This is an old revision of the document!
Table of Contents
Alzheimer's Disease Powerpoint
Alzheimer's Disease
INTRODUCTION
Alzheimer’s disease is a type of dementia specifically targeting areas of the brain responsible for memory, behaviour and language. Throughout the progression of this disease, neurons lose their ability to properly communicate, causing cell death in these regions. Alzheimer’s is a progressive disease where its symptoms accumulate very slowly, and worsen over time (da Silva, 2017). Beginning stages are typically very mild, however eventually individual’s lose the ability to engage in everyday tasks such as having meaningful conversation and responding to environmental cues. Though Alzheimer’s is not a normal side effect of aging, its greatest risk factor is age. However, approximately five percent of people between the ages of 40-50 develop early-onset Alzheimer’s (da Silva, 2017).
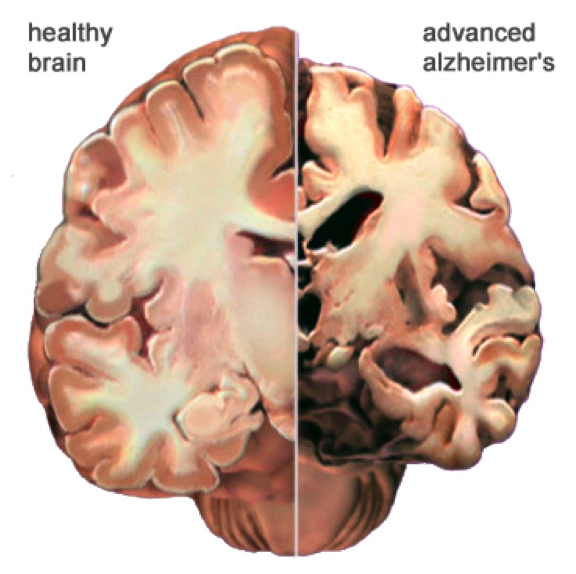
Alzheimer’s is the most common neurodegenerative disease. Individuals diagnosed with this disease live approximately eight years after symptoms become noticeable (Alzheimer's Association, 2011). This is a result of the progressive nerve cell death and tissue loss within the brain. Consequently, as an individual continues to age the size of the brain drastically shrinks, causing loss of function to almost all areas.
SIGNS & SYMPTOMS
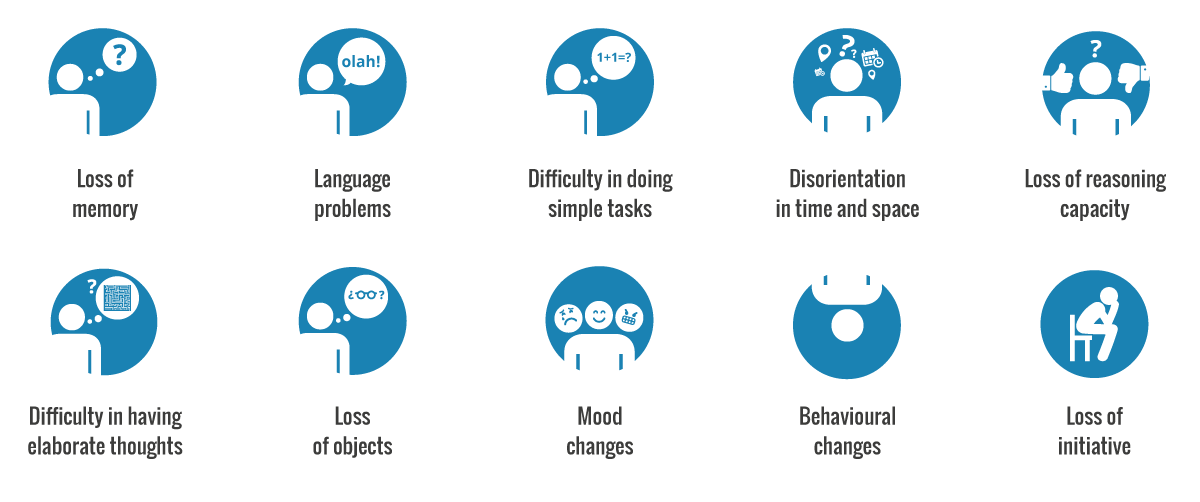
Though there are many signs of early onset Alzheimer’s, five of the major symptoms include:
- Memory loss: I.e. forgetting important dates or repeatedly asking the same question
- Difficulty with familiar tasks: I.e. not being able to drive to familiar locations
- Confusion with time or place: I.e. losing track of dates/seasons
- New issues with speaking or writing: I.e. trouble following/ joining a conversation
- Changes in mood and personality: I.e. becoming confused, depressed and anxious
EPIDEMIOLOGY
Key Points:
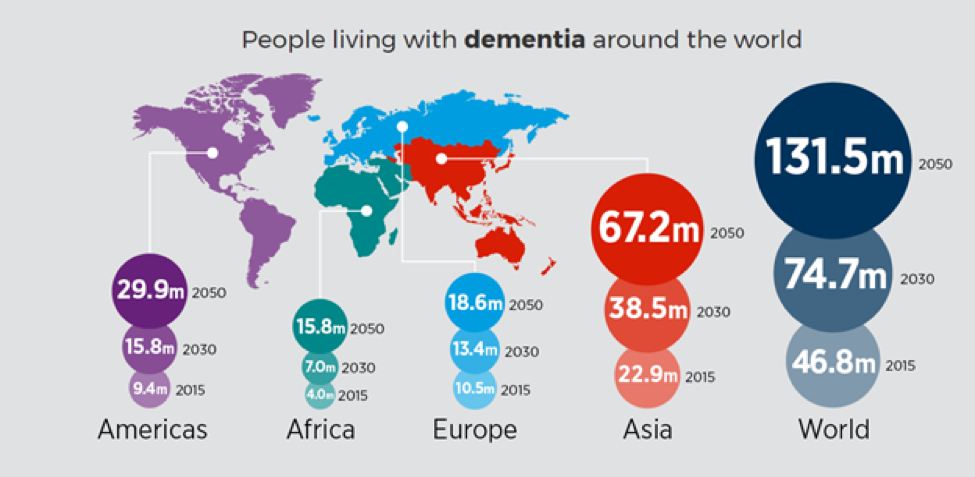
- Worldwide, nearly 44 million people have Alzheimer’s or a related dementia.
- Only 1-in-4 people with Alzheimer’s disease have been diagnosed.
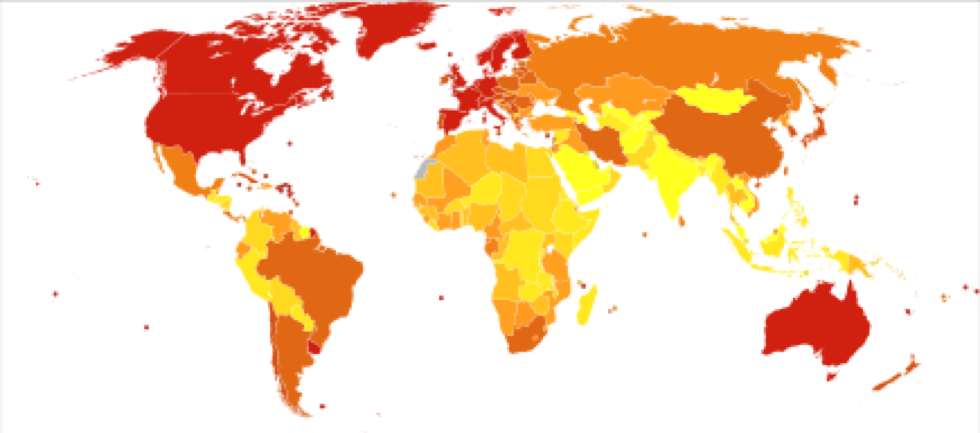
- Alzheimer’s and dementia is most common in Western Europe (North America is close behind).
- Alzheimer’s is least prevalent in Sub-Saharan Africa.
- Alzheimer’s and other dementias are the top cause for disabilities in later life. (Alzheimer’s Disease International, 2015)
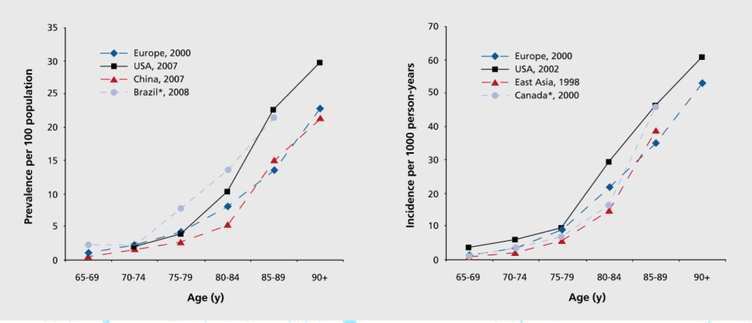
The incidence of AD is approximately 5–8 for per thousand person, which represents half of new dementia cases each year are AD (Bermejo-Pareja et al, 2008). One of the primary risk factor for AD, where there is a correlation of the incidence rate of every five years after the age of 65, the risk of acquiring AD approximately doubles (Di Carlo et al, 2002). Furthermore, there is also higher incidence rates in women compared to men of developing AD particularly in the population older than 85 (Andersen et al, 1999). In the United States, Alzheimer prevalence was estimated to be 1.6% in 2000 both overall and in the 65–74 age group, with the rate increasing to 19% in the 75–84 group and to 42% in the greater than 84 group. (Hebert et al, 2003) Prevalence rates of AD is less in developed countries compared to developing countries (Ferri et al, 2006). AD accounts to 50-70% of all forms of dementia, making it the leading cause of this neurodegenerative disease (7). Furthermore, due to the rapid increase of dementia, individuals would also be at increased risk of AD. Another study estimated that in 2006, 0.40% of the world population were afflicted by AD, and that the prevalence rate would triple and the absolute number would quadruple by 2050. (Brookmeyer et al, 2007)
PATHOPHYSIOLOGY
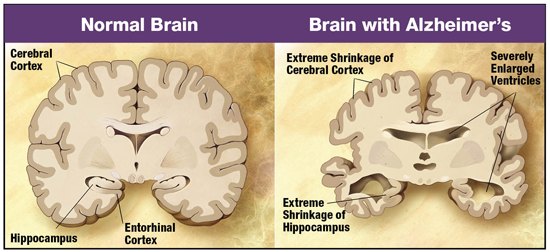
The early onset of Alzheimer’s disease (AD) is marked by neuronal loss occurring within the entorhinal cortex of the brain, which is located under the cerebral cortex (Columbia University Medical Center, 2013). The major function of this region of the brain is to submit information to and from structures in the hippocampus. The hippocampus then accumulates this information and stores it as long-term memory. Any pathology in these areas of the brain would affect an individual’s memory, being as they are so closely associated with memory processing (Saylor, 2017). Additionally, pathology is also seen in the amygdala, a region accountable for emotional responses and behaviour to various stimuli (Saylor, 2017). During the moderate onset of AD, cortical and hippocampal atrophy continues, alongside the widening of cerebrospinal fluid-filled ventricles within the brain (Salcudean, 2010). This ventricle widening causes further damage to surrounding tissues in the brain. A patient affected by AD may experience an inability to accomplish daily tasks, increased anxiety and emotional outbursts, and language impairment among several other symptoms as their memory loss continues. The severe (late) stage of dementia is characterized by the presence of two major neuropathological trademarks including intracellular neurofibrillary tangles and extracellular amyloid plaques (Salcudean, 2010). At this point, irreversible neuronal loss is evident throughout the brain, causing extensive dysfunction and extreme atrophies to affected areas (see figure XX). This individual would experience severe loss of autonomic functions and would require complete dependence on others for care.
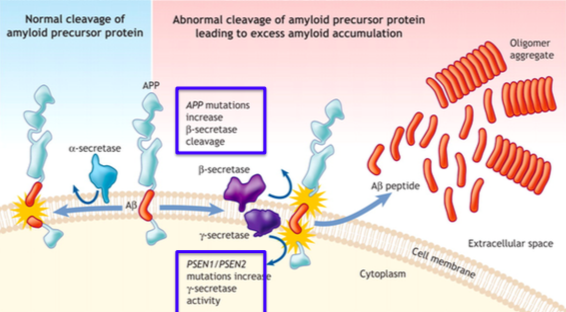
Amyloid precursor proteins (APP) are constantly released during normal metabolism in the brain. Little is understood about the functioning of these proteins, especially regarding its part in the pathogenesis of AD (Lin, 2001). APP can be cleaved by α-secretase proteases, which would result in normal functioning of the brain, and no buildup of extracellular amyloid plaque (Lichtenthaler, 2012) (See left hand side of figure XX). However, if the APP molecule is cleaved by β-secretase and/or γ-secretase, amyloid-β peptide fragments are released into the extracellular space due to an abnormal cleavage of the protein by these enzymes (Salcudean, 2010). In small amounts, this procedure is not harmful and may even be desirable for normal synaptic functioning. More often than not, however, the over activity of these β- and γ-secretase enzymes cause a large, toxic amount of amyloid-β peptide fragments. In excessive amounts, these fragments aggregate and form plaque in the extracellular space (see right hand side of figure XX). PSEN1 and PSEN2 (Presenilin 1 and 2 respectively) play a part in regulating APP processing through γ-secretase, but mutuations in these protein molecules increase γ-secretase activity, which can lead to even more plaque formation (Salcudean, 2010). A huge component of these plaques are found throughout the brains of patients affected by AD, showing that they may play a huge role in the loss of functioning of the brain in these individuals (Lin, 2001).
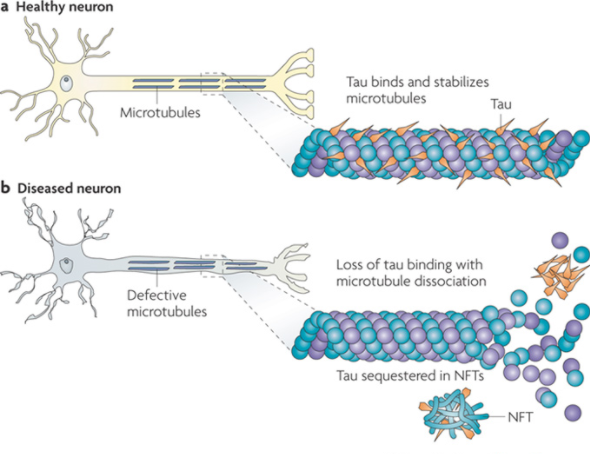
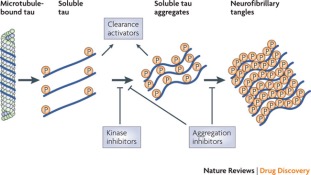
Similarly, the brains of patients with AD seem to have an abundant amount of intracellular neurofibrillary tangles (Salcudean, 2010). Microtubules run along the length of axons, simulating tracks for motor proteins like kinesin and dynein to use in transporting their neuron signals to other areas of the brain. The Tau protein binds to this microtubule to stabilize it and prevent its depolymerization (Salcudean, 2010) (See Figure XX a). In normal physiology of a healthy neuron, protein kinases phosphorylate tau, lifting them off the microtubule and allowing the passage of the motor proteins along the track. After this passage, the tau dephosphorylates and reattaches to the microtubule, stabilizing it once again (Salcudean, 2010). This procedure is much different in pathology of a diseased neuron. Hyperphosphorylation of tau proteins occur, which causes tau to aggregate with other taus, forming tangles in the brain (See figure XX b). Neuron signaling is not able to function as normal when this occurs, and it seems to have a choking affect on nerve cells, killing them in abundance (Ballatore, 2007).
Thus, both these processes are seen in many patients that suffer from AD. However, there are some patients with AD that do not seem to have mutations in their APP, PSEN1 and PSEN2 genes. This insinuates that some forms of this dementia can be associated with alterations in other genes that have yet to be identified by scientific research (Mayo Foundation for Medical Education and Research, 2016).
DIAGNOSIS
Alzheimer’s Disease can be categorized into three stages; Early, Mild-to-severe, and Advanced. The clinical diagnosis is most often made during the mild-to-severe stage. Nonetheless, definitive diagnosis of Alzheimer’s can only be made post-mortem by examining brain tissues (Dubois et al., 2014).
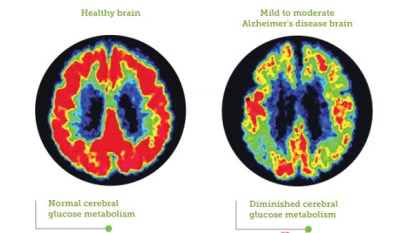
Clinicians can diagnose the presence of Alzheimer’s disease with an adequate amount of certainty using several factors. These factors include the individual’s medical history, history from relatives, behaviour observations, presence of characteristic neurological and neuropsychological features, and the absence of alternate conditions (Dubois et al., 2014). This probable diagnosis of Alzheimer’s Disease can be made with approximately 90% confidence based on the stated criteria. Clinicians focus heavily on a combination of both brain imaging and clinical assessments for diagnosis, particularly focusing on declining mental ability (Dubois et al., 2014). In order to identify the presence of neurological features, a number of brain imaging techniques are used, such as, computed tomography (CT), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET). These can not only be used to identify presence of neurological features characteristic of Alzheimer’s disease, but also exclude other pathology or subtypes of dementia that may be mistaken for Alzheimer’s Disease (McKhann et al., 2011) . Figure 8 shows a PET scan in which we can compare a healthy brain to a mild-to-moderate Alzheimer’s Disease brain. Not only is there a shrinkage in mass of the Alzheimer’s Disease brain, but glucose metabolism, indicated by the red probe, seems to be significantly decreased in the Alzheimer’s Disease brain relative to the healthy brain (Landau et al., 2014). In addition, assessment of the individual’s intellectual functioning can further help support a diagnosis. The Mini-Mental State Examination (MMSE) is the most common administered test to evaluate cognitive impairments such as problems with memory, attention and language. Further examinations are crucial to diagnose Alzheimer’s with sufficient certainty, including, interviews with family members and caregivers, who can provide important information about daily living abilities (McKhann et al., 2011).
It is vital to also identify biomarkers that will help assess risk, diagnosis, and monitor the progression of the disease (Hansson et al., 2006). They can provide valuable indications of health and disease, and contain high sensitivity and specificity that defines their diagnostic utility (Humpel, 2011). Biomarkers can help distinguish between different types of dementias that may appear very similar to Alzheimer’s Disease. There are no specific biomarkers that can confirm Alzheimer’s Disease with 100% certainty. However, it is possible to use measurements of B-amyloid (1-42), total tau, and phosphorylated-tau—181 in the cerebrospinal fluid as biomarkers for Alzheimer’s Disease (Humpel, 2011). The combination of all three can significantly increase the diagnosis of Alzheimer’s Disease (Humpel, 2011). In patients with Alzheimer’s, due to aggregation of B-Amyloid (1-42) in the brain, B-amyloid (1-42) would essentially be reduced in the cerebrospinal fluid, whereas total tau and phosphorylated tau would be enhanced (Humpel, 2011).
Candidate gene studies for Alzheimer’s Disease do not allow conclusive evidence for the presence of disease beyond a simple hypothesis. The Genome-wide association studies (GWAS) test many genetic markers to detect the presence of diseases related to Alzheimer’s Disease. This form of study is most often used in clinical trials rather than clinical diagnosis (Bertram & Tanzi, 2009).
CURRENT TREATMENTS
Similar to the diagnosis of the disease, there has yet to be an effective cure for Alzheimer’s Disease. Although there are several treatments available that help treat the symptoms of the disease, they provide relatively few benefits. They have not shown to delay the onset of AD or halt the progression of the disease. Current treatments can be pharmaceutical, psychosocial and caregiving in nature (Yiannopoulou & Papageorgiou, 2013).
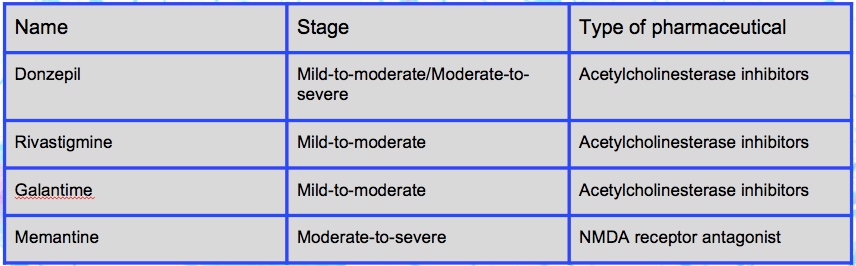
Pharmaceutical treatments include acetylcholinesterase inhibitors and as well as a NMDA receptor antagonist. Donzepil, Rivastigmine, and Galantime are acetylcholinesterase inhibitors most often used in the mild-to-moderate stage of the disease (Lahiri, Greig, & Sambamurti, 2002). Alzheimer’s Disease is correlated with a decrease in the activity of cholinergic neurons, and these drugs inhibit the activity acetylcholinesterase (breakdown of acetylcholine), therefore increasing the concentration of acetylcholine in the brain. Approximately 20% of patients taking one of these three drugs may show common side effects associated with cholinergic excess such as, nausea and vomiting (Lahiri, Greig, & Sambamurti, 2002). Memantine is a NMDA receptor antagonist, which is predominantly used in the moderate-to-severe stages of the disease (Reisberg et al., 2003). In Alzheimer’s Disease the excitatory neurotransmitter Glutamate is overstimulated, ultimately causing cell death though a process known as excitotoxicity. Memantine acts on the glutamatergic system by inhibiting the overstimulation of glutamate. Common side effects of this drug include, hallucinations, confusion, fatigue, headache, and dizziness (Reisberg et al., 2003).
In addition to pharmaceutical treatments, many Alzheimer’s patients will undergo psychosocial and caregiving treatments. Psychosocial treatments specifically target the cognition, emotion, behaviour, and stimulation-oriented aspects of the disease (Brodaty, Green, & Koschera, 2003). A common example of a psychosocial intervention is known as Reminiscence Therapy, in which patients are involved in a discussion of past experiences with photographs, music, and other familiar items. This type of intervention tends to show minor improvements in the cognition and mood of the patients (Sitzer, Twamley, & Jeste, 2006). Caregiving is essential to help patients who are incapable of carrying out common day-to-day activities. Both have received minimal scientific support but seem to be significant in helping patients adjust to the illness (Brodaty, Green, & Koschera, 2003).
FUTURE TREATMENTS
Researchers are continually looking at possible ways to delay and halt the progression of Alzheimer’s Disease. Current treatments only provide minimal relief to the symptoms of the disease and not the underlying disease itself.
Some possible therapeutics include drugs that decrease the production of B-amyloid plaques and tau tangles, removing the toxic aggregates, or preventing the aggregation (Yiannopoulou & Papageorgiou, 2013).
Figure 10 depicts therapeutics that target Tau aggregates through kinase inhibitors, which block the formation of the tau aggregates (Yiannopoulou & Papageorgiou, 2013).
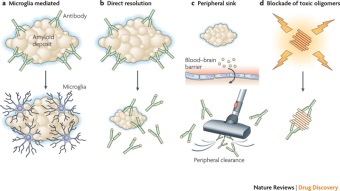
Figure 11 shows four distinct ways amyloid specific antibodies may be able to degenerate the B-amyloid plaques (Yiannopoulou & Papageorgiou, 2013).
In addition to future treatments, it is important to focus on advancing research and development in biomarkers that can help assess risk and monitor the underlying disease.
References
Alzheimer's Association. (2011). Healthy Brain Versus Alzheimer's Brain. Retrieved March 03, 2017, from http://www.alz.org/braintour/healthy_vs_alzheimers.asp
Alzheimer's: Is it in your genes? (n.d.). Retrieved March 4, 2017, from http://www.mayoclinic.org/diseases-conditions/alzheimers-disease/in-depth/alzheimers-genes/art-20046552?pg=2
Alzheimer's: Type 3 Diabetes? (2014, March 10). Retrieved March 4, 2017, from http://www.wholehealthinsider.com/newsletter/alzheimers-type-3-diabetes/
Ballatore, C., Lee, V. M. Y., & Trojanowski, J. Q. (2007). Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nature Reviews Neuroscience, 8(9), 663-672.
Bertram, L., & Tanzi, R. E. (2009). Genome-wide association studies in Alzheimer's disease. Human molecular genetics, 18(R2), R137-R145.
Brodaty, H., Green, A., & Koschera, A. (2003). Meta‐analysis of psychosocial interventions for caregivers of people with dementia. Journal of the American Geriatrics Society, 51(5), 657-664.
Brunden, K. R., Trojanowski, J. Q., & Lee, V. M. Y. (2009). Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nature reviews Drug discovery, 8(10), 783-793.
Cephalicvein. (2016, September 11). Alzheimer's Symptoms. Retrieved March 06, 2017, from http://cephalicvein.com/2016/07/alzheimers-symptoms/
Da Silva, R. (2017) Module 1 Lecture 4: Alzheimer’s Disease [PowerPoint Slides]. Retrieved from McMaster University Human Pathophysiology.
Dubois, B., Feldman, H. H., Jacova, C., Hampel, H., Molinuevo, J. L., Blennow, K., … & Cappa, S. (2014). Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. The Lancet Neurology, 13(6), 614-629.
F Lichtenthaler, S. (2012). Alpha-secretase cleavage of the amyloid precursor protein: proteolysis regulated by signaling pathways and protein trafficking. Current Alzheimer research, 9(2), 165-177.
Hansson, O., Zetterberg, H., Buchhave, P., Londos, E., Blennow, K., & Minthon, L. (2006). Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. The Lancet Neurology, 5(3), 228-234.
Humpel, C. (2011). Identifying and validating biomarkers for Alzheimer's disease. Trends in biotechnology, 29(1), 26-32.
Lahiri, D. K., Farlow, M. R., Greig, N. H., & Sambamurti, K. (2002). Current drug targets for Alzheimer's disease treatment. Drug Development Research, 56(3), 267-281.
Landau, S. M., Thomas, B. A., Thurfjell, L., Schmidt, M., Margolin, R., Mintun, M., … & Alzheimer’s Disease Neuroimaging Initiative. (2014). Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. European journal of nuclear medicine and molecular imaging, 41(7), 1398-1407.
Lin, H. A. I., Bhatia, R., & Lal, R. (2001). Amyloid β protein forms ion channels: implications for Alzheimer’s disease pathophysiology. The FASEB Journal, 15(13), 2433-2444.
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., … & Mohs, R. C. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia, 7(3), 263-269.
Patterson, C., Feightner, J. W., Garcia, A., Hsiung, G. Y. R., MacKnight, C., & Sadovnick, A. D. (2008). Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. Canadian Medical Association Journal, 178(5), 548-556.
Reisberg, B., Doody, R., Stöffler, A., Schmitt, F., Ferris, S., & Möbius, H. J. (2003). Memantine in moderate-to-severe Alzheimer's disease. New England Journal of Medicine, 348(14), 1333-1341.
Salcudean, A., & Todoran, A. M. (2010). Pathophysiology of Alzheimer’s disease. Romanian Journal of Psychopharmacology, 10, 1-8.
Saylor, D., & Allen, J. (2017, January 31). What is the Entorhinal Cortex? Retrieved March 5, 2017, from http://www.wisegeek.com/what-is-the-entorhinal-cortex.htm
Sitzer, D. I., Twamley, E. W., & Jeste, D. (2006). Cognitive training in Alzheimer's disease: a meta‐analysis of the literature. Acta Psychiatrica Scandinavica, 114(2), 75-90.
Study Shows Where Alzheimer's Starts and How It Spreads. (2013, December 23). Retrieved March 4, 2017, from http://newsroom.cumc.columbia.edu/blog/2013/12/22/how-alzheimers-spreads/
Yiannopoulou, K. G., & Papageorgiou, S. G. (2013). Current and future treatments for Alzheimer’s disease. Therapeutic advances in neurological disorders, 6(1), 19-33.