This is an old revision of the document!
Table of Contents
Schizophrenia
 Retrieved from: http://fcscortland.org/schizophrenia
Retrieved from: http://fcscortland.org/schizophrenia
Overview
Introduction
The term Schizophrenia means ‘a splitting of the mind’ and is a mental disorder that affects about 0.5% of the population (van Os & Kapur, 2018). It is said to be chronic, which means that it lasts for an extremely long time and can recur frequently. Patients with Schizophrenia often experience altered realities, affecting the way they feel, behave and act. Patients can experience positive symptoms or negative symptoms or both. Positive symptoms are ones that healthy patients do not experience, while negative symptoms are deficiencies in emotions and behaviours (NIMH, 2018). This disease is extremely debilitating, and no known cures are present at this time. Official diagnosis is usually made from medical personnel observing the patient; however, differing cultures may lead to misdiagnosis. The DSM-5 or Diagnostic and Statistical Manual of Mental Disorders is usually used as reference for practitioners. It is common for diagnosed individuals to also have other mental health conditions, such as: anxiety, substance-use or depressive disorders (Schizophrenia Society of Canada, 2018). Antipsychotic medication and psychosocial therapies are generally the most common forms of treatment currently (CMHA, 2018).
History
Emil Kraepelin classified schizophrenia as a mental disorder in 1887. He first named the disorder “dementia praecox” which means early dementia in order to distinguish it from other forms of dementia which occur later on in life (Jablensky, 2010). Kraepelin first isolated schizophrenia from other forms of psychosis in 1887 but this is not to say that this form of mental illness did not exist before Kraepelin’s day (Burton, 2012). In 1911 a swiss psychiatrist by the name of Eugen Bleular created the term schizophrenia. He was the first person who described these symptoms as both “positive” or “negative. He decided to change the name to schizophrenia because Kraepelin’s name was misleading due to the fact that this illness was not a dementia as it did not always lead to mental deterioration (Burton, 2012).
The word “schizophrenia” is derived from the Greek roots schizo which means split and phrene which means mind. This name has influenced the misunderstanding that describes schizophrenia as a split personality disorder but rather those with schizophrenia are instead said to have fragmented thinking (Jablensky, 2010). Bleuler and Kraepelin both further grouped schizophrenia into categories based on the symptoms and prognosis. Five types of schizophrenia are outlined in the DSM III: disorganized, catatonic, paranoid, residual, and undifferentiated (Jablensky, 2010).
Epidemology
Epidemiology is the study of the frequency and the determinants of an illness in the population. A systematic review that looked at epidemiological data which showed that the lifetime prevalence and incidence of schizophrenia is 0.30 - 0.66% and that 10.2-22.0 per 100 000 people/year respectively is considered in isolation. A more narrow definition of schizophrenia looking at patients with an illness duration of at least six month and negative symptoms has a lower rate compared to a more broad definition with less specific criteria (Bromet & Fennig, 1999).
The lifetime prevalence (the proportion of people who have had the disorder at some time in their life) estimate was 4.0/1000 people, while lifetime morbid risk (the proportion of people who will eventually develop the disorder at some time in their life) estimates 7.2 per 1000 people. The median male to female ratio is 1.4:1.2. Symptomatic expression of schizophrenia and related diagnoses is more severe in men than in women and it has also been seen that an earlier onset is found more in men compared to women (McGrath & Sasser, 2009).
Risk Factors
Previous studies have indicated that the condition was solely genetically determined however genome wide association studies have revealed weak associations in just genetic interactions (McDonald & Murray, 2000). There may be genetic risk factors, as well as having an autoimmune disease, a severe infection, and environmental and developmental factors (McDonald & Murray, 2000).
Perinatal brain damage and being born preterm may affect having the disease as well as having low birth weight (McDonald & Murray, 2000). Prenatal exposure to rubella may also be a risk factor for later onset of schizophrenia (McDonald & Murray, 2000). These rubella studies have not been replicated however (McDonald & Murray, 2000). Prenatal exposure to infection could contribute to these findings as they may affect the growth of the brain throughout childhood (McDonald & Murray, 2000). Hypoxic- ischemic damage to the brain may be linked to obstetric complications, which later lead to schizophrenia (McDonald & Murray, 2000).
Childhood risk factors such as having postural and upper limb movement abnormalities were more likely to develop schizophrenia (McDonald & Murray, 2000). Behavioural and cognitive risk factors in children who played alone between the age of 4-6 and those who lacked confidence were more likely to develop schizophrenia (McDonald & Murray, 2000). These children also scored poorly on cognitive tests (McDonald & Murray, 2000). Other developmental risk factors may include urban birth and growth as well as migration (McDonald & Murray, 2000). Lastly, studies have indicated that stressful life events in the few weeks before the onset or relapse of schizophrenia may act as a trigger (McDonald & Murray, 2000). The use of stimulants and cannabis may cause transient psychotic symptoms that can cause the relapse of an existing psychotic illness such as schizophrenia (McDonald & Murray, 2000).
Etiology
Through the complex diagnosis of schizophrenia, the causes are not entirely known as to why one would directly be linked to the disorder. Genes and environment have been attributed to a probable cause as 10% of individuals who have a first-degree relative with the individual with schizophrenia. In the case of twins, the likelihood increases to a range from 10-65%. Through research it is shown that schizophrenia is not linked to a single gene in the biology thereby leading into the complexity of the disorder. Many genes act synergistically resulting in changed neurotransmitter interactions (Harrison, 2014). Genetic risk for schizophrenia comes from changes in the DNA sequences, more specifically single nucleotide polymorphisms (SNPs) and copy number variants (CNVs). Several loci have been observed including CACNA1C which is a L- type calcium channel, DRD2, the dopamine D2 receptor, GRIA1, an AMPA receptor subunit and GRIN2A, a NMDA receptor subunit to name a few (Harrison, 2014). This leads into the argument of genetic changes such as mutations as a cause for the illness. Other causes include pregnancy and birth complications such as low birth weight, premature labour and asphyxia during birth.
Diagnosis
Schizophrenia diagnosis begins with a physical examination through a physician that will include initial tests and screenings that will rule out of the possibility of other conditions (“Schizophrenia- Diagnosis and treatment- Mayo Clinic”, 2018). It can also include psychiatric evaluation through which the mental status of the individual will be assessed by observing their appearance, demeanour, mood, if they have had delusions, hallucinations and the individual’s family history (“Schizophrenia- Diagnosis and treatment- Mayo Clinic”, 2018). The two widely used tools for schizophrenia diagnosis are the Diagnostic and Statistical Manual of Mental Disorders (DSM V), which is published by the American Psychiatric Association and the International Classification of Disease (ICD) (Spencer & Campbell, 1994). The DSM V serves as a universal method for the diagnosis of psychiatric disorders and is used for its treatment recommendations (Spencer & Campbell, 1994). In the United Kingdom and Europe, the International Classification of Disease (ICD) is used which is a medical classification list created by the World Health Organization (Spencer & Campbell, 1994).
Previous studies have indicated that functional neuroimaging techniques also serve as a direct way of visualizing the pathophysiology associated with schizophrenia in vivo (McGuire, Howes, Stone & Fusarpoli, 2008). Neurotransmitters such as dopamine and glutamate are affected in this condition and can be assessed through molecular imaging techniques such as Positron emission tomography (PET) scans; single-photon emission tomography (SPET) scans as well as magnetic resonance spectroscopy (MRS) (McGuire, Howes, Stone & Fusarpoli, 2008). In addition, brain activity that associated with cognitive processes can be studied using functional magnetic resonance imaging (fMRI) (McGuire, Howes, Stone & Fusarpoli, 2008).
Symptoms
However, the truly problematic risk factors are the ones that are sociological in nature for which there are no easy fixes. For example, it has been shown that a combination of poor nutrition and lack of physical activity can lead to the development of obesity which can greatly increase one’s risk for developing CAD (Eckel & Krauss, 1998). It can seem obvious to state that if an obese individual wanted to lower their risk factors for CAD that they should lose weight. This simple statement is extremely difficult to implement as long term clinically significant weight loss is seldomly achieved (Wing & Phelan, 2005).
Pathophysiology
Anatomic Abnormalities
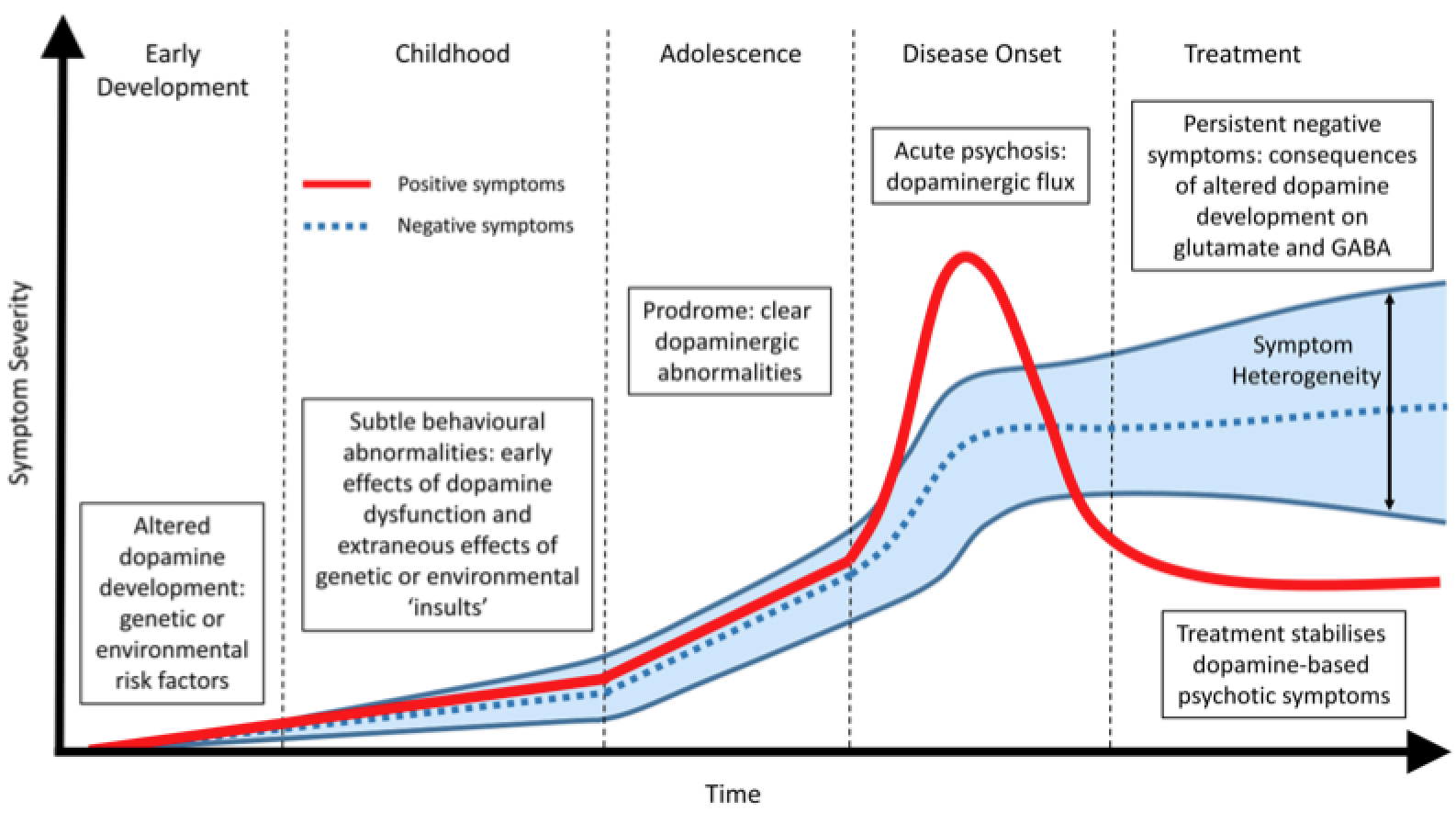
Both structural and functional abnormalities are common in patients with schizophrenia. The changes occur in several key brain systems, including prefrontal and medial temporal lobe regions, as well as in the cortex and in the connections between different cortical regions (Karlsgodt et al. 2010). Individuals with schizophrenia have smaller volume in the hippocampus, thalamus, amygdala, nucleus accumbens and intracranial space, and larger pallidum and ventricle volumes (McIntosh et al. 2011). At illness onset, prefrontal hypoactivity and hippocampal and subcortical hyperactivity represent a core illness pathophysiology (Gong et al. 2015). Over the course of the illness, changes in the brain progress and differ from the brain deficits at the onset of the illness, which has correlations with clinical symptoms (Gong et al. 2015). It is commonly seen in drug-free patients with schizophrenia that the functions and anatomic brain abnormalities overlapped in the default mode (DMN) and auditory networks (AN) however, the pattern of changes differed between the two (Gao et al. 2017). Decreased grey matter was associated with decreased activation within the DMN, whereas it was associated with increased activation within the AN (Gao et al. 2017). Changes in grey matter have been most pronounced in the thalamo-cortical networks, whereas altered brain activity has been seen in fronto-parietal and default-mode networks (Gong et al. 2015). White and grey matter volumes are found to be 5-6% smaller in individuals with schizophrenia (Sigmundsson et al. 2001). Similarly, to the grey matter changes, white matter changes are present by the time of the first episode and are an indicator that the subject is at risk for the disorder (Karlsgodt et al. 2010).
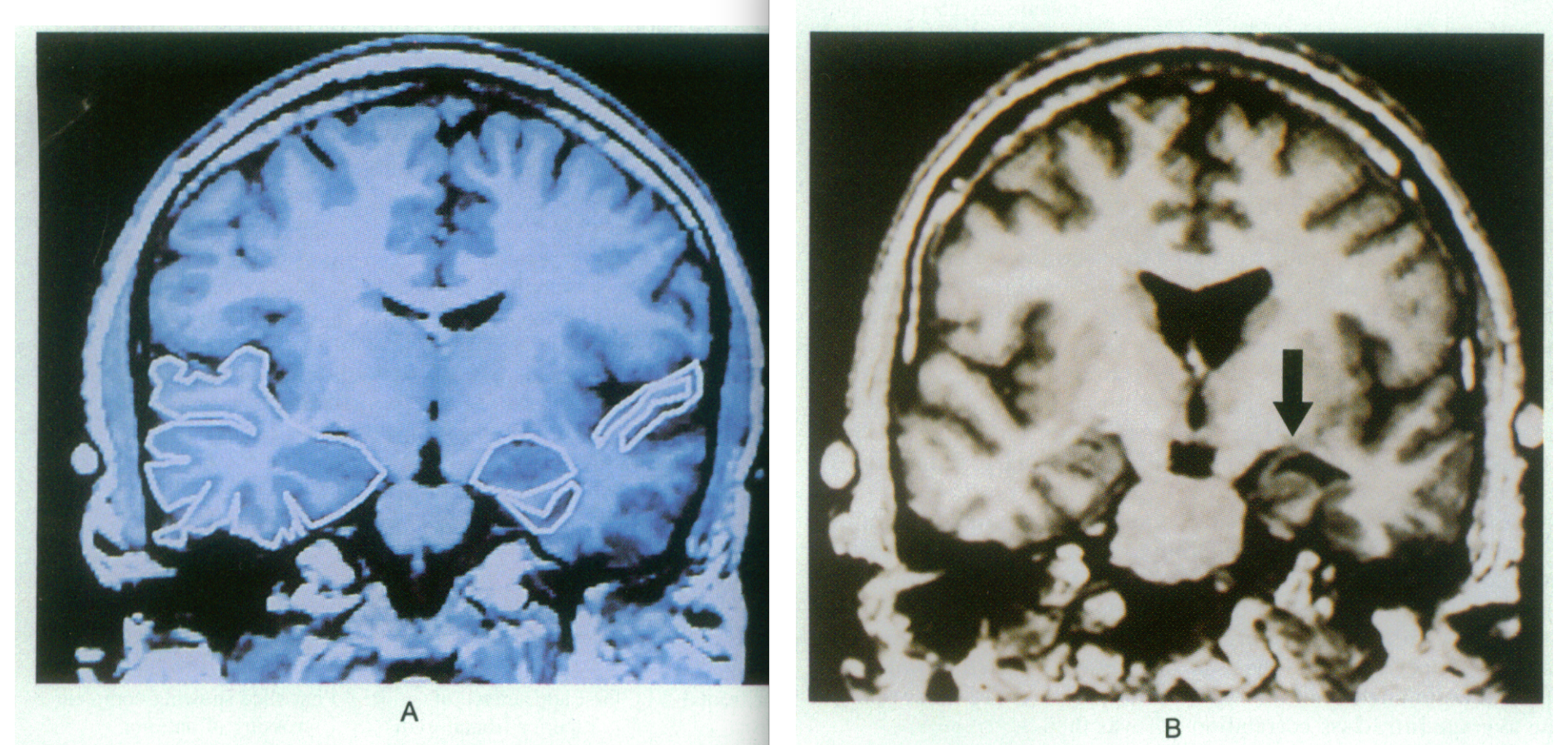
Figure 4: Coronal slide of the temporal lobe in control (A) and one of a patient with schizophrenia (B). Regions of interest in A include the grey matter of the superior temporal gyrus (right) as well as the amygdala- hippocampal complex (medial) which is almond- shaped. In Image B, the cerebrospinal fluid (CSF) surrounding the left superior temporal gyrus has increased compared to the control. Arrow points to tissue loss in the parahippocampal gyrus and increase in the temporal horn surrounding the amygdala- hippocampal complex. Retrieved from : http://www.nejm.org/doi/pdf/10.1056/NEJM199208273270905 Abnormalities of the left temporal lobe and thought disorder in schizophrenia
Neurotransmitter System Abnormalities
Currently, it is a common belief that both positive and negative symptoms are associated with neurotransmitter imbalances in patients’ brain (Frankenburg, 2018). Neurotransmitters are chemicals in the brain that are responsible for communication within the brain (Snyder, 2018). The neurotransmitters that may be imbalanced include: dopamine, serotonin, GABA and glutamate. In schizophrenic patients, dopamine is thought to be in excess in the brain, and as a result antipsychotic drugs are the gold standard medication to lower levels. Breaking down symptoms, positive symptoms are usually attributed to excess dopamine activity in the mesolimbic system (midbrain to the nucleus accumbens). Negative symptoms on the other hand are attributed to low dopamine activity in the mesocortical system (Ventral tegmentum to prefrontal cortex). Lastly, it is thought that lower glutamate levels are also found in schizophrenic patients, which produce psychotic symptoms (Frankenburg, 2018).
Inflammation and Immune Function
Schizophrenia has been found to have many immunological effects. Observations have been made of a diffuse non-specific overactivation of the immunological response system, of a T-helper cell type 1 immune activation and of a T-helper cell type 2 immune activation in subgroups of schizophrenia patients (Strous et al. 2006). Cytokines play an important role in infection and inflammation and are crucial mediators between the brain and the immune system (Ptovin et al. 2008). An imbalance in inflammatory cytokines is common in individuals with schizophrenia leading to a decrease in Th1 and an increase in Th2 cytokine secretion (Ptovin et al. 2008). The cytokines exhibit a number of critical and overlapping regulating functions in the immunological system and interact in a highly complex fashion with a range of neurotransmitters including; increased levels of interleukin (IL) 2 and soluble IL-2 receptor, increased cerebrospinal fluid levels IL-4, increased levels of IL-6 and soluble IL-6 receptor, increased levels of IL-10, increased levels on tumor necrosis factor alpha, as well as decreased mitogen stimulated levels of interferon-y (Strous et al. 2006).
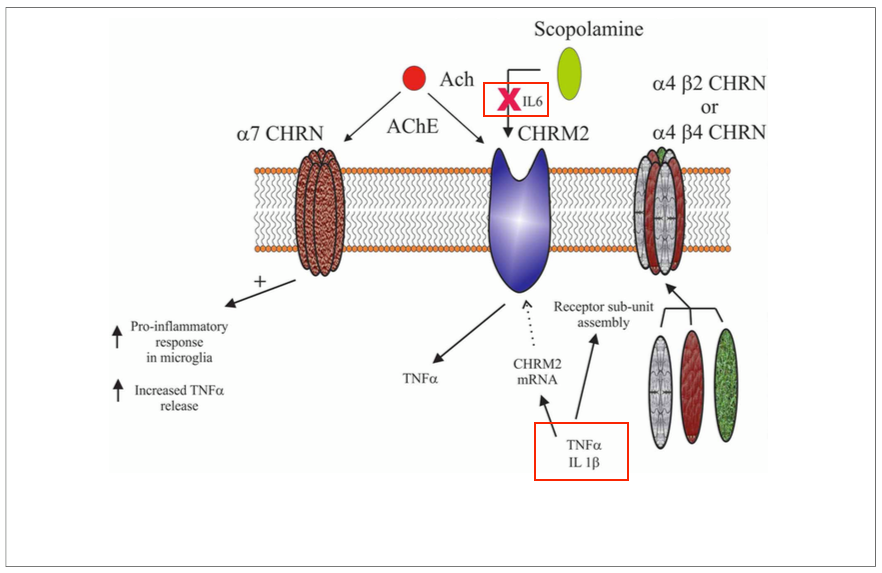
There are three prominent lines in the overactivation of the immune system: 1. Elevated serum levels of the pleiotropic cytokine IL-6, released from astrocytes, lymphocytes, and macrophages; 2. Increased levels of serum IL-2 and soluble IL-2 receptor (sIL-2) levels have been commonly noted in the illness (Gaughran et al. 2998); 3. Decreased mitogen-stimulated IL-2 production by overloaded activated lymphocytes (Muller et al. 1999 & Ginestet et al. 19890 (Strous et al. 2006). The elevated levels of pro-inflammatory cytokines activate the kynurenine pathway, by which tryptophan is metabolized into kynurenic and quinolinic acids; these acids regulate NMDA receptor activity and may be involved in dopamine regulation (Strous et al. 2006). The kynurenine pathway, when dysfunctional, is commonly associated with a number of disorders one being schizophrenia. Dysregulation of molecules in individuals with schizophrenia is associated with inflammation/immune responses and centered around disrupted interactions between the central cholinergic system (Deng et al. 2013).
Treatments
Schizophrenia is a lifelong disorder without a cure however there are treatments dedicated to manage the everlasting symptoms. Various therapies have been developed over the century including pharmacological drugs and behavioural interventions.
First a plan is set in order to target an optimal strategy for the patient to ensure their symptoms are regulated. The Care Programme Approach is initiated and used by professionals within the mental health services. This plan caters to the symptoms of the individual affected with schizophrenia. Second generation antipsychotics are typically administered as the initial therapy as opposed to first generation as the former presents fewer side extrapyramidal side effects however there are increased metabolic symptoms (Patel et al. 2014). Second generation antipsychotics include aripiprazole, olanzapine, paliperidone, quetiapine, risperidone and ziprasidone delivered through injection or tablet. Metabolic side effects are an increase in weight, fatigue, cholesterol and blood sugar (Healthwise Staff, 2011).
Future Direction
Currently, many experts believe there will not be a universal cure for schizophrenia, but rather progress will amount in stages. Clozapine, the most effective antipsychotic to this date, has significant side effects and thus causes patients to be uncompliant. Researchers now are investigating whether other antipsychotic medications can have potent efficacy, and reduced side effects, specifically atypical ones (Beaumont, 2000). It should be noted that atypical medications target receptors other than dopamine D2 receptors. Additionally, clozapine and typical current antipsychotics do not target negative symptoms of schizophrenia, and thus further research is being targeted at those symptoms (Miyamoto, Miyake, Jarskog, Fleischhacker & Lieberman, 2012).
While treatment through medication has been relatively successful at relieving the burden of positive symptoms, not much is known about this condition. Researchers emphasize the importance of first understanding the pathophysiology, then attempting to find the solution. It is believed that both genetics and environment play a role in developing this condition, as well as symptom severity, and as a result, more funding should be directed into this field (van Os, Rutten & Poulton, 2008). Although our progress in this disease appears to be superficial, physicians and researchers both believe that more effective treatments are in the near future. Through discoveries, not only will schizophrenia be better managed, but also other mental conditions - the once “black box” will be more understood.
References
Beaumont, G. (2000). Antipsychotics – The Future of Schizophrenia Treatment. Current Medical Research And Opinion, 16(1), 37-42. http://dx.doi.org/10.1185/0300799009117006
Burton, N. (2012). A Brief History of Schizophrenia. Retrieved January 24, 2018, from https://www.psychologytoday.com/blog/hide-and-seek/201209/brief-history-schizophrenia
Bromet, E. J., & Fennig, S. (1999). Epidemiology and natural history of schizophrenia. Biological psychiatry, 46(7), 871-881.
Cardno, A. G., & Gottesman, I. I. (2000). Twin studies of schizophrenia: from bow‐and‐arrow concordances to star wars Mx and functional genomics. American Journal of Medical Genetics Part A, 97(1), 12-17.
Deng, C., & Dean, B. (2013). Mapping the pathophysiology of schizophrenia: interactions between multiple cellular pathways. Frontiers in cellular neuroscience, 7.
Frankenburg, F. (2018). Schizophrenia: Practice Essentials, Background, Pathophysiology. Emedicine.medscape.com. Retrieved 23 January 2018, from https://emedicine.medscape.com/article/288259-overview#a3
Gao, X., Zhang, W., Yao, L., Xiao, Y., Liu, L., Liu, J., … & Sweeney, J. A. (2017). Association between structural and functional brain alterations in drug-free patients with schizophrenia: a multimodal meta-analysis. Journal of psychiatry & neuroscience: JPN, 43(1), 160219-160219.
Gaughran, F., O'Neill, E., Cole, M., Collins, K., Daly, R. J., & Shanahan, F. (1998). Increased soluble interleukin 2 receptor levels in schizophrenia. Schizophrenia research, 29(3), 263-267.
Ginestet, D., Loo, H., & Zarifian, E. (1989). Aberrant T cell-mediated immunity in untreated schizophrenic patients: deficient interleukin-2 production. Am J Psychiatry, 1(46), 609.
Gong, Q., Lui, S., & Sweeney, J. A. (2015). A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. American Journal of Psychiatry, 173(3), 232-243.
Healthwise Staff (2011). Second-Generation Antipsychotics for Treating Schizophrenia. Michigan Medicine.
Karlsgodt, K. H., Sun, D., & Cannon, T. D. (2010). Structural and functional brain abnormalities in schizophrenia. Current directions in psychological science, 19(4), 226-231.
Kay, S., Fiszbein, A., & Opler, L. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261-276. Jablensky, A. (2010). The diagnostic concept of schizophrenia: its history, evolution, and future prospects. Dialogues in clinical neuroscience, 12(3), 271. Learn about what is schizophrenia and schizophrenia treatment options. (2018). Schizophrenia.ca. Retrieved 23 January 2018, from http://www.schizophrenia.ca/learn_more_about_schizophrenia.php Lichtenstein, Paul, et al. “Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study.” The Lancet 373.9659 (2009): 234-239. McDonald, C., & Murray, R. (2000). Early and late environmental risk factors for schizophrenia. Brain Research Reviews, 31(2-3), 130-137. McGuire, P., Howes, O., Stone, J., & Fusarpoli, P. (2008). Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends In Pharmacological Sciences, 29(2), 91-98. McGrath, J. J., & Sasser, E. S. (2009). New directions in the epidemiology of schizophrenia. Medical Journal of Australia, 190(4), S7.
McIntosh, A. M., Owens, D. C., Moorhead, W. J., Whalley, H. C., Stanfield, A. C., Hall, J., … & Lawrie, S. M. (2011). Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biological psychiatry, 69(10), 953-958.
Miyamoto, S., Miyake, N., Jarskog, L., Fleischhacker, W., & Lieberman, J. (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Molecular Psychiatry, 17(12), 1206-1227. http://dx.doi.org/10.1038/mp.2012.47
NIMH » Schizophrenia. (2018). Nimh.nih.gov. Retrieved 23 January 2018, from https://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml
Muller, N., Riedel, M., Hadjamu, M., & Schwarz, M. J. (1999). Increase in expression of adhesion molecule receptors on T helper cells during antipsychotic treatment and relationship to blood-brain barrier permeability in schizophrenia. The American Journal of Psychiatry, 156(4), 634.
Patel, K. R., Cherian, J., Gohil, K., & Atkinson, D. (2014). Schizophrenia: overview and treatment options. Pharmacy and Therapeutics, 39(9), 638. Potvin, S., Stip, E., Sepehry, A. A., Gendron, A., Bah, R., & Kouassi, E. (2008). Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological psychiatry, 63(8), 801-808.
Reichenberg, A., Rieckmann, N., & Harvey, P. (2005). Stability in schizophrenia symptoms over time: Findings from the Mount Sinai Pilgrim Psychiatric Center Longitudinal Study. Journal Of Abnormal Psychology, 114(3), 363-372. Schizophrenia - CMHA National. (2018). CMHA National. Retrieved 23 January 2018, from https://cmha.ca/mental-health/understanding-mental-illness/schizophrenia Schizophrenia - Diagnosis and treatment - Mayo Clinic. (2018). Mayoclinic.org. Retrieved 21 January 2018, from https://www.mayoclinic.org/diseases-conditions/schizophrenia/diagnosis-treatment/drc-20354449 Sigmundsson, T., Suckling, J., Maier, M., Williams, S. C., Bullmore, E. T., Greenwood, K. E., … & Toone, B. K. (2001). Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. American Journal of Psychiatry, 158(2), 234-243.
Snyder, S. (2018). What dopamine does in the brain. PNAS. Retrieved 23 January 2018, from http://www.pnas.org/content/108/47/18869.full Spencer, E., & Campbell, M. (1994). Children with Schizophrenia: Diagnosis, phenomenology, and pharmacotherapy. Schizophrenia Bulletin, 20(4), 713-725. Strous, R. D., & Shoenfeld, Y. (2006). Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. Journal of autoimmunity, 27(2), 71-80.
van Os, J., & Kapur, S. (2018). Schizophrenia. ScienceDirect. Retrieved 23 January 2018, from http://www.sciencedirect.com/science/article/pii/S0140673609609958?via%3Dihub
van Os, J., Rutten, B., & Poulton, R. (2008). Gene-Environment Interactions in Schizophrenia: Review of Epidemiological Findings and Future Directions. Schizophrenia Bulletin, 34(6), 1066-1082. http://dx.doi.org/10.1093/schbul/sbn117