This is an old revision of the document!
Table of Contents
5. Regeneration in Humans
Regeneration in humans encompasses a wide range of clinical and research areas, from the use of stem cells to organ transplantation and therapeutic interventions. Generally, regeneration in humans refers to the repair of a body structure, specifically tissues and organs, after a loss of function. During this process, stem cells can differentiate in order to replace lost tissue. They can also proliferate and transdifferentiate to assist in the regeneration process (Stark, 2018).
Current research in biomedicine focusses on animal regeneration and its application to humans. The vast majority of mammals, including humans, cannot regenerate whole tissue and complex organs. In contrast, other non-mammalian organisms can regenerate full organs and limbs, as shown in the planaria and salamander examples (Li et al., 2016). Research into model organisms can provide insight into the various processes involved in regeneration on a molecular level and aid in the discovery of interventions for human medicine. What is currently known, however, is that some organs in the human body have some regenerative properties.Tissue regeneration is part of regular maintenance, where biochemical signals and stem cells create a regenerative environment for the tissue. However, this process is largely unknown. Other tissues have been shown to regenerate once stem cells are injected into the body. Biomedical research is currently focused in this area, as it can lead to the use of stem cell transplants and implanted materials for regeneration in humans (Stark, 2018).
Liver
The liver is the most well-known example of an organ with natural regenerative capabilities. Given the rising number of liver disease patients, regenerative mechanisms can be used to improve treatment given the cost, side effects, and lack of transplant options. The liver can regenerate from less than half of its original tissue because all liver cell types can proliferate, specifically hepatocytes and progenitor cells. When there is a large loss of liver tissue, rapid regenerative response will begin (Abdellatif & Shiha, 2017).
Mechanism - Compensatory
Hepatocytes are differentiated liver cells that have many metabolic functions. In response to tissue loss, existing hepatocytes enter the cell cycle under the influence of various growth factors such as AP-1 and STAT-3 (Fabrikant, 1968). Hepatocyte proliferation is initiated in the outer region of the liver and then moves towards the center (Abdellatif & Shiha, 2017). After removing two-thirds of the pancreas in rodents, it has been found that hepatocytes undergo 1.4 rounds of replication on average to restore normal liver size, taking 5-7 days. In humans, this process takes 3-4 weeks (Michalopolous, 2007).
The liver also has hepatic progenitor cells (HPCs) that can be activated during chronic liver damage. The HPCs are can fully differentiate into hepatocytes and bile duct cells. Other studies have demonstrated that these cells can also transdifferentiate into hepatocytes when they are unable to proliferate during an injury (Evarts et al., 1996).
Spinal Cord
The spinal cord is an example of induced regeneration and does not occur naturally in the human body. The aim of spinal cord regeneration is to reconnect damaged nerves and reverse paralysis. Currently, there is no cure for spinal cord injuries. This is because the nerves become blocked by severe scar tissue that does not allow a natural molecular regeneration process (Kabu et al., 2015). Research conducted by Li et al., (2017) has shown promising results, using synthetic and mesenchymal stem cells for scar tissue removal. However, these cells rapidly degrade and cannot withstand high temperatures.
One of the most widely researched therapies is the use of olfactory ensheathing cells (OECs), which are cells involved in axon extension and promote receptor signalling for the human sense of smell. They have been found to regulate neuroinflammation and promote axon growth. Thus, they have been used in animal transplant studies as a treatment for spinal cord injury (Chehrehasa et al., 2010). Though many studies have reported neurological benefits, the research is inconsistent. Furthermore, there are many populations of OECs and purification methods must be put in place (Deumans et al., 2018).
6. Medical Applicants / Treatments
Regenerative medicine is an ongoing interdisciplinary field of research that aims to develop treatments that replace- or restore organ functions and tissue damages from the effects of diseases, injuries, aging, or congenital defects [23]. Since the early 1950s, regenerative medicine has been studied and practiced within the health and science industries in hopes to fully restore the functions of human tissues and organs. The first cell transplantation: bone marrow transplantation, and the first organ transplantation: kidney transplantation, has sparked further insight and discovery within this field of medicine [25]. With the rising demand for healthy tissues and organs, many regenerative medicine therapies have found its way into clinical settings and commercial usage.
a. Current Commercial Usage
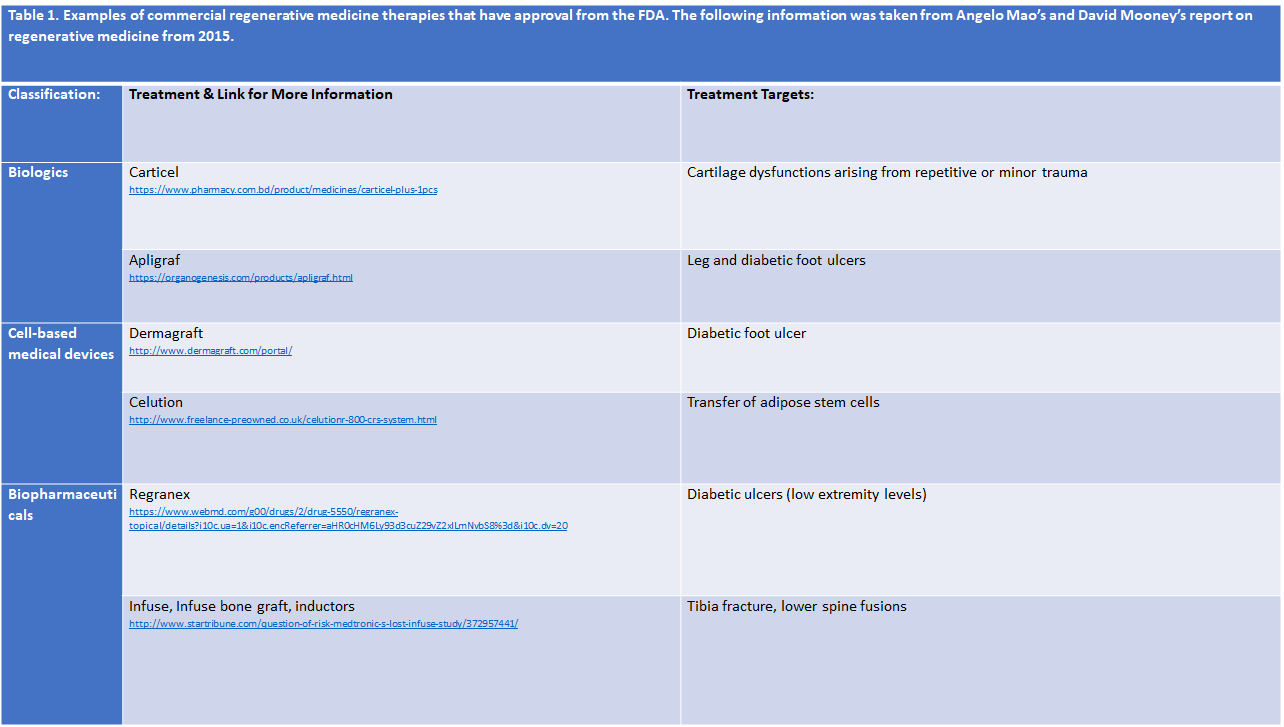
An increased amount of regenerative medicine therapies has received either clearance or approval from the FDA- Food and Drug Administration (Table 1). Carticel is one of the earlier products that made its way into commercial usage. To treat defects within the focal articular cartilage, Carticel uses autologous, from the same individual, chondrocytes. Essentially, cells that produce cartilage in the knees are applied to the body to combat the chondral defect [20]. Apligraf serves as a living, bi-layered substitute for human skin [2]. Apligraf uses allogeneic, genetically similar but not identical, stem cell transplantation, a treatment where stem cells from a healthy individual is applied to the damaged skin of the recipient [18]. Similarly, Dermagraft uses human cells, fibroblasts, to treat damaged cells or skin tissue caused by foot ulcers [24]. Celution is a machine that efficiently produces adipose-derived regenerative cells (ADRCs) which are aimed to treat wounds or assist in wound healing [8]. Both Regranex and Infuse, infuse bone graft, and inductors use growth factors that allow for the regeneration into biomaterials [18]. Regranex specifically uses platelet derived growth factors while Infuse, infuse bone graft, and inductors use bone morphogenetic protein 2- growth factors [18]. Though these products have been approved by the FDA and have shown evidence for assisting in healing and regeneration, they still fail to fully resolve the issue, whether it be an injury, disease, or congenital defect. Complications and critiques have also came about concerning some of these treatments. Thus, research in regenerative medicine therapies remains on-going with the aim to improve upon current strategies and develop more reliable and efficient treatments.
b.===Addressing Limitations and Steps Towards Complete Human Organ or Tissue Recovery===
Regenerative medicine continues to develop as there are variety of limitations that hinder the full treatment of non-functional tissues and organs. For example, the behavior of stems cells needs to be strictly controlled for in order to improve safety and efficacy post-transplantation treatments [18]. To do this, researchers are looking into building microenvironments that include specific cues that allow for direct manipulation of target cells [18]. One of the biggest limitations hindering the complete treatment of dysfunctional or damaged tissues and organs is immune reactions, specifically the rejection of regenerative treatments from immune responses [18]. Further knowledge and expertise in the human immune system, along with a clearer understanding of how age, disease, and one’s biological environment affect regeneration is needed to enhance existing regenerative treatments. As development continues, our health care system may be able to provide tissue-engineered heart muscles for damaged heart tissue, infuse cells into a patient’s existing matrix to improve lost functionality, regenerate insulin-producing pancreatic islets to assist with the effects of diabetes, and many more [23].
References
[1] Abdellatif, H., & Shiha, G. (2017). Liver Regeneration: Summary of Involved Cell Types. Journal of Stem Cell and Regenerative Biology, 3(1), 0-0.
[2] Apligraf. (2010). What is Apligraf? Retrieved from http://www.apligraf.com/professional/what_is_apligraf/index.html
[3] Bosch, T. C. (2007). Why polyps regenerate and we don't: towards a cellular and molecular framework for Hydra regeneration. Developmental biology, 303(2), 421-433.
[4] Cai, S. A., Fu, X., & Sheng, Z. (2007). Dedifferentiation: a new approach in stem cell research. AIBS Bulletin, 57(8), 655-662.
[5] Chehrehasa, F., Windus, L. C., Ekberg, J. A., Scott, S. E., Amaya, D., Mackay-Sim, A., & St John, J. A. (2010). Olfactory glia enhance neonatal axon regeneration. Molecular and Cellular Neuroscience, 45(3), 277-288.
[6] Deumens, R., Van Gorp, S. F. J., Bozkurt, A., Beckmann, C., Führmann, T., Montzka, K., … & Brook, G. A. (2013). Motor outcome and allodynia are largely unaffected by novel olfactory ensheathing cell grafts to repair low-thoracic lesion gaps in the adult rat spinal cord. Behavioural brain research, 237, 185-189.
[7] Evarts, R. P., Hu, Z., Omori, N., Omori, M., Marsden, E. R., & Thorgeirsson, S. S. (1996). Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis, 17(10), 2143-2151.
[8] Fraser, J. K., Kicok, K. C., Shanahan, R., Zhu, M., Miller, S., & Arm, D. M. (2014). The Celution System: Automated Processing of Adipose-Derived Regenerative Cells in a Functionally Closed System. Adv Wound Care, 3(1), 38-45.
[9] Galliot, Brigitte(Nov 2013) Regeneration in Hydra. In: eLS. John Wiley & Sons Ltd, Chichester. Retrieved from http://www.els.net [doi: 10.1002/9780470015902.a0001096.pub3]
[10] Godwin, J. (2014). The promise of perfect adult tissue repair and regeneration in mammals: learning from regenerative amphibians and fish. BioEssays, 36(9), 861-871.
[11] Godwin, J. W., Pinto, A. R., & Rosenthal, N. A. (2013). Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences, 110(23), 9415-9420.
[12] Gilbert, Scott F.; Barresi, Michael J. F. (2016). Developmental Biology (11th ed.), 701–702.
[13] Heinrich, A. (2016, April 08). Humans could get salamander-like tissue regeneration abilities in “a few years”. Retrieved from https://newatlas.com/reprogramming-stem-cells/42697/
[14] King, R. S., & Newmark, P. A. (2012). The cell biology of regeneration. J Cell Biol, 196(5), 553-562.
[15] Kragl, M., Knapp, D., Nacu, E., Khattak, S., Maden, M., Epperlein, H. H., & Tanaka, E. M. (2009). Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature, 460(7251), 60.
[16] Li, J., Zhang, S., & Amaya, E. (2016). The cellular and molecular mechanisms of tissue repair and regeneration as revealed by studies in Xenopus. Regeneration, 3(4), 198-208.
[17] Lu, J., Féron, F., Mackay‐Sim, A., & Waite, P. M. (2002). Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain, 125(1), 14-21.
[18] Mao, A. S., & Mooney, D. J. (2015). Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U.S.A., 112(47), 14452-14459. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4664309/
[19] Metcalfe, A., & Waugh, N. (2017). Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technology Assessment, 21.6.
[20] Mistry, H., Connock, M., Pink, J., Shyangdan, D., Clar, C., Royle, P., Court, R., Biant, L. C., Michalopoulos, G. K. (2007). Liver regeneration. Journal of cellular physiology, 213(2), 286-300.
[21] Morrison, J. I., Lööf, S., He, P., & Simon, A. (2006). Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. The Journal of cell biology, 172(3), 433-440.
[22] Muneoka, Ken; Han, Manjong; and Garinder, David M. “Regrowing Human Limbs.” Scientific American. April 2008. (Oct. 28, 2008). Retrieved from http://www.sciam.com/article.cfm?id=regrowing-human-limbs
[23] NIH. (2018). Regenerative Medicine. Retrieved from https://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=62
[24] Organogensis inc. (2013). Treating Your Diabetic Foot Ulcer. Retrieved from http://www.dermagraft.com/patient/about-dermagraft/
[25] Sampona, G., Guraya, S. Y., Forgione, A. (2015). Regenerative medicine: Historical roots and potential strategies in modern medicine. Journal of Microscopy and Ultrastructure, 3(3), 101-107.
[26] Singh, V. K., Saini, A., Kalsan, M., Kumar, N., & Chandra, R. (2016). Describing the stem cell potency: the various methods of functional assessment and in silico diagnostics. Frontiers in cell and developmental biology, 4, 134.
[27] Stark, J. F. (2018). Perspectives on human regeneration. Palgrave communications, 4(1), 66.
[28] Slack, J. M. (2007). Metaplasia and transdifferentiation: from pure biology to the clinic. Nature reviews Molecular cell biology, 8(5), 369.
[29] Solomon, E., Berg, l., Martin, D. (2002) Biology 6th edition. Brooks/Cole Publishing.
[30] Zamaraev, V. N. (1956). Distortion of polarity in hydra. Bulletin of Experimental Biology and Medicine, 42(6), 1051-1053.