful history (i.e. nocturia and bedwetting, increased frequency and/or unusually wet diapers, and persistent thirst). Weight loss is a symptom in about half of children.
If hyperglycaemia goes untreated, your body resorts to using its fats and protein stores as an alternate source of energy. As fat breakdown continues, certain byproducts, known as ketone bodies, accumulate in the blood, resulting in ketosis. When ketone build up to dangerously high levels, a life threatening condition called diabetic ketoacidosis results. If one’s blood glucose level remains high over time, long term health problems can occur, such as atherosclerosis, nerve damage, blindness, and kidney disease.
The reported frequency of DKA as the initial presenting symptom for childhood T1DM is approximately 30 percent, but varies from 15 to 67 percent (Wolfsdorf, Glaser & Sperling 2006)
Diagnosis
The complete diagnosis of T1DM is based on your symptoms and blood tests. Blood tests are a measure of blood sugar levels. Higher blood sugar levels coupled with the aforementioned symptoms is an indication of DM.
T1DM is characterized primarily by insulin deficiency, whereas T2DM is characterized primarily by insulin resistance with relative insulin deficiency. As the incidence of T2DM increases in children and adolescents, it becomes increasingly important to distinguish type 1 from type 2 disease, because long-term management differs.
Diabetes mellitus is diagnosed based upon one of the following four signs of abnormal glucose metabolism (America Diabetes Association, 2011)
● Fasting plasma glucose ≥126 mg/dL (7 mmol/L) on more than one occasion. Fasting is defined as no caloric intake for at least eight hours.
●Random venous plasma glucose ≥200 mg/dL (11.1 mmol/L) in a patient with classic symptoms of hyperglycemia
Although there is no specific test to distinguish between the two types of diabetes, T1DM is suggested by the presence of circulating, islet-specific, pancreatic autoantibodies against glutamic acid decarboxylase (GAD65), the 40K fragment of tyrosine phosphatase (IA2), insulin, and/or zinc transporter 8 (ZnT8). However, the absence of these autoantibodies does not rule out the possibility of T1DM. A recent study has found that up to 30 percent of individuals with the classical appearance and presentation of T2DM have positive autoantibodies and may have a slowly progressive type of autoimmune diabetes (Klingensmith, Pyle, Arslanian et al. 2010).
Causes
T1DM is a chronic medical condition that occurs when the pancreas produces little to no insulin. Insulin is a hormone that helps the body absorb and use glucose and other nutrients from food, store fat, and build up protein. Without insulin, blood glucose levels become elevated. T1DM normally starts in childhood or young adulthood, but can develop at any age.
T1DM usually develops when the immune system destroys the insulin-producing cells, called the beta cells, in the pancreas. Hence, the pancreatic beta cells loose their ability to produce insulin. In T1DM, your immune system, specifically your WBCs, mistake your pancreatic beta cells for foreign invaders. In an autoimmune response, your WBC secrete autoantibodies that destroy your own beta cells. Thus the pancreas produce little to no insulin. This is an autoimmune response.
Pathogenesis
Genetic predisposition is considered to be a primary factor involved in the development of Type-1 diabetes. Children and siblings of Type-1 diabetics are at a much greater risk of carrying genes associated with the disease (5-6%) as opposed to individuals with no direct family history link (0.4%) (Diabetes Care, 2005).
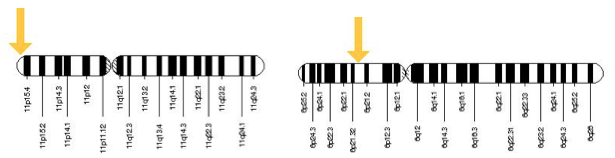
Type-1 diabetes has been observed to have a direct correlation with the Human Leukocyte Antigen (HLA), the human equivalent of the Major Histocompatibility Complex (MHC). MHC allows for the binding of antigens in order to present select cells to specific antigen receptors located on T lymphocytes (T-cells) (Lie et al., 1999). These major effector cells in the body allow for receptor mediated destruction in the immunological response (Brusko, Wasserfall, Clare-Salzler, Schatz, & Atkinson, 2005). Specific to Type-1 diabetes, irregularities of the HLA gene on chromosome 6 at the IDDM1 locus accounts for approximately fifty-percent of unique genetic content for this pathological disease (Lie et al., 1999). Candidate gene studies conducted display a ninety-percent association rate in regards to two identified haplotype combinations of HLA; DR4-DQ8 and DR3-DQ2 (Lie et al., 1999). A third haplotype, DR15-DQ6 is more prevalent in non-diabetics and thus can be inferred as a protective sequence from autoimmune response towards pancreatic beta-cells (Gillespie, 2006).
Figure 4: Location of the INS and HLA gene on chromosome 11 and chromosome 6, respectively. Retrieved from http://ghr.nlm.nih.gov/gene/INS and http://ghr.nlm.nih.gov/gene/HLA-B.
An additional genetic factor contributing to Type-1 diabetes can be attributed to sequencing differences of the insulin production gene (INS) located on chromosome 11. Size variations of repeating units in the non-coding region of INS lead to either predisposing or protective attributes of the disease, carrying up to ten percent of genetic content specific to Type-1 diabetic individuals. The number of non-coding repeated units on the INS gene has been classified by a system termed as Variable Number of Tandem Repeats (VNTR) (Nakamura et al., 1987). VNTR can be divided into three categories based on repeating unit length; class 1 containing 26 to 63 repeat units, class 2 containing approximately 80 repeat units, and class 3 containing 141 to 209 repeat units (Vafiadis et al., 2001). Risk percentage has been observed to be inversely proportional to sequencing size, such that class 1 individuals are more highly predisposed to Type-1 diabetes while class 3 individuals exhibit more protective properties against the disease (Vafiadis et al., 2001). After the correlation between VNTR and risk percentage had been identified, influence of VNTR in regards to insulin production had been explored. Speculation has arisen that the effects of VNTR influence insulin production via the transcription of the INS gene as opposed to directly altering the protein sequence itself due to its placement in the non-coding region (Pugliese et al., 1997).
Pathophysiology
The failure to produce insulin due to the autoimmune destruction of pancreatic beta-cells result in the inability of peripheral muscles and adipose tissue to uptake available glucose in the blood stream (Kilpatrick, Alan, & Atkin, 2006). Upon the destruction of 80-90% of pancreatic beta-cells, individuals begin to display overt symptoms of hyperglycemia, a phenomenon of increased blood sugar levels (Kilpatrick, Alan, & Atkin, 2006). With growing trends of obesity found in the general population, Type-1 diabetics experiencing insulin deficiency may still be prone to exhibiting insulin resistance experienced by individuals having Type-2 diabetes.
A significant long-term impact held by hyperglycemic patients is the accumulation of glycosylated tissue and serum proteins. Glycosylation is the process under which adduction of glucose to protein molecules occurs (Stanaway & Gill, 2000). In the initial reaction, an unstable aldimine molecule is formed when the protein’s amino terminal reacts with the carbonyl group of the sugar. The concentration of aldimine is proportional to the concentration of the surrounding glucose such that a decrease in the glucose concentration leads to a fall in the aldimine concentration due to uncoupling. The unstable aldimine undergo a process termed the Amadori transformation, forming more stable products known as Early Glycation Products (EGP) (Stanaway & Gill, 2000). Like aldimine, EGP can dissociate and reduce the overall concentration if there is a decrease in surrounding glucose levels. When certain macromolecules such as DNA and collagen undergo glycolysis, the resulting Amadori products will transform into Advanced Glycation End-products (AGEs) as opposed to forming EGP (Stanaway & Gill, 2000). This is due to the fact that the parent molecules have increased longevity in contrast with other glycated molecules. AGEs form and accumulate throughout one’s life and cannot dissociate unlike EGPs and aldimine molecules.
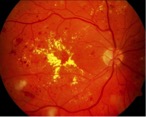
The accumulation of AGEs can cause or accelerate the onset of various diseases in the body including atherosclerosis, retinopathy and nephropathy. The binding of AGEs to Low Density Lipoproteins (LDL) can accelerate atherosclerosis regardless of the level of cholesterol in the serum (Kilpatrick, Alan, & Atkin, 2006). Similarly, the binding of AGEs to blood vessel proteins can compromise the structure of the vessel itself, allowing for the leakage of surrounding plasma and serum proteins. This ultimately leads to the damaging of major organs such as the eye and the kidney, causing diabetic retinopathy and nephropathy respectively (Kilpatrick, Alan, & Atkin, 2006).
Figure 5: Aneurysm development in retinal blood vessels causes fluid leakage to surrounding tissue due to AGEs accumulation in hyperglycaemic individuals. Retrieved from http://www.diabetes.ca/diabetes-and-you/complications/eye-damage-diabetic-retinopathy.
Management of Type 1 Diabetes
Monitoring of Blood Glucose Levels
Blood glucose levels must be tested a minimum of 4 times/day when the patient is in a fasted state and before bed (Levitsky et al., 2015). The blood glucose meter is the instrument used to monitor blood glucose levels and only requires a small sample of blood of around 0.3-1 microliter that is taken by a fingerstick (Levitsky et al., 2015).
Frequent monitoring of blood glucose levels is essential for those suffering from T1DM and has shown to optimize glycemic control and decrease the severity of hypoglycaemia.T1DM Leads to increased production of ketoacids (Levitsky et al., 2015). These small molecules have the effect of reducing the pH of whatever solution they reside in leading to an acidic environment (Levitsky et al., 2015). In order to prevent diabetic ketoacidosis, it is essential for the patient to check urine and blood ketones when blood glucose levels reach optimal levels of more than or equal to 250 mg/dL. Periods of acute episodes such as increases in stress may also lead to ketoacidosis (Levitsky et al., 2015). When hypoglycemic levels and blood ketones increase in a patient, it is essential for the patient to be treated with additional insulin and strict monitoring precautions must be put in place of their ketone concentrations and blood glucose levels (Levitsky et al., 2015).
Devices known as “continuous glucose monitoring (CGM) systems” use glucose sensors that continuously measure interstitial fluid glucose levels (Levitsky et al., 2015). There are several different types of CGM devices: The first device is the CGM without real-time feedback, in which a patient needs to go to a physician in order to obtain the blood glucose data (Levitsky et al., 2015). The second device is the CGM with real-time feedback, in which a patient can receive instant information about his/her blood glucose levels within each time interval (Levitsky et al., 2015). Lastly, sensor-augmented insulin pump devices are used in combination with an insulin pump. The insulin dosage is adjusted based on the blood glucose data that the CGM shows on the screen (Levitsky et al., 2015).
Possible Treatments
Insulin/ Insulin Pump
Insulin can be taken through multiple daily injections (as a shot several times a day), or infused through a small needle known as a catheter, which is attached to an insulin pump (Levitsky et al., 2015). Insulin pumps consist of rapid or short acting insulin delivered in small increments every few minutes (Levitsky et al., 2015). Therefore, a patient can easily increase the dosage after a meal (Levitsky et al., 2015). The instructions for the dosage are entered into the pump’s small computer and the appropriate amount of insulin is then injected into the body (Levitsky et al., 2015). Insulin is delivered by inserting a catheter into the abdominal fat and is replaced every 2 to 3 days (Levitsky et al., 2015).
Islet Transplantation
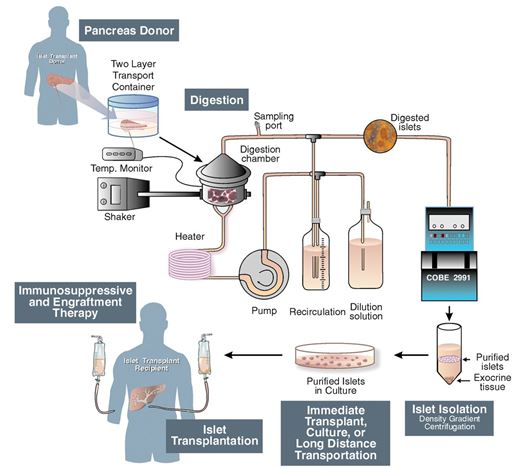
Islet cell transportation is a procedure that is performed to transplant the islets taken from a donated pancreas usually from a deceased donor, into a diabetic patient’s liver (Shapiro, 2006). 1) a thin tube is introduced into the pancreatic duct which runs throughout the whole pancreas carrying with it a mixture of highly purified enzymes known as collagenases 2) This leads to the enlargement and distention of the organ, due to the internal pressure 3)The swollen pancreas is chopped into small pieces and further transferred into a machine called Ricordi's chamber 4) Inside, the Ricordi’s chamber, digestion takes place in order to isolate the islets and prepare for their removal from the solution 5) Purification proceeds in order to separate the isolated islets from the exocrine tissue 6) Transplantation starts with placing a small catheter through a tiny incision into the upper abdomen and into the portal vein of the liver 7) The patient receives a local anesthetic, and then the islets are infused through the catheter allowing them to enter the diabetic patient’s liverFinding the proper placement of the catheter is very risky, and requires the use of an ultrasound and radiography techniques 8) After the islets are successfully transplanted in the liver, insulin starts to be produced, resulting in the immediate release of beta cells Immunosuppressant drugs are prescribed to patients after the transplant in order to ensure that their immune system doesn’t attach the transplanted islets (Shapiro, 2006).
Figure 6: Schematic of islet transplantation procedure
Bi-hormonal Bionic Pancreas
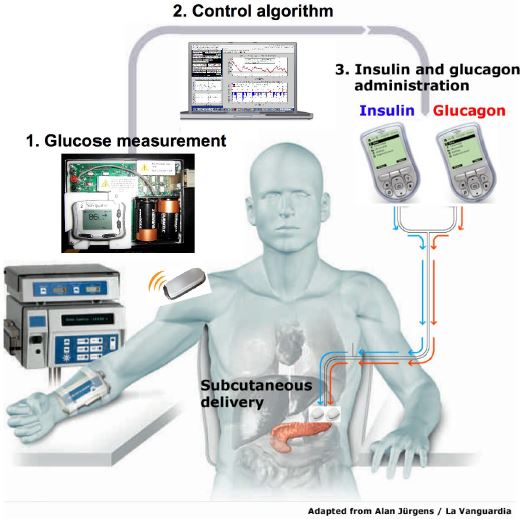
A bionic pancreas that would automatically control blood glucose levels for type 1 diabetes patients is proposed to be brought to the market by 2017 (Enriquez, 2015). The Bionic Pancreas is worn externally and the insulin is infused inside the skin. This device functions similarly to the biologic pancreas (physiological function) with a push/pull mechanism combining insulin and glucagon. The device consists of a Dexcom continuous glucose monitor (CGM), two infusion pumps and mathematical algorithms which runs on a smartphone (Enriquez, 2015). The Dexcom continuous glucose monitor (CGM) device checks glucose levels every 5 min, in order to make decisions whether or not to release insulin or glucagon (Enriquez, 2015). The dual pump (insulin pump and glucagon pump) is commanded by the smartphone’s app to deliver either insulin if glucose levels rise or glucagon if glucose levels drop (Enriquez, 2015). There are several advantages to using this device. This device has the ability in removing human error and lack of precision out of diabetes management. This treatment option will not only ease the burden of therapy for the insulin-dependent patients, but will also improve insulin therapy until glycemic control becomes normal.
The first human study of a Bi-Hormonal Bionic endocrine Pancreas was performed by Russel et al, 2008 in 2008 (Russell et al., 2012). The researchers tested a closed-loop system that took venous plasma glucose (PG) measurements every 5 minutes (Russell et al., 2012). These measurements went into a computer algorithm that was run on a laptop (Russell et al., 2012). Nurses would take the insulin and glucagon doses delivered by the algorithm, and using dual pumps, they would manually enter the doses every 5 minutes over a course of 24 hours (Russell et al., 2012). These insulin pumps delivered the insulin and glucagon through subcutaneous infusion sets (Cleo® 90, Smiths Medical) inserted into the abdomen (Russell et al., 2012). The study only included T1DM subjects with undetectable C-peptides (Russell et al., 2012). Undetectable C-peptides allowed the researchers to ensure that the device is completely controlling their glucose levels, and that the subjects were not making any of their own insulin (Russell et al., 2012). A closed-loop control was performed for 24 hours, including three high carbohydrate meals (Russell et al., 2012). Blood samples were taken every 10 minutes to measure insulin and glucagon levels (Russell et al., 2012). This gave researchers insight in how these drugs were managed in real life (Russell et al., 2012). It was found that glucose control without hypoglycemia is feasible with a bi-hormonal bionic pancreas (Russell et al., 2012). Moreover, the micro dose glucagon was effective and well tolerated (Russell et al., 2012). However, the system still required an accurate continuous glucose monitor (CGM) that was able to make blood glucose measurements every 5 minutes (Russell et al., 2012).
Figure 7: The bihormonal bionic endocrine pancreas used in the clinical trial. (Russell, 2012)
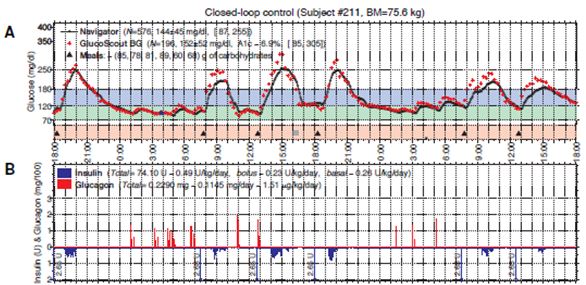
The second part of the study tested the accuracy and reliability of three different continuous glucose monitors (CGMs) (Russell et al., 2012). During the study the subjects wore three CGMs across their abdomen and they compared the results from each of the CGM’s blood glucose values to measure the accuracy of the devices (Russell et al., 2012). The three CGM devices included the Dexcom Seven (2nd generation), Abbott Navigator and the Minimed Gaurdian RT (Russell et al., 2012). It was found that there was a huge difference in their accuracy (Russell et al., 2012). The mean absolute relative deviation (MARD) measured the average error in each of the devices (Russell et al., 2012). It was found that the average error for the Abbott Navigator device was 90%, the Dexcom Seven device was 56% and the Minimed Gaurdian RT was 69% (Russell et al., 2012). The researchers decided to move forward with a 48 hour CGM driven closed-loop control (Russell et al., 2012). The researchers used the Abbott Navigator CGM device that ran automatically every 5 minutes (Russell et al., 2012). A portable system was developed. Since, there was a delay/lag in picking up blood glucose levels with the CGM; the researchers announced the meals to the controlled, which would give a very small priming bolus of insulin at the exact same time the subjects started to eat (Russell et al., 2012). The bolus of insulin was intended to be half of the insulin required to manage the meal (Russell et al., 2012). The closed loop control lasted for 48 hours, and subjects had 6 meals with the incorporation of exercise (Russell et al., 2012). It was found that the mean blood glucose bolus was 152 mg/dl with most of the blood glucose values between the 70–180 mg/dl range (refer to figure below) (Russell et al., 2012). These results indicate that a hypoglycemic range was rare, and the subjects were able to manage the disease (Russell et al., 2012). It was also found that the incorporation of exercise allowed the subjects to uptake glucose into the muscle, independent of insulin (Russell et al., 2012). This study showed that a CGM driven, bihormonal blood glucose control is effective for 2 continuous days in the face of exercise and large meals. However, optimizing meal priming boluses may have improved control (Russell et al., 2012).
Figure 8: Typical 48-hour closed-loop experiment using a meal priming bolus of 0.035 U/kg. Figure 2A shows the blood glucose values (high carb meals) over the course of 48 hours. Figure 2B shows the insulin levels in blue and glucagon level is in red. (Russell, 2012)
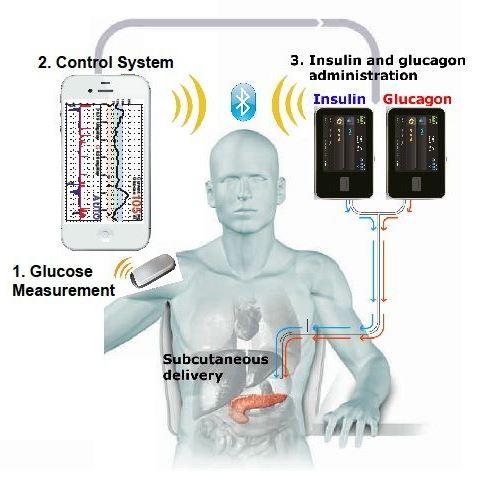
Since, the bionic pancreas was still in its experimental stages, the study used a laptop driven system, instead of a phone driven system. Nowadays, the algorithm is controlled using a smartphone through a custom app. Bluetooth technology allows the pumps and the smartphone to communicate and calculate the required doses of insulin and glucagon needed. This allows the smartphone to control the pumps wirelessly, instructing them to release either insulin or glucagon.
Figure 9: The bihormonal bionic endocrine pancreas, with the incorporation of a smartphone. (Philip Elmer-DeWitt, 2014)
References
Alberti, K. G. M. M., & Zimmet, P. F. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation.Diabetic medicine, 15(7), 539-553.
American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care 2011; 34 Suppl 1:S11.
Atkinson, M. A., Eisenbarth, G. S., & Michels, A. W. (2014). Type 1 diabetes.The Lancet, 383(9911), 69-82.
Atkinson, M. A., & Eisenbarth, G. S. (2001). Type 1 diabetes: new perspectives on disease pathogenesis and treatment. The Lancet,358(9277), 221-229.
Brusko, T. M., Wasserfall, C. H., Clare-Salzler, M. J., Schatz, D. A., & Atkinson, M. A. (2005). Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes, 54(5), 1407-1414.
Daneman, D. (2006). Type 1 diabetes. The Lancet, 367(9513), 847-858.
Devendra, D., Liu, E., & Eisenbarth, G. S. (2004). Type 1 diabetes: recent developments. Bmj, 328(7442), 750-754.
Diabetes Care. (2005).Diagnosis and classification of diabetes mellitus.Diabetes care, 28, S37.
Elmer-DeWitt, P. (2014). Apple-powered bionic pancreas one step closer. Fortune. Retrieved January 20, 2016, from http://fortune.com/2014/06/16/apple-powered-bionic-pancreas-one-step-closer/
Enriquez, J. (2015). Could A Bionic Pancreas Be Ready By 2017?. Yale News. Retrieved January 20, 2016, from http://www.meddeviceonline.com/doc/could-a-bionic-pancreas-be-ready-by-0001
Gillespie, K. M. (2006). Type 1 diabetes: pathogenesis and prevention.Canadian Medical Association Journal, 175(2), 165-170.
Karvonen, M., Viik-Kajander, M., Moltchanova, E., Libman, I., LaPorte, R. O. N. A. L. D., & Tuomilehto, J. (2000). Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes care,23(10), 1516-1526.
Kilpatrick, Eric S., Alan S. Rigby, and Stephen L. Atkin. “The effect of glucose variability on the risk of microvascular complications in type 1 diabetes.” Diabetes care 29, no. 7 (2006): 1486-1490.
Klingensmith GJ, Pyle L, Arslanian S, et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care 2010; 33:1970.
Levitsky, L., Misra, M. (2015). Management of type 1 diabetes mellitus in children and adolescents.UptoDate.
Lie, B. A., Todd, J. A., Pociot, F., Nerup, J., Akselsen, H. E., Joner, G., & Undlien, D. E. (1999). The predisposition to type 1 diabetes linked to the human leukocyte antigen complex includes at least one non–class II gene.The American Journal of Human Genetics, 64(3), 793-800.
Nakamura, Y., Leppert, M., O'Connell, P., Wolff, R., Holm, T., Culver, M., & Kumlin, E. (1987). Variable number of tandem repeat (VNTR) markers for human gene mapping. Science, 235(4796), 1616-1622.
Pugliese, A., Zeller, M., Fernandez, A., Zalcberg, L. J., Bartlett, R. J., Ricordi, C., & Patel, D. D. (1997). The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nature genetics,15(3), 293-297.
Robertson R (2004). Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med 350 (7): 694–70
Roche EF, Menon A, Gill D, Hoey H. Clinical presentation of type 1 diabetes. Pediatr Diabetes 2005; 6:75.
Russell, S. et al. (2012). Blood Glucose Control in Type 1 Diabetes with a Bihormonal Bionic Endocrine Pancreas. Diabetes Care, 35(11), 2148-55.
Shapiro, J., Merani, S. (2006). Current Status of Pancreatic Islet Transplantation. Clinical Science, 110(6), 611-625.
Stanaway, S. E. R. S., & Gill, G. V. (2000). Protein glycosylation in diabetes mellitus: biochemical and clinical considerations. Practical Diabetes International, 17(1), 21-25.
Vafiadis, P., Ounissi-Benkalha, H., Palumbo, M., Grabs, R., Rousseau, M., Goodyer, C. G., & Polychronakos, C. (2001). Class III alleles of the variable number of tandem repeat insulin polymorphism associated with silencing of thymic insulin predispose to type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism, 86(8), 3705-3710.
Wolfsdorf J, Glaser N, Sperling MA, American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care 2006; 29:1150