This is an old revision of the document!
Table of Contents
Add intro image
Alzheimer's Disease Powerpoint
Alzheimer's Disease
INTRODUCTION
blah
SIGNS & SYMPTOMS
blah
EPIDEMIOLOGY
Key Points:
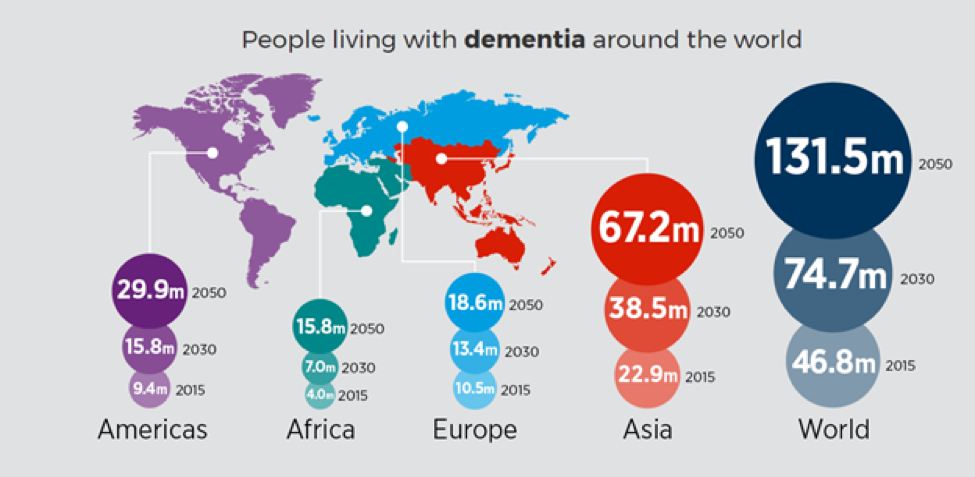
- Worldwide, nearly 44 million people have Alzheimer’s or a related dementia.
- Only 1-in-4 people with Alzheimer’s disease have been diagnosed.
- Alzheimer’s and dementia is most common in Western Europe (North America is close behind).
- Alzheimer’s is least prevalent in Sub-Saharan Africa.
- Alzheimer’s and other dementias are the top cause for disabilities in later life. (Alzheimer’s Disease International, 2015)
The incidence of AD is approximately 5–8 for per thousand person, which represents half of new dementia cases each year are AD (Bermejo-Pareja et al, 2008). One of the primary risk factor for AD, where there is a correlation of the incidence rate of every five years after the age of 65, the risk of acquiring AD approximately doubles (Di Carlo et al, 2002). Furthermore, there is also higher incidence rates in women compared to men of developing AD particularly in the population older than 85 (Andersen et al, 1999). In the United States, Alzheimer prevalence was estimated to be 1.6% in 2000 both overall and in the 65–74 age group, with the rate increasing to 19% in the 75–84 group and to 42% in the greater than 84 group. (Hebert et al, 2003) Prevalence rates of AD is less in developed countries compared to developing countries (Ferri et al, 2006). AD accounts to 50-70% of all forms of dementia, making it the leading cause of this neurodegenerative disease (7). Furthermore, due to the rapid increase of dementia, individuals would also be at increased risk of AD. Another study estimated that in 2006, 0.40% of the world population were afflicted by AD, and that the prevalence rate would triple and the absolute number would quadruple by 2050. (Brookmeyer et al, 2007)
ETIOLOGY
The exact aetiology of breast cancer is unknown; however, what scientists know for sure is that it is caused by some damage to a cell’s DNA (National Breast Cancer, 2016). It is also known that there are certain risk factors that make some women more likely than others to develop breast cancer. Many of these factors can be controlled, such as a person’s diet and whether or not they smoke and/or consume high levels of alcohol. Consuming a diet high in fats for example, especially trans fats, can induce breast cancer in some individuals, as these fats promote free radicals that can damage DNA and mutate cells (Farvid et al., 2016).
It is also essential for individuals that smoke to change their smoking habits. Smoking does not only damage an individual’s lungs but it also increases blood clot risks when taking exogenous hormones (Breast Cancer, 2014). As well, smoking cigarettes are seen to cause high levels of toxic material accumulation in the breast tissue. A study by Gaudet et al. assessed the effects of tobacco smoke in lab rodents and found that at least 20 lipophilic chemical compounds contained in cigarettes induced tumours in mammary adipose tissues, which can have similar effects in humans (2013).
In terms of alcohol consumption, although higher intakes are seen to be associated with increased risk of breast cancer, studies have shown that an individual consuming a moderate intake of alcohol before and/or after diagnosis experienced better survival than non-drinkers (Newcomb, 2013). Yet, alcohol consumption and breast cancer observations are varied in each study; in some studies, the risk of breast cancer from consuming about two or more drinks per day (>24g) compared to non-drinkers is higher; a lighter alcohol intake is associated with a weaker risk of breast cancer, and moderate and heavy drinking both contribute to greater risks of developing the disease than that of non-drinkers (Longnecker, 1988). In this way, comparing studies regarding alcohol consumption and breast cancer risk show inconclusive results.
Furthermore, women that are taking hormones or using contraceptives like birth control put themselves at a higher risk of developing breast cancer than non-users (Chlebowski, 2009). Estrogen is shown to facilitate cell proliferation and high levels of estrogen can aid in the spreading of cancerous cells if the hormone binds to a mutated cell receptor (Kaczmarczyk, 2015). Exogenous hormones that can be taken include progesterone and estrogen; where estrogen can be taken by itself or together with progesterone or progestin (a synthetic hormone that mimics effects of progesterone) (Kaczmarczyk, 2015). These hormones are similar to, but not exact in, chemical composition to the hormones an individual produces naturally, thus different users may experience different side effects. Some studies have shown that women on certain hormone therapies like these had a higher chance of developing breast cancer than women that are not using these forms of treatment (Chlebowski, 2008).
Other modifiable risk factors are associated with an individual’s exercise habits and weight, as obesity puts an individual at a higher risk of becoming diagnosed with the disease (National Cancer Institute, 2009). Estrogen can be produced in fat tissues, so the more fatty tissue an individual has, the more estrogen they will produce.
Unfortunately, some risk factors cannot be controlled, such as an individual’s age. Girls that reach menarche at a young age (<12 years old), and women that reach menopause at a late age (>55 years old) are at higher risk of developing the disease as they produce more estrogen during their lifespan (National Breast Cancer, 2016). Genetics plays a role as well, as mutations in some genes are known to predispose an individual to breast cancer. These include BRCA1 and BRCA2 (Breast cancer gene 1 and 2 respectively), and they put carriers at a 65-85% chance of developing breast cancer by the age of 70 (da Silva, 2015).
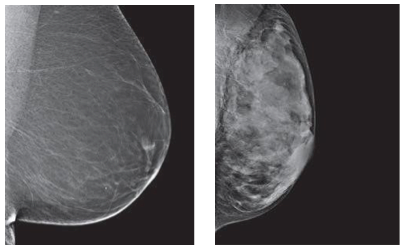
Breast density is another contributing factor, as women with denser breasts have a higher chance of developing breast cancer (American Cancer Society, 2016). Breasts that have more duct and lobular tissue and less fatty tissue are considered to be “more dense.” When doctors look at x-rays like mammograms, dense breasts make them harder to read and thus harder to locate any tumors (see Figure 4) (American Cancer Society, 2016). Thus, denser breasts pose a greater risk of having breast cancer.
Similarly, individuals with a history of breast cancer, higher endogenous sex hormone levels, as well as more environmental exposure to carcinogens also have a higher risk of developing the disease. Environmental carcinogen exposure may play a role in breast cancer development as the breast anatomy makes breasts a susceptible target for chemical carcinogens (Li, 1996). A study by Li et al. showed that the total aromatic DNA adduct levels are higher in adjacent tissues of breast cancer patients (individuals with 100% risk of having breast cancer) than are the levels in normal breast tissue of participants without the disease (Li, 1996). Thus, all these risk factors contribute in increasing the likelihood of some individuals getting breast cancer over others.
DIAGNOSIS
Prior to being diagnosed with breast cancer there are ways to test for an individual’s genetic risk factors for developing the disease. Majority of inherited cases of breast cancer is a result of mutations within the BRCA1 or BRCA2 genes. BRCA1 is found on chromosome 17, whereas BRCA2 is found on chromosome 13 (Radford & Zehnbauer, 1996). This is an important gene to consider as an individual only needs one mutated copy in order to possess increased chances of developing breast cancer. If one parent has this mutation, their offspring will automatically have a 50% chance of inheriting it and developing specific cancers (Radford & Zehnbauer, 1996). Un-mutated BRCA genes produce tumor suppressor proteins that aid in the repair of damaged DNA (Radford & Zehnbauer, 1996). As a result, when mutations occur within either BRCA gene, the damaged DNA is left un-repaired, resulting in higher chances of future cancer development.
Though majority of breast cancer cases are sporadic, approximately ten percent of cases result from an inherited autosomal dominant mutation within the BRCA genes (Radford & Zehnbauer, 1996). However, once a mutation is inherited, there is a 65-85% chance that an individual will develop breast cancer by the time they are 70 years old (Radford & Zehnbauer, 1996). Genetic screening options are available with the purpose of identifying individuals at risk of developing certain cancers within their lifetime. There are two main methods used when screening for inherited breast cancer-causing mutations. The first form of genetic screening searches for every known mutation that is linked to cancer. This is done by collecting a sample of DNA, typically a buccal sample, and examining it for cellular mutations. The second method collects a DNA sample in a similar way, however it also requires the collection of DNA from at least four family members who are known to have a BRCA mutation (Radford & Zehnbauer, 1996). By doing this, the test screens for those particular mutations that are currently present within the family. Once the tests have been completed an individual is then identified as being positive or negative. A positive test implies that they possess a known BRCA mutation (Radford & Zehnbauer, 1996). However, it is important to understand that carrying a mutation does not necessarily mean an individual will develop the disease, it just informs them that they have an increased chance of future development.
There are also a variety of indirect examinations to test for the presence of breast cancer without collecting DNA samples. One such test is a mammography. This type of screening makes use of breast imaging via low-dose X-rays (Canadian Cancer Society, 2017). Using this technique it is possible to detect the presence of breast cancer at its earliest stages, when it is most treatable and mortality rates are low. During this procedure, each breast is individually compressed by parallel plates. In doing so, the breast thickness is reduced, making it easier to recognize abnormal masses. X-rays are then used to create images for examination (Canadian Cancer Society, 2017). Additionally, in the province of Ontario it is recommended that females between the ages of 50-74 get mammograms done every two years, in order to detect early development of breast cancer.
A second procedure is a breast biopsy which is used to remove patches of tissue or fluid from abnormal lumps within the breast. There are three types of breast biopsies when trying to discover the presence or absence of breast cancer. The first is fine needle aspiration. This is the least commonly used method out of the three with regards to breast cancer. However, this method is used when a lump is easily reachable and expected to be fluid-filled (Ballo & Sneige, 1996). The extremely thin needle allows for extraction of the fluid to be used for examination. The second method is core needle biopsy. This method makes use of a slightly larger, hollow needle. Using this technique technicians are able to remove layers of tissue from suspicious areas within the breast in order to determine how far cancer has penetrated (Ballo & Sneige, 1996). The final method is called surgical biopsy. This is done by making an incision, ranging from one to two inches on the breast (Ballo & Sneige, 1996). Following this, part or all of the abnormal mass is removed, along with a small amount of surrounding tissue. All three methods are used to determine if cancer cells are present in abnormal masses within the breast.
PATHOPHYSIOLOGY
Breast Cancer is similar to other cancers in which abnormal cell growth can potentially invade and spread to other areas of the body. In normal cell growth, cells can divide as necessary and have functioning signals that can stop cell division. These cells stay attached and localized in their respective sites and do not spread or invade other areas. Normal cells can also undergo programmed cell death, which keeps a balance in the cell population and discards unnecessary cells (Hartwell & Kastan, 1994).
The P13K Pathway and HER2 Gene
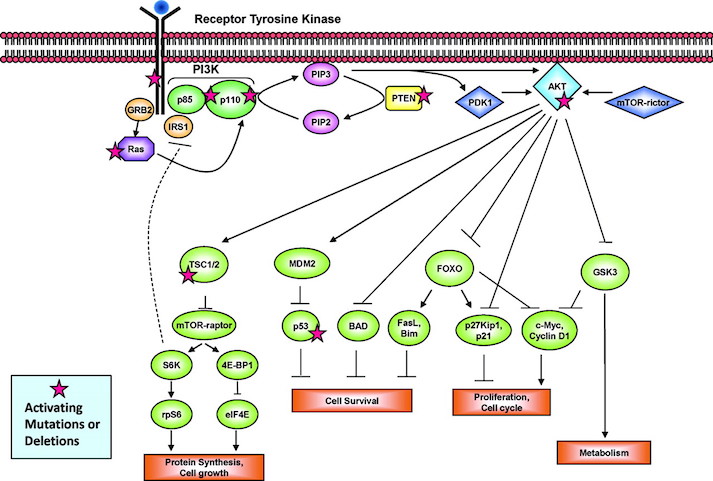
The P13K pathway is an example of one of the most important pathways that protect cells by controlling functions such as cell growth, differentiation, motility, survival, and proliferation. P13Ks are lipid kinases that phosphorylate phosphoinositides. The P13Ks involved in breast cancer are Class 1A, which are comprised of a regulatory subunit (p85) and a catalytic subunit (p110). The activation of the P13K is initiated from growth factor or ligand binding to its respective receptor tyrosine kinase (RTK). These receptors contain two important growth factor receptors; the human epidermal growth factor receptor (HER) family and the insulin-like growth factor 1 receptor (IGF-1R). Once activated by a receptor, the P13K heterodimer interacts with a portion of the p85. This binding removes the inhibitory effect of p85 on p1110 rendering the P13K pathway active. The activation leads to a cascade of events downstream of the pathway, resulting in diverse functions such as cellular metabolism, proliferation, differentiation and survival (Liang & Slingerland, 2003).
In breast cancer this pathway is aberrantly hyperactive, as shown in Figure __. This results from mutations in various parts of the pathway but most predominantly in PIK3CA, which encodes for the catalytic p110a subunit. Mutations in these promote downstream signaling elements such as Akt that increase enzymatic function. The overexpression of p110a subunit, therefore leads to robust activation of the pathway (Berns et al., 2007). P13K pathway also involves tumor suppressors such as PTEN, which turn off the pathway and induce cell death. However, in cases such as breast cancer, the PTEN protein may be mutated and unable to turn off the pathway. Therefore, there is excessive cell proliferation and the cell does not undergo programmed cell death. In this case, low expression of PTEN leads to excessive cell growth (Depowski, Rosenthal, & Ross, 2001).
The HER2 Gene
The ErbB is a family of receptor tyrosine kinases, which include the Her2 (ErbB2) receptor-tyrosine-protein kinase. Both the P13K/AKT pathway and Ras-Raf-MAPK pathway are important signalling routes for the ErbB protein. In normal cell activity, the ErbB protein forms homodimers and heterodimers upon activation by growth factor ligands. Upon dimerization these proteins become autophosphorylated and act as binding sites for intracellular signal activators. This triggers a signal cascade leading to increased cell proliferation and inhibition of apoptosis (cell death). Mutation in the HER2 gene leads to amplification of this oncogene and excessive ErbB signalling. Therefore, this increased signalling leads to aberrant cell proliferation and increased inhibition of cell apoptosis (Mitri, Constantine, & O’Regan, 2012).
Types of Abnormalities
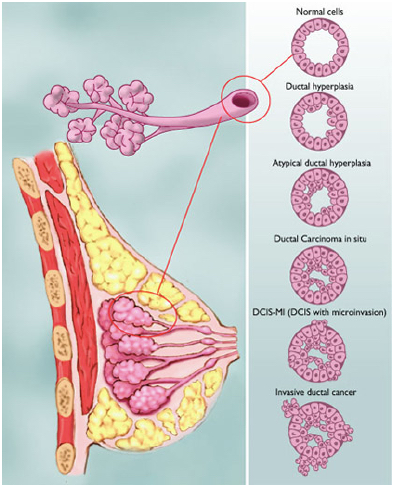
Figure shows the various types of abnormalities that can result from abnormal cell growth. Ductal hyperplasia contains lesions in the breast that are not cancerous but have active growth and division in the breast tissue cells. Atypical Ductal Hyperplasia and Ductal Carcinoma In Situ (DCIS) occurs when breast tissue is beginning to take an abnormal appearance, however the abnormal cells are still localized in within the tissue. In DCIS-microinvasion and Invasive ductal cancer, the excessive cell growth spreads outside of the tissue and invades other areas of the body (Hüsemann et al., 2008).
Spread of Malignant Tumours
The spread of the tumour varies with different stages in breast cancer. Usually, the least progressive is when atypical cells are in the breast tissue and there are no signs of spread to lymph nodes. As the tumour size increases it begins to spread to lymph nodes and there is metastases in axillary lymph nodes. From here the tumours of any size may spread into the skin, chest wall, and eventually the cancer spreads to other organs and tissues (Hüsemann et al., 2008).
The cancer cells are able to spread to various areas of the body by penetrating the extracellular matrix and blood vessels. Cells are constrained in their respective organs by cell-cell adhesion and basement membrane. However, cancer cells can penetrate the basement membrane by using invadopodia. Invadopodia is the process in which cells form actin-rich protrusions in the plasma membrane and subsequently degrade the extracellular matrix. Genes involved in these protrusions and invasions are upregulated in malignant cancer cells, allowing increased cell invasion and motility. This way, some cells contained in the tumour can separate from their parent mass and enter vascular and lymphatic circulation (Linder, 2007).
CURRENT TREATMENTS
There are many procedures to treat breast cancer ranging from invasive to non-invasive treatments. One of the least invasive treatments is a lumpectomy, which is considered breast conserving surgery. This form of treatment aims to remove any abnormal masses within the breast, without removing all breast tissue (Fisher et al, 1993). Along with the mass itself, a layer of surrounding tissue, specifically regions near the axillary nodes, are also extracted to ensure cancer has not spread to neighboring regions (Fisher et al, 1993). However, due to the conservation of most breast tissue, this method is most effectively used at the earliest stages of breast cancer, before it has spread to multiple layers of tissue.
One of the most commonly used treatments is a mastectomy. This is because a lot of breast cancer cases are reported late, after cancer has already spread to multiple layers of tissue. Using this method all of the breast tissue, including the nipple and areola is removed on the affected breast(s) (Canadian Cancer Society, 2017). Once removed, the skin is stitched closed with a temporary tube attached to an opening in the wound, allowing for any fluid post-surgery to be removed safely (Canadian Cancer Society, 2017). After surgery, a pathologist examines the removed tissue to determine the extent of cancer penetration, and detect whether it has spread to neighboring regions. Since the axillary nodes have the highest likelihood for cancer to spread from the breasts, a few lymph nodes are also removed during surgery for examination (Canadian Cancer Society, 2017). If cancer has appeared to spread to other regions, then other treatments may be used in conjunction with a mastectomy, such as radiation therapy, in order to kill all remaining cancer cells.
Radiation therapy is a form of treatment that targets specific suspected regions. This type of therapy uses high-energy X-rays to assist in targeting and killing cancer cells (Canadian Cancer Society, 2017). Due to its ability to target cells, this form of treatment is typically used post-surgery in order to kill any remaining cancer cells that were missed during surgical procedures. This form of treatment used alone is an option for those experiencing early stage breast cancer, when all the cancer cells are contained within a specific region. However, radiation therapy is also an option for individuals with more advanced breast cancer if used in combination with other forms of treatment such as surgery or chemotherapy (Canadian Cancer Society, 2017).
Majority of lumpectomy procedures are followed by radiation therapy to ensure the cancer is killed from remaining breast tissue (Fisher et al, 1993). By doing this it will lower the chance of breast cancer recurrence by 50 percent, along with death due to breast cancer events by approximately 20 percent (Fisher et al, 1993). In terms of a mastectomy, radiation therapy has not shown to be a beneficial post-procedure (Canadian Cancer Society, 2017). However, in rare cases when radiation therapy is used after mastectomy, its purpose is to treat neighboring regions that are highly susceptible to the spread of cancer, such as the chest wall and axillary nodes (Canadian Cancer Society, 2017).
One of the most common forms of treatment for general cancer cases is chemotherapy. During this form of treatment tumors are treated with high levels of radiation to kill the rapidly diving cells (Canadian Cancer Society, 2017). This form of treatment targets cells non-specifically, and is therefore highly invasive as it kills both healthy and cancerous cells within the body. The radiation from chemotherapy causes the cells within the body to remain in the metaphase-anaphase stage during cell division, leading to apoptosis instead of replication (Canadian Cancer Society, 2017). Due to the killing of these cells there are high toxicity levels associated with this form of treatment which cause side effects such as hair loss, fatigue and nausea (Canadian Cancer Society, 2017).
Chemotherapy is divided into two categories depending on when it is used. Chemotherapy done prior to surgery is referred to as Neoadjuvant therapy. This method is used to shrink large tumors before attempting surgery to increase the chances of successful lump extraction, preventing breast removal (Kuerer et al, 1999). Neoadjuvant therapy can also be used in those with larger tumors in need of a mastectomy. In this case it would be used to increase the chances of tumor shrinkage, and promote a lumpectomy breast conserving procedure instead. Treatment this way also monitors how the tumor responds to treatment, making it safer for tumor removal (Kuerer et al, 1999). Chemotherapy used post surgery is referred to as Adjuvant therapy. This method is given to those who have no current evidence of containing cancer cells in their body (Kuerer et al, 1999). The purpose of adjuvant therapy is to kill the cancer cells that may potentially have been left behind or have metastasized, to reduce the risk of recurrence.
Patients who have been diagnosed with estrogen-receptor positive tumors often experience beneficial results from undergoing endocrine therapy (Lumachi et al, 2011). This type of therapy is usually done through oral contraceptives containing specific estrogen-receptor modulators (SERMs) (Lumachi et al, 2011). A few of the most commonly used SERMs are aromatase inhibitors, GnRH agonists and tamoxifen. Aromatase inhibitors decrease estrogen levels, whereas tamoxifen blocks estrogen receptors, both of which aim to either decrease the tumor’s size or delay its growth (Lumachi et al, 2011). Since endocrine therapy is used amongst those with estrogen-receptor positive tumors, women are classified as premenopausal or postmenopausal in order to determine which method of treatment to undergo. Women in the premenopausal stage who have the estrogen/progesterone receptor are normally treated with tamoxifen for a length of five years (Lumachi et al, 2011). This drug’s ability to block estrogen receptors lowers the risk of breast cancer recurrence by approximately 50 percent (Lumachi et al, 2011). Alternatively, women who are postmenopausal and estrogen-receptor positive have higher beneficial rates from adjuvant therapy, using an aromatase inhibitor (Lumachi et al, 2011). Since postmenopausal women still produce low levels of estrogen in specific fatty tissues, such as the breast tissue, this estrogen-lowering drug reduces the amounts of hormones which contribute to breast cancer growth.
FUTURE TREATMENTS
To continue declining mortality rates, some future treatments and screening technologies that are in the process of being introduced, focus on more micro areas of the disease. A new screening technology called Scintimammography, which is molecular breast imaging, uses a radioactive drug called Tracer which is injected into the patient. The drug travels to the breasts via circulation and attaches to breast tissue cells (American Cancer Society, 2016). The radioactive substance is then detected by a special camera called a cadmium-zinc-tullerida gamma camera. The significance of this piece of technology is its beneficial detection of small tumours which other current screening technologies lack in advancement (Rhodes, 2005). This screening technology is able to give an early diagnosis to certain cases that show no symptoms of the disease in earlier stages, leading to declines of mortality rates. (Rhodes, 2005).
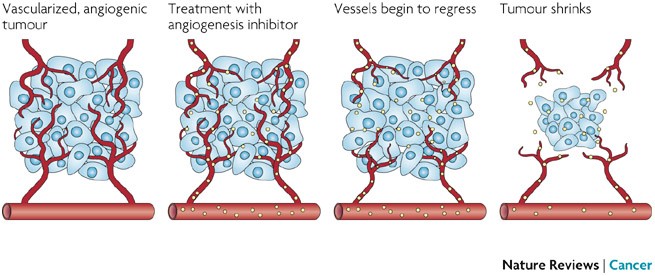
Future treatments are underway. These specific treatments are called target therapies and are a group of drugs which target specific changes in genes of cancer cells, a preventative measure in cancer cell proliferation. Some of these target therapies include PARP inhibitors. PARP inhibitors target the BRCA mutations (American Cancer Society, 2016). These target drugs have shown success in some types of breast cancers and are further being studies to determine exactly when these drugs have their highest potential to be helpful in treatment. Another target therapy in current research is anti-angiogenesis drugs, which refers to the blocking process of angiogenesis. Fast growing cancer cells require blood cells to nourish their cell bodies. Therefore preventing the proliferation and rapid growth of the cancer cells, blocking angiogenesis may be helpful in slowing down the disease (American Cancer Society, 2016).
References
Ballo, M. S., & Sneige, N. (1996). Can core needle biopsy replace fine‐needle aspiration cytology in the diagnosis of palpable breast carcinoma: A comparative study of 124 women. Cancer, 78(4), 773-777.
Berns, K., Horlings, H. M., Hennessy, B. T., Madiredjo, M., Hijmans, E. M., Beelen, K., … & Beijersbergen, R. L. (2007). A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer cell, 12(4), 395-402.
Breast Density and Your Mammogram Report. (n.d.). Retrieved January 25, 2017, from https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/mammograms/breast-density-and-your-mammogram-report.html
Canadian Cancer Society. (2017). Retrieved January 26, 2017, from http://www.cancer.ca/
Causes of Breast Cancer :: The National Breast Cancer Foundation. Retrieved January 24, 2017, from http://www.nationalbreastcancer.org/causes-of-breast-cancer
Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. New England Journal of Medicine 2009; 360(6):573–587
Da Silva, R. (2015) Module 3 Lecture 2: Breast Cancer[PowerPoint Slides]. Retrieved from McMaster University Health and Disease.
Depowski, P. L., Rosenthal, S. I., & Ross, J. S. (2001). Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Modern Pathology, 14(7), 672-676.
DeSantis, C., Bray, F., Ferlay, J., Lortet-Tieulent, J., Anderson, B., & Jemal, A. (2015). International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiology Biomarkers & Prevention, 24(10), 1495-1506. http://dx.doi.org/10.1158/1055-9965.epi-15-0535
Farvid, M. S., Eliassen, A. H., Cho, E., Liao, X., Chen, W. Y., & Willett, W. C. (2016). Dietary fiber intake in young adults and breast cancer risk. Pediatrics, 137(3), 1-11.
Fisher, B., Costantino, J., Redmond, C., Fisher, E., Margolese, R., Dimitrov, N., … & Mamounas, E. (1993). Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. New England Journal of Medicine, 328(22), 1581-1586.
Gaudet, M. M., Gapstur, S. M., Sun, J., Diver, W. R., Hannan, L. M., & Thun, M. J. (2013). Active smoking and breast cancer risk: original cohort data and meta-analysis. Journal of the National Cancer Institute, djt023.
Hartwell, L. H., & Kastan, M. B. (1994). Cell cycle control and cancer. Science, 266(5192), 1821.
Hüsemann, Y., Geigl, J. B., Schubert, F., Musiani, P., Meyer, M., Burghart, E., … & Klein, C. A. (2008). Systemic spread is an early step in breast cancer. Cancer cell, 13(1), 58-68.
Kaczmarczyk, T. (n.d.). Estrogen-Induced Cancer. Retrieved January 24, 2017, from http://www.cumc.columbia.edu/publications/in-vivo/Vol2_Iss10_may26_03/
Kuerer, H. M., Newman, L. A., Smith, T. L., Ames, F. C., Hunt, K. K., Dhingra, K., … & Buchholz, T. A. (1999). Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. Journal of Clinical Oncology, 17(2), 460-460.
Li, C. (Ed.). (2010). Breast cancer epidemiology (p. 401). New York: Springer.
Li, D., Wang, M., Dhingra, K., & Hittelman, W. N. (1996). Aromatic DNA adducts in adjacent tissues of breast cancer patients: clues to breast cancer etiology. Cancer Research, 56(2), 287-293.
Liang, J., & Slingerland, J. M. (2003). Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell cycle, 2(4), 336-342.
Linder, S. (2007). The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends in cell biology, 17(3), 107-117.
Longnecker, M. P., Berlin, J. A., Orza, M. J., & Chalmers, T. C. (1988). A meta-analysis of alcohol consumption in relation to risk of breast cancer. Jama, 260(5), 652-656.
Lumachi, F., Luisetto, G., Basso, S., Basso, U., Brunello, A., & Camozzi, V. (2011). Endocrine Therapy of Breast Cancer. PubMed, 18(4), 513-522. doi:10.2174/092986711794480177
McPherson, K. (2000). ABC of breast diseases: Breast cancer—epidemiology, risk factors, and genetics. BMJ, 321(7261), 624-628. http://dx.doi.org/10.1136/bmj.321.7261.624
Mitri, Z., Constantine, T., & O'Regan, R. (2012). The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemotherapy research and practice, 2012.
Newcomb, P. A., Kampman, E., Trentham-Dietz, A., Egan, K. M., Titus, L. J., Baron, J. A., … & Willett, W. C. (2013). Alcohol consumption before and after breast cancer diagnosis: associations with survival from breast cancer, cardiovascular disease, and other causes. Journal of clinical oncology, 31(16), 1939-1946.
Physical Activity and Cancer. (n.d.). Retrieved January 24, 2017, from https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/physical-activity-fact-sheet#q5
Radford, D. M., & Zehnbauer, B. A. (1996). Inherited breast cancer. Surgical Clinics of North America, 76(2), 205-220.
Rhodes, D., O'Connor, M., Phillips, S., Smith, R., & Collins, D. (2005). Molecular Breast Imaging: A New Technique Using Technetium Tc 99m Scintimammography to Detect Small Tumors of the Breast. Mayo Clinic Proceedings, 80(1), 24-30. http://dx.doi.org/10.4065/80.1.24
Smoking and Breast Cancer Risk. (n.d.). Retrieved January 25, 2017, from http://www.breastcancer.org/risk/factors/smoking
Tao, Z., Shi, A., Lu, C. et al. Cell Biochem Biophys (2015) 72: 333. doi:10.1007/s12013-014-0459-6
Zetter, B. (2008). The scientific contributions of M. Judah Folkman to cancer research. Nature Reviews Cancer, 8(8), 647-654. http://dx.doi.org/10.1038/nrc2458