Table of Contents
Glucocorticoid Hyperfunction: Cushing's Syndrome
Adrenal Glucocorticoid Hyperfunction: Cushing's Syndrome PowerPoint
Introduction
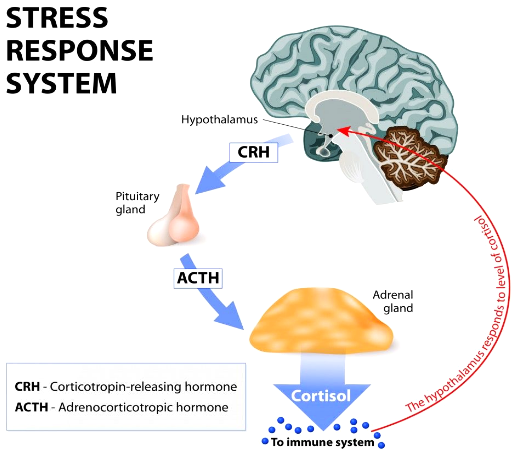
Cushing’s syndrome results from excessive, long-term exposure to endogenous or exogenous forms of glucocorticoids (Orth, 1995). Glucocorticoids are a class of steroids, with cortisol being the most important. Glucocorticoids are particularly important in mediating the stress response of the body, and they are also involved in anti-inflammatory actions (Marshall, Bangert & Lapsley, 2012). Normally, the hypothalamic-pituitary-adrenal axis (HPA Axis) releases cortisol from the adrenal glands in response to a stressor (Figure 1). Activation of the HPA axis initiates a cortisol cascade. Various stressors will result in the secretion of corticotropin-releasing hormone (CRH) from the hypothalamus, which then stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH). Circulating ACTH acts on the adrenal cortex to release cortisol and exert negative feedback control to restore homeostasis.
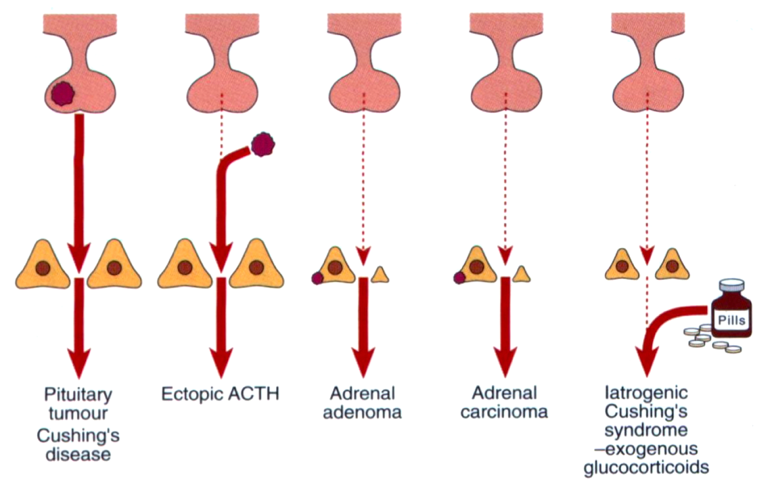
However, in Cushing's Disease (the most prevalent endogenous subtype of this syndrome), a tumour in the pituitary causes excessive release of ACTH, therefore acting on the adrenal cortex to produce abnormally high levels of cortisol (Orth, 1995). The excess release of glucocorticoids present life-threatening conditions and a host of clinical manifestations. Other endogenous causes of Cushing’s syndrome include a tumour of the adrenal cortex over-secreting cortisol, an ectopic non-pituitary ACTH-secreting tumour, or a tumour of the hypothalamus secreting excessive CRH leading to activation of the cortisol cascade. Potential sources of an ectopic tumour include the lungs, pancreas, or thymus (Marshall, Bangert & Lapsley, 2012).
History
The history of Cushing’s disease dates back to 1912 (Medvei, 1991). Harvey Cushing was an American neurosurgeon born in Cleveland, Ohio. He received his education at Yale and Harvard Medical School. Cushing was a professor of surgery at Harvard Medical School, and he was the surgeon-in-chief at the Peter Brent Brigham Hospital (Medvei, 1991). In 1931, he published “The basophilic adenomas of the pituitary body and their clinical manifestations”. In this paper, he mentioned a patient with the name of Minnie whose symptoms included amenorrhea (abnormal periods) and abnormal hair growth. He claimed that her symptoms resembled the symptoms seen with adrenal tumours due to dysfunction of the pituitary glands (Medvei, 1991). Cushing hypothesized that if acidophil hyperpituitarism (excess acidophil cells) caused acromegaly, then excess basophil cells would be involved with a pituitary disorder that involves sexual function (amenorrhea in women and impotence in men) (Medvei, 1991). In 1932, Cushing published “A Case of Basophil Adenoma of the Anterior Lobe of the Pituitary: Cushing’s Syndrome”, which led to the introduction of the disease (Medvei, 1991).
Epidemiology
The most common exogenous cause of Cushing’s Syndrome (CS) is iatrogenic CS, which occurs through the consumption of prescribed medications for inflammatory, autoimmune, and neoplastic disorders, as well as the surreptitious use of corticosteroids (Hopkins and Leinung, 2005). The most common cause of endogenous CS, also known as Cushing’s Disease (CD), is much rarer. Currently, there is not any concrete report on the epidemiology of iatrogenic CS, however, there are some epidemiological studies reviewing CD.
A Spanish study analyzed the prevalence of CD cases until 1992, and found a total 39.1 cases per million people; reported also was a rate of 2.4 new cases per million people per year, and a CD frequency of 15:1 ratio of women to men (Extabe and Vasquez, 1994). From this CD population, 38.7% had diabetes mellitus and 55.1% had hypertension (Extabe and Vasquez, 1994).
A Danish study obtained patient information for an 11 year period, and found rates of 1.2-1.7 cases per million per year of CD, 0.6 cases per million per year with an adrenal adenoma, and 0.2 cases per million per year with an adrenal carcinoma (Lindholm, Juul, Jørgenson, et al., 2001). Adrenal adenomas have been reported to be at least twice as prevalent as adrenal carcinomas.
Very little has been published on mortality rates of people with CS, but a retrospective meta-analysis was done in Stoke-on-Trent, UK, for CD’s mortality rates. The median age-range for diagnosis was 36-46 years old, and 85% of these patients were women (Clayton, Raskauskiene, Reulen, et al., 2011). The median follow-ups were 15 years after diagnosis, and 13 people died within that period - 9 of them due to cardiovascular problems (Clayton, Raskauskiene, Reulen, et al., 2011). Mortality rates reported of CD patients are nearly double that of the general population, but those in remission have mortality rates close to equal to the general population (Clayton, Raskauskiene, Reulen, et al., 2011).
The prevalence of Cushing’s Syndrome may be heavily underestimated as there are limited studies in its epidemiology. Also, the cyclic nature of CS, the high variability of intensity, remission phases, and comorbid symptomology leaves many people misdiagnosed and treated just for symptoms like hypertension or diabetes. Evidently, more research must be done in this field.
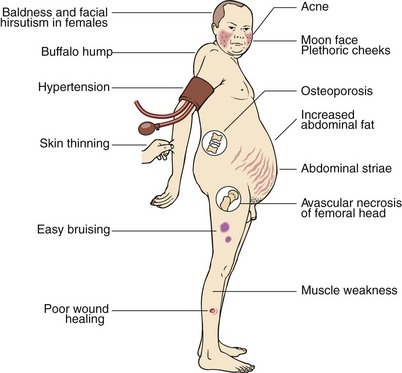
Signs and Symptoms of Cushing's
A general diagnosis of Cushing’s Syndrome can be categorized into one of four classes:
- Primary (a tumour of the adrenal cortex)
- Secondary (a tumour of the pituitary)
- Tertiary (a tumour of the hypothalamus)
- Ectopic (a non-pituitary ACTH secreting tumour)
Regardless of the class of diagnosis, patients with Cushing’s exhibit a multitude of similar symptoms with some sex specific.
Most Common Symptoms:
- High-Blood Pressure
- Weight Gain
- Fatigue
- Poor-Short Term Memory
- Irritability
- Poor Concentration
- Muscle Weakness
- Sore Joints
- Bone Weakness
- Weakened Skin
- Poor Wound Healing
- Recurrent Infections
- Moon Face (Fat deposits around the face)
- Irregular Menstruation Cycle (Women)
Least Common Symptoms:
- Insomnia
- Acne
- Edema of the Exteremities
- Erectile Dysfunction (Men)
- Excessive Body Hair Growth (Women)
Hypothalamic-Pituitary Adrenal Axis
Several stimuli can perturb homeostatic mechanisms resulting in the secretion of glucocorticoids, mainly cortisol in humans, from the adrenal cortex. The most common stimulus, stress, is a potent activator of the HPA axis. Our body's stress system coordinates the adaptive responses of organisms to different types of stressors resulting in changes that improve our ability to adjust homeostasis and increase chances of survival (Tsigos & Chrousos, 2002). Cortisol is the end product of the activation of the hypothalamic-pituitary adrenal (HPA) axis. The HPA axis consists of excitatory signals and negative feedback loops to regulate its activity (Nicolaides, Kyratzi, Lamprokostopoulou, Chrousos, & Charmandari, 2015).
Physiology of Glucocorticoids: Cortisol
In response to a stressor, the hypothalamus secretes corticotrophin releasing hormone (CRH) which acts on the anterior pituitary gland to secrete adrenocorticotrophin hormone (ACTH). Circulating ACTH is a key regulator of cortisol secretion from the adrenal cortex. The release of glucocorticoid hormones help regulate homeostatic imbalances and mediate the body's response to stress (Tsigos & Chrousos, 2002).
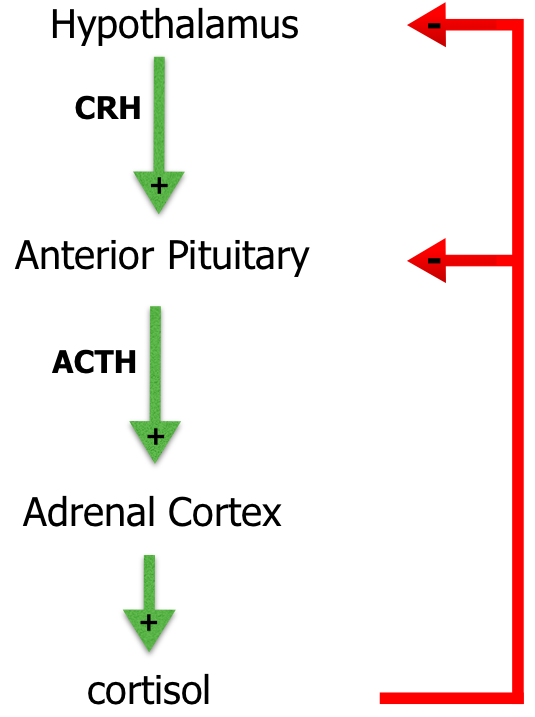
The most important glucocorticoid hormone is cortisol. Glucocorticoids inhibit the immune response, have anti-inflammatory and catabolic effects, maintain blood pressure by sensitizing the arterioles to norepinephrine and increase glucose levels in the blood (Marshall, Bangert & Lapsley, 2012). Figure 4 shows cortisol exerting negative feedback control on ACTH release through the inhibition of CRH as well as inhibiting CRH release itself (Marshall, Bangert & Lapsley, 2012).
Pathophysiology of Glucocorticoids: Excess Cortisol
Regulation of the HPA axis is critical, otherwise disorders of the adrenal cortex can arise and present life-threatening conditions. In Cushing's Syndrome, there is overproduction of glucocorticoids. The various causes of Cushing's Syndrome, illustrated in figure 5 below, include a pituitary adenoma over-secreting ACTH, ectopic ACTH secretion, an adrenal adenoma over-secreting glucocorticoids, or an exogenous supply of glucocorticoids (Marshall, Bangert & Lapsley, 2012). The most common endogenous cause is a pituitary adenoma which accounts for 60-70% of diagnosis (Marshall, Bangert & Lapsley, 2012).
Figure 5: The possible causes of Cushing's Syndrome (Marshall, Bangert & Lapsley, 2012)
Pathophysiology of Cushing's Disease
Cushing’s disease, a subtype of Cushing’s syndrome, is a disease primarily due to excessive secretion of adrenocorticotropic hormone (ACTH) by a pituitary adenoma. The pituitary tumours are usually microadenomas, which are 10 mm or less in diameter. These tumours are often discovered by doing tests for other reasons, since these microadenomas do not have mass effects. (Kirk and Jones, 2000). Cushing’s disease accounts for about two thirds of the cases of endogenous Cushing’s syndrome. It mainly affects women of reproductive age, but can also affect males and females in general (Kirk and Jones, 2000). In Cushing’s disease, ACTH secretion from the pituitary is increased because the pituitary becomes less sensitive to glucocorticoid effects and negative feedback mechanisms become faulty. Consequently, excess cortisol will be required in order to suppress the release of ACTH from the pituitary through negative feedback. Therefore, as cortisol continues accumulating due to a failure in negative feedback mechanisms, it begins causing negative effects throughout the body (Marshall, Bangert & Lapsley, 2012).
Presentation of clinical features
The clinical features of Cushing's Syndrome are primarily due to excess production of glucocorticoids. Taking into account the actions of glucocorticoids, high levels in the bloodstream will lead to easy bruising, poor wound healing, glucose intolerance, truncal obesity and purple stretch marks among other features. Interestingly, glucocorticoids also have mineralcorticoid activity. This means that cortisol can also act as aldosterone, an important mineralcorticoid, and present some of the clinical features characteristic of a patient with high levels of aldosterone in their bloodstream. This explains why patients present with high blood pressure (hypertension) due to the sodium retention, and metabolic alkalosis due to potassium wasting. Metabolic alkalosis is an increase in serum bicarbonate concentrations due to loss of hydrogen ions in the body, or a gain in bicarbonate ions. Since glucocorticoids have mineralocorticoid effects, an excess amount of glucocorticoids will increase the retention of sodium, and also dump hydrogen ions into the renal tubules. This loss of hydrogen ions creates a state of metabolic alkalosis.
Lab Abnormalities
Due to the actions of glucocorticoids and their mineralcorticoid activity, blood tests of patients with Cushing's Syndrome are quite distinct. Some abnormalities in the lab results are outlined below (Marshall, Bangert & Lapsley, 2012):
Increased
- Cortisol
- Glucose
- This is due to the increased hepatic gluconeogenesis that occurs with high amounts of glucose, causing the liver to produce glucose from proteins, lipids, and other metabolic sources such as lactate and pyruvate. A high concentration of plasma glucose may cause insulin resistance due to the pancreas' inability to keep up with insulin production.
- Bone turnover markers
- Cortisol is a catabolic hormone. It triggers bone resorption of amino acids in order to be used as an energy source in gluconeogenesis. This is achieved indirectly through cortisol by blocking calcium absorption in order to decrease bone growth.
Decreased
- Potassium
- Cortisol promotes sodium absorption in the small intestine, while at the same time acting to excrete potassium in the urine. This results in a state of hypokalemia, which occurs when the body experiences a decrease in the level of potassium.
- Eosinophils and Lymphocytes
- Eosinophils and lymphocytes are decreased because excess cortisol weakens the immune system. T-cells respond to cytokine molecules (called interleukins)via a signalling pathway. Cortisol blocks the proliferation of T-cells by preventing them from recognizing interleukin signals. More specifically some individuals with Cushings' may also be needed to be treated for severe parasitic infections, since eosinophils are responsible for clearing parasitic infections, declined levels leave affected individuals more susceptible to parasites.
Diagnosis of Adrenal Hyperfunction: Glucocorticoids
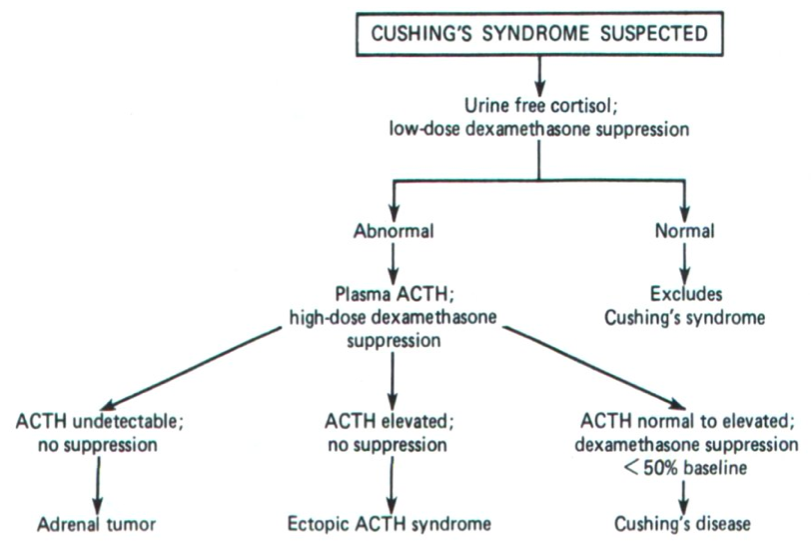
One of the goals of diagnosis is to distinguish Cushing’s disease from Cushing’s syndrome, as the disease is a variation of Cushing's syndrome. Secondary adrenal hyperfunction results from a pituitary tumour leading to excessive production of ACTH. Elevated ACTH stimulates the adrenal cortex to produce high levels of cortisol giving rise to the disease state. Testing for Cushing's is outlined below and random plasma cortisol levels are unacceptable due to the variations of cortisol levels throughout the day.
Figure 2: Suspected Cushing's syndrome algorithm to determine the origin of excess glucocorticoids in the blood (Marshall, Bangert & Lapsley, 2012)
24 Hour Urine Free Cortisol Excretion
The best screening test for Cushing's is 24 hour urinary free cortisol, as urine cortisol levels are representative of blood cortisol levels. A 24 hour urine collection test is done to collect urine over a full daily cycle of the patient. 24 hour urinary cortisol excretion have normal levels of less than 300 nmol/24 hours (Marshall, Bangert & Lapsley, 2012). Values exceeding the normal range are characteristic of Cushing's.
Low Dose Dexamethasone Suppression Test
Dexamethasone is a synthetic glucocorticoid that binds to cortisol receptors in the pituitary and suppresses ACTH release. This ultimately suppresses the secretion of cortisol by the adrenal gland in normal, healthy individuals (Marshall, Bangert & Lapsley, 2012). A 1mg dosage is given at night and blood is drawn for measurement of cortisol the next morning. In healthy individuals this level should be less than 50 nmol/L. (Marshall, Bangert & Lapsley, 2012). Failure to suppress ACTH levels are suggestive of Cushing’s.
Once increased cortisol levels have been established, the measurement of plasma ACTH is used to determine the cause of increased cortisol levels in the blood. Low levels of ACTH, a key regulator of glucocorticoid activity in the blood, suggests an adrenal tumour over-secreting cortisol. On the other hand, very high levels of ACTH suggest excessive secretion of ACTH from a pituitary tumour or an ectopic non-pituitary ACTH secreting tumour (Marshall, Bangert & Lapsley, 2012). In order to distinguish between the two, a high dose dexamethasone test is needed.
High Dose Dexamethasone Suppression Test
A 2mg dosage is given to patients every 6 hours for a total of 48 hours. Plasma cortisol levels are then measured again at 9am the next morning. Patients with Cushing's Disease would have their cortisol concentration levels drop by at least 50% of pre-treatment value. This indicates that high cortisol concentrations due to a pituitary tumour over-secreting ACTH is because of faulty negative feedback mechanisms. As a result, a higher dose is required to counteract the faulty negative feedback mechanisms. On the other hand, failure to suppress ACTH levels is suggestive of an ectopic ACTH secreting tumour that will continue to stimulate the adrenal cortex to release cortisol irregardless of the negative feedback loops.
CRH Dynamic Testing
Dynamic testing on patients is normally not performed unless absolutely necessary as it puts strain on the patient. In Cushing's, dynamic testing is used to distinguish pituitary adenomas from ectopic ACTH secreting tumours or adrenal secreting tumours. Corticotropin Releasing Hormone (CRH), which is released by the hypothalamus in response to stress, stimulates the entire cortisol release cascade. Providing CRH and measuring the ACTH levels helps to further diagnose the type of Cushing's. An exaggerated elevated response of excessive ACTH levels suggest a pituitary tumour (secondary class diagnosis) while no response after the administration of CRH indicates a primary class disorder (i.e., an adrenal secreting tumour).
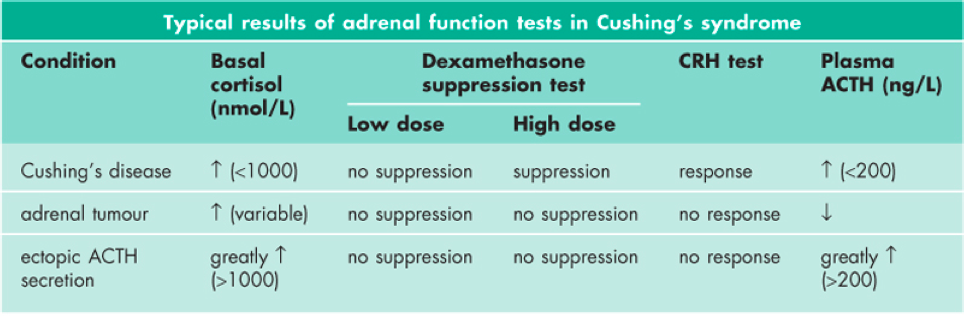
Figure 3: Typical results of Adrenal Hyperfunction tests in Cushing's syndrome (Marshall, Bangert & Lapsley, 2012)
Screening for Tumours
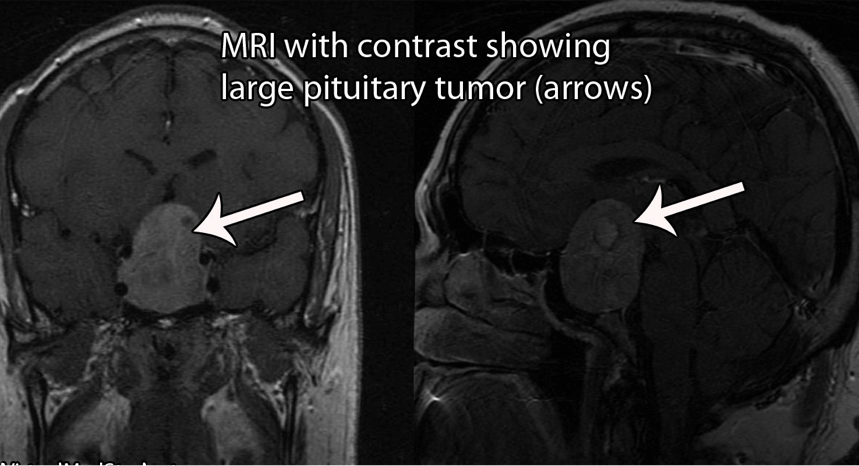
Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) of the pituitary helps to visualize the regions of the brain where an ACTH-secreting tumour is suspected to be localized. These visualization tests are often ordered when the patient has failed the low-dose dexamethasone test and has exhibited an exaggerated ACTH response. This test is done to confirm the existence of an ectopic tumour.
Treatment
The mode of management or treatment selected for Cushing's syndrome depends on the type of Cushing's and its root cause. For those with iatrogenic Cushing's syndrome, the safest and least invasive way to treat the patient involves a gradual decrease in glucocorticoid medication intake, along with conscious efforts to treat the condition originally targeted by the medication (i.e. gradually weaning off of cortisone and incorporating a diet beneficial for arthritis). For Cushing's disease, the primary form of treatment is trans-sphenoidal hypophysectomy, which is the surgical removal of small pituitary adenomas (Marshall, Bangert & Lapsley, 2012). If the adenoma cannot be identified, then surgeons remove 50-90% of the pituitary gland. However, the problem with removing too much of the gland is that it reduces the effectiveness of the pituitary and causes problems with ovulation in women and sperm production in men. Cure rates for this approach range from 65-90% for microadenomas and much lower for macroadenomas (Fleseriu and Petersenn, 2012).
Another surgery that is performed occasionally is bilateral adrenalectomy, which is the surgical removal of the adrenal glands. This procedure stops cortisol production, but the pituitaries must still be dealt with in order to not secrete too much ACTH which can lead to Nelson's syndrome (Marshall, Bangert & Lapsley, 2012). After both surgical procedures have been performed, respective steroid replacement therapy is necessary for the patient's lifetime (Marshall, Bangert & Lapsley, 2012).
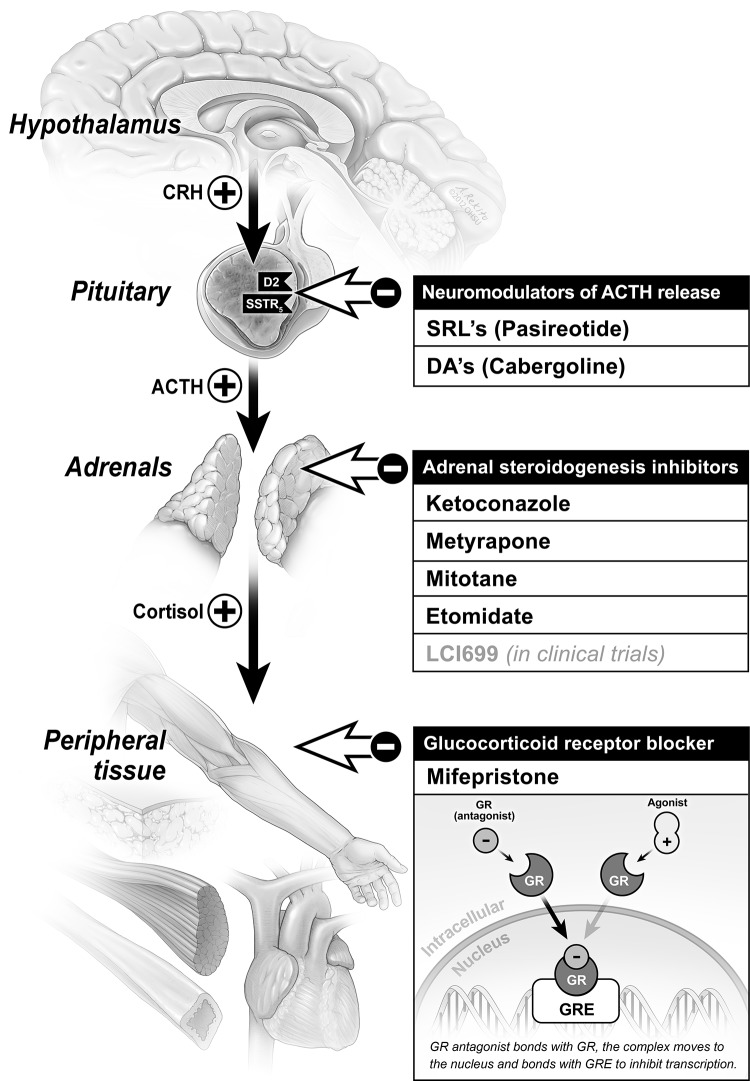
Ketoconazole
Ketoconazole is a drug that inhibits the process of steroidogenesis in the adrenal glands. This limits the production of steroids such as cholesterol. A study done with 200 patients with Cushing’s disease were treated with ketoconazole. At the last follow-up for the experiment, 49.3% of the patients showed normalized urinary free cortisol (UFC) levels and 25.6% had at least a 50% decrease (Castinetti et al, 2014). However, this drug is shown to have some adverse side effects among patients that take it. Gastrointestinal issues, such as diarrhea and nausea, seem to be most common. One major concern for the drug, although rare, is that it can increase liver enzyme levels and cause hepatotoxicity(Fleseriu, 2014).
Pasireotide (Signifor)
Pasireotide acts as an analog to somatostatin, which is known as a growth hormone-inhibiting hormone. The drug inhibits adrenocorticotropin (ACTH) secretion from the pituitary gland, a hormone that eventually leads to the secretion of cortisol (McKeage, 2013).
A study done by researchers looked at patients with Cushing’s disease and prescribed them with pasireotide for 6 months. At the end of the trials, 25% of all patients have normalized UFC levels and a significant decrease in the mean UFC levels of all patients. Patients that saw a reduction in their UFC levels also saw improvements in clinical symptoms as well, such as blood pressure and body weight. The drug, however, has shown to cause hyperglycemia in patients and is still under further testing (McKeage, 2013).
Cabergoline
Cabergoline is a dopamine receptor agonist and initially just used to inhibit the production of prolactin in patients that had hyperprolactinemia. However, studies have shown that Cabergoline might have some potential as a treatment for Cushing’s disease. A study looked at 30 patients with Cushing's disease and treated them with Cabergoline at varying doses. Around 40% of the patients treated with a maximum dose of 6 mg/week achieved normalized UFC levels in little over 4 months. Around 30% of the patients that were prescribed long-term treatments at doses of 2.1 mg/week for 37 months achieved normalized UFC levels (Godbout, 2010).
Mitotane
Mitotane can be used to as a treatment of adrenocortical carcinoma, but it can also be used to limit cortisol production by inhibiting cholesterol side-chain cleavage and 11 β-hydroxylase, which is used in the final phase of cortisol synthesis as a catalyst. It has shown some prospect in managing hypercortisolemia, however, there are better options such as ketoconazole and metyrapone as they are easier on the body of patients. Some of the common side effects of this drug are diarrhea, nausea, and anorexia (Trainer, 2013).
Metyrapone
Metyrapone is an adrenal steroidogenesis inhibitor. Specifically, it inhibits the enzyme 11β-hydroxylase, which converts 11-deoxycortisol into cortisol. Metyrapone is used to treat adrenal insufficiencies, but also hypercortisolism in Cushing’s syndrome. Using this medication for the long-term results in side-effects, such as an increase in ACTH production, acne, hirsutism, hyperkalemia, and edema (Daniel, Aylwin, Mustafa, et al., 2015). Currently, it is used for short-term treatment before surgeries, but it shows some promise as a long-term treatment, especially when combined with other drugs (Daniel, Aylwin, Mustafa, et al., 2015). Metyrapone’s long-term use on its own is still in question as its depletion of cortisol levels seem to affect memory formation and retrieval (Marin, Hupbach, Maheu, et al., 2011). Its long-term usage, however, should not be ruled out, rather it should be approached with caution.
Mifepristone
Mifepristone is a progesterone receptor antagonist, but at higher doses it also acts as a glucocorticoid receptor antagonist (Fleseriu, Biller, Findling, et al. 2012). It is most known for its use in abortions, but it is also used to treat Cushing’s disease. As a glucocorticoid receptor antagonist, mifepristone blocks cortisol’s mechanism of action. Studies do show benefits in its use for people with Cushing’s disease, especially for those with more fat and abnormal glucose metabolism, but there are still side effects worth examining. A few studies of mifepristone used for Cushing’s disease have exhibited symptoms such as increased blood pressure due to lowered high density lipoprotein (HDL), low potassium, alkalosis, vaginal bleeding, and uterine contractions (Castinetti, Conte-Devolx, Brue, et al., 2010; Fleseriu, Biller, Findling, et al. 2012). Mifepristone has its benefits, but more research has to be done on its effects on blood pressure and deteriorative effects on the female reproductive system.
Etomidate
Etomidate is an adrenal steroidogenesis inhibitor that is administered intravenously, which enables it to be a fast-acting drug to control hypercortisolism and psychosis in emergency situations (Davies, 2015). It is also a good option for those who cannot ingest their medication. Etomidate inhibits 11-deoxycortisol ß-hydroxylase, which is necessary for the conversion of cholesterol into cortisol (Heyn, Geiger, Hinske, et al., 2015). It is administered in low doses and does not seem to be a long-term solution; it commonly serves as an anesthetic.
Conclusions and Future Implications
Cushing’s syndrome is an umbrella term used to describe excessive, long term exposure to glucocorticoids, such as cortisol, in the blood. The most common subtype is Cushing's Disease, which is a result of a pituitary tumour over-secreting ACTH leading to excessive secretion of cortisol from the adrenal cortex. Several treatments target various mechanisms in order to prevent high levels of cortisol in the blood from having life threatening conditions. However, the future of treating Cushing’s syndrome may lie within a combination of different drug therapy rather than a single drug.
Future Research
A study including 17 patients diagnosed with Cushing’s disease was given pasireotide as a treatment. After some time, five of those patients showed normalized UFC levels. The addition of cabergoline to the treatment normalized UFC levels in four further patients, and the addition of ketoconazole normalized six more patients. At the end of the trials, only 2 out of 17 patients had elevated UFC levels (Feelders and Hofland, 2013)
Another study looked at a combination of cabergoline and ketoconazole for treating Cushing’s disease. Initially, 12 patients were prescribed cabergoline for 6 months. Nine of these patients did not respond well to the treatment, however the addition of the ketoconazole to the treatment led to six of the nine patients having normalized UFC levels and the remaining patients showed a reduction in UFC levels of 44-52% (Vilar et al, 2010)
Combination therapy is on the rise and has a lot of potential as it has shown to increase the effectiveness of different drugs while reducing their side effects.
References
Castinetti, F., Conte-Devolx, B., Brue, T. “Medical treatment of Cushing's syndrome: glucocorticoid receptor antagonists and mifepristone.” Neuroendocrinology. 2010; 92(1): 125–130.
Castinetti, F., Guignat, L., Giraud, P., Muller, M., Kamenicky, P., Drui, D., et al. “Ketoconazole in Cushing’s Disease: Is It Worth a Try?” The Journal of Clinical Endocrinology & Metabolism 99, no. 5 (May 1, 2014): 1623–30. doi:10.1210/jc.2013-3628.
Cushing’s Disease: Clinical Manifestations and Diagnostic Evaluation. (n.d.). Retrieved September 23, 2017, from https://www.drplace.com/Cushings_Disease_Clinical_Manifestations_and_Diagnostic_Evaluation.16.25787.htm
Daniel, E., Aylwin, S., Mustafa, O., et al. “Effectiveness of metyrapone in treating Cushing’s syndrome: a retrospective multicenter study in 195 patients.” The Journal of Clinical Endocrinology & Metabolism. 2015;100(11):4146–4154.
Davies, T.F. “ A Case-Based Guide to Clinical Endocrinology.” Springer 2015; pp. 11. ISBN 978-1-4939-2059-4.
Disorders of Cortisol Secretion. (n.d.). Retrieved September 23, 2017, from https://courses.washington.edu/conj/bess/feedback/cortisol/cortisoldisorders.html
Etxabe, J., Vazquez, JA. “Morbidity and mortality in Cushing’s disease: an epidemiological approach.” Clin Endocrinol (Oxf) 1994; 40:479–484.
Feelders, Richard A., and Leo J. Hofland. “Medical Treatment of Cushing’s Disease.” The Journal of Clinical Endocrinology and Metabolism 98, no. 2 (February 2013): 425–38. doi:10.1210/jc.2012-3126.
Fleseriu, M. “Recent Advances in the Medical Treatment of Cushing’s Disease.” F1000Prime Reports 6 (March 3, 2014). doi:10.12703/P6-18.
Fleseriu, M. & Petersenn, S. (2012). Medical management of Cushing’s disease: what is the future? Pituitary, 15(3), 330–341. http://doi.org/10.1007/s11102-012-0397-5
Fleseriu, M., Biller, B.M., Findling, J.W., Molitch, M.E., Schteingart, D.E., Gross, C. “Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome.” The Journal of Endocrinology & Metabolism. 2012; 97(6): 2039–2049.
Godbout, Ariane, Marcos Manavela, Karina Danilowicz, Hugues Beauregard, Oscar Domingo Bruno, and André Lacroix. “Cabergoline Monotherapy in the Long-Term Treatment of Cushing’s Disease.” European Journal of Endocrinology 163, no. 5 (November 2010): 709–16. doi:10.1530/EJE-10-0382.
Heyn, J., Geiger, C., Hinske, C.L., et al. “ Medical suppression of hypercortisolemia in Cushing’s syndrome with particular consideration of etomidate.” Pituitary. 2015; 15(2): 117-125. https://doi.org/10.1007/s11102-011-0314-3
HPA Axis and Pain - Dr. Joe Tatta, DPT, CNS-. (n.d.). Retrieved September 21, 2017, from http://www.drjoetatta.com/how-your-brain-changes-with-pain/
Clayton, R.N., Raskauskiene, D., Reulen, RC., Jones, P.W. “Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature.” The Journal of Clinical Endocrinology and Metabolism. 2011; 96(3):632-42. doi: 10.1210/jc.2010-1942
Lindholm, J., Juul, S., Jørgenson, J.O., et al. Incidence and late prognosis of Cushing’s syndrome: a population-based study. The Journal of Clinical Endocrinology and Metabolism. 2001; 86:117–123.
Marin, M.F., Hupbach, A., Maheu, F.S., Nader, K., Lupien, S.J. “Metyrapone Administration Reduces the Strength of an Emotional Memory Trace in a Long-Lasting Manner”. Journal of Clinical Endocrinology & Metabolism.2011; 96(8): 1221–1227. https://doi.org/10.1210/jc.2011-0226
Marshall, W. J., Bangert, S. K., Lapsley, M. (2012). Clinical Chemistry Seventh Edition. Mobsy Ltd.
McKeage, Kate. “Pasireotide: A Review of Its Use in Cushing’s Disease.” Drugs 73, no. 6 (May 2013): 563–74. doi:10.1007/s40265-013-0052-0.
Medvei, V. C. (1991). The history of Cushing’s disease: a controversial tale. Journal of the Royal Society of Medicine, 84(6), 363–366.
Nicolaides, N. C., Kyratzi, E., Lamprokostopoulou, A., Chrousos, G. P., & Charmandari, E. (2015). Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation, 22(1–2), 6–19. https://doi.org/10.1159/000362736
pituitary_adenoma_MRI.jpg (1259×682). (n.d.). Retrieved September 25, 2017, from http://www.virtualmedstudent.com/images/pituitary_adenoma_MRI.jpg
Trainer, Peter J. “New Options for the Medical Treatment of Cushing’s Syndrome.” Indian Journal of Endocrinology and Metabolism 17, no. 2 (2013): 245–48. doi:10.4103/2230-8210.109685.
Tsigos, C., & Chrousos, G. P. (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. https://doi.org/10.1016/S0022-3999(02)00429-4
Vilar, L., Naves, L. A., Azevedo, M. F., Arruda, M. J., Arahata, C. M., Moura E Silva, L., … Canadas, V. (2010). Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary, 13(2), 123–129. https://doi.org/10.1007/s11102-009-0209-8