This is an old revision of the document!
Table of Contents
Genetically Modified Organisms
Introduction
History
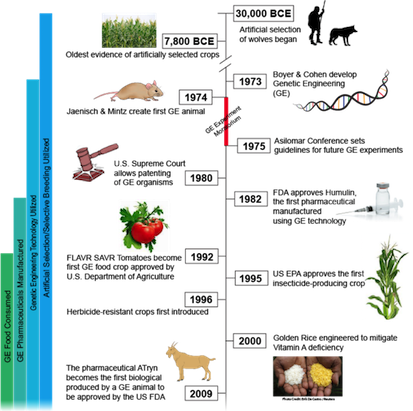
Humans have been domesticating and improving plants and animals since 30,000 BCE (Rangel, G., 2015). Initially, ancestors began genetically modifying dogs through selective breeding of traits (Rangel, G., 2015). This is achieved by only allowing dogs with desirable characteristics to reproduce until such trait is amplified. Modern genetic modifications did not begin until 1973 when two scientists, Herbert Boyer and Stanley Cohen, designed a method to splice a gene from one organism and insert it into the genome of another (Rangel, G., 2015). Bacteria were the initial organisms manipulated to have antibiotic resistance, followed by inserting foreign DNA into mice a year later (Jaenisch, R. & Mintz, B.,1974). The field continued to grow exponentially with many foreseeable possibilities.
As GMOs began to be used in industry by farmers and countries they were thought of as the answer to world hunger. GMOs were used to achieve many different results; a more visually attractive organism, one that is easier to cultivate and breed, pest resistance, drought resistance and an increase in nutritional value were all goals of the developing GMO market. Agencies such as the government, natural resources, environmental groups, and the media worried about the effect of GMOs on the environment and the food chain (Rangel, G., 2015). There have also been changes in public opinion as many of the long-term side effects have yet to be determined both from an environmental and biological health perspective.
Procedure
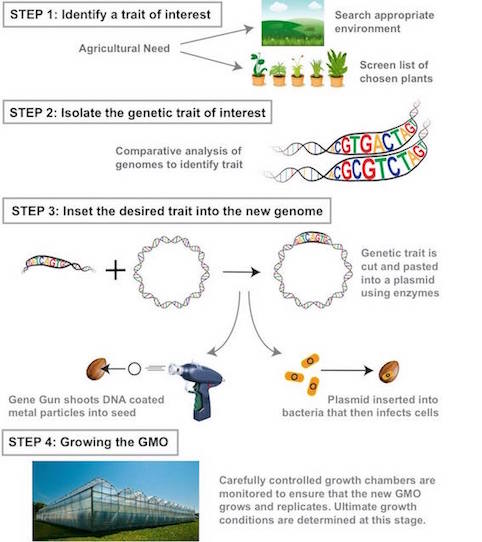
Step 1: Identifying the trait of interest. Scientists identify the trait they wish to include. For example, increased resistance in an environment and look for an organism that is naturally resistant/ has that trait (Powell, C., 2015). Then they must identify that gene sequence responsible.
Step 2: Isolate the genetic trait of interest Comparative analysis is used to decode what part of the genetic makeup contains the gene (Powell, C., 2015). Then genomes of the same species with and without the trait are compared to identify varying regions containing the gene of interest (Boyle, R., 2011). If no database exists scientists will knock out parts of the genome until the gene of interest is lost. Seed chipping is a method where a piece of the seed is shaved off before it is planted so that its DNA can be studied. Then plants are allowed to grow and the one with the desired traits are traced back to initial DNA harvested (Boyle, R., 2011).
Step 3: Insert the desired genetic trait into a new genome Gene guns are the most common mechanisms of gene transfer (Powell, C., 2015). A metal particle coated with DNA is inserted into a plant (Powell, C., 2015). Enzymes insert DNA of interest into a plasmid, then shuck the plasmid to guarantee replication and incorporation of the gene into the organism's genome.
Step 4: Growing the GMO Must monitor the organism to ensure proper growing conditions with the correctly modified genome. A huge effort is made to keep desired plants alive and reproducing once working to monitor optimal growing conditions for seeds (Powell, C., 2015).
Biological Effects: Benefits
In the past few decades, the technologies employed used edible biomass material such as corn to produce ethanol. However, current methods are being utilized to harness energy from non-edible biomass to conserve our edible resources.
Lignocellulosic biomass is the non-edible portion of the plant material. It is the dry plant matter consisting of cellulose, hemicellulose, and encased in lignin. This type of biomass is the most the most abundant raw material on earth for the production of biofuels (Sukumaran, Singhania, Mathew, and Pandey, 2009).
Cellulose is coated with hemicellulose. The most abundant type of hemicellulose is xylan; a polymer of β-1,4-linked xylose which may have branches containing other sugars such as arabinose. The hydrolysis of both cellulose and hemicellulose releases glucose which can be then be fermented into ethanol (Sukumaran, Singhania, Mathew, and Pandey, 2009).
Lignin is a complex polymer consisting of hydroxylated and methoxy-lated phenylpropanoids. These phenylpropanoids cross-link plant secondary cell walls and provide mechanical strength and a physical barrier to invasive pathogens. The percentage of lignin content in cell wall varies between plants and is a crucial parameter affecting the decomposition efficiency of the polysaccharides (Sukumaran, Singhania, Mathew, and Pandey, 2009).
Ethanol Production
The production of ethanol from lignocellulosic biomass begins with several pretreatment steps, hydrolysis of cellulose polymers into glucose, and followed by ethanol recovery. (Van Zessen et al., 2003).
The purpose of the pretreatment is to remove lignin and hemicellulose, reduce cellulose crystallinity, and increase the porosity of the materials. The pretreatment should achieve the following requirements:
- improve the formation of sugars or the ability to subsequently form sugars by enzymatic hydrolysis
- avoid the degradation or loss of carbohydrate
- avoid the formation of byproducts inhibitory to the subsequent hydrolysis and fermentation processes
- cost effective
There are numerous methods to pre-treat the lignocellulosic biomass. Physical treatments such as grinding and drying the lignin can be employed. Drying is the removal of volatile compounds such as water from a material to a gaseous phase by thermal evaporation. Grinding reduced the size of the solid material by impact and compression. Chemical and enzymatic treatment can be also used to degrade and digest the lignin (Kumar, Parveen et al., 2009).
The combination of biological pretreatment by a white rot fungus Echin- odontium taxodii or a brown rot fungus Antrodia sp. 5,898 with mild acid pre- treatment (0.25 % sulfuric acid at varied temperature) were evaluated by Ma et al. (2010), under different pretreatment conditions for enzymatic hydrolysis and ethanol production from water hyacinth (E. crassipes). The reducing sugar yield from enzymatic hydrolysis of co-treated water hyacinth increased 1.13-2.11-fold than that of acid-treated water hyacinth at the same conditions. The following study on separate hydrolysis and fermentation with Saccharomyces cerevisiae indicated that the ethanol yield from co-treated water hyacinth achieved 0.192 g/g of dry matter, which increased 1.34-fold than that from acid-treated water hyacinth (0.146 g/g of dry matter). The discussion and conclusion of this research was that one pretreatment is often not the most efficient pathway as combining different types of pre-treatment and the order in which they occur are directly affect its efficiency.
Genetically modify the lignin component of plant cell wall by induced mutation or genetic manipulation exercising transcriptional control over the biosynthetic pathway genes. Genetic manipulation of the composition and amount of lignin in the cell walls to modify the material properties of biomass making it susceptible to thermochemical deconstruction followed by enzymatic degradation. A novel modification strategy that enables the synthesis of new phenolic molecules that can, when incorporated into the lignin polymer, can greatly improve its degradation. Research shows an increase in the number of total cellulose products when lignin is genetically modified (Fu et al., 2011)
Biological Effects: Risks
Genetic manipulation of plant cell wall polymer lignin is an effective route to ensure increased biomass production that can be translated to higher quality raw materials for industrial production of biofuels (Ciesielski et al., 2014). Lignin is a phenylpropanoid polymer component of the plant cell wall structure which has a natural tendency of biomass recalcitrance to resist microbial, chemical or enzymatic degradation (Himmel, 2007). Genetic engineering techniques are aimed at overcoming this barrier as the hydrophobic network of cross links that lignin forms with the polysaccharides inhibits their enzymatic degradation (Zeng, Zhao, Yang, & Ding, 2014).
There are various genetic engineering routes to overcoming biomass recalcitrance including:
- Obtaining localized lignification to promote accessibility to enzymes (Chen & Dixon, 2007).
- Genetic modification of lignin via mutation or transcriptional manipulation of biosynthetic pathway genes to bypass costly pretreatments and result in facilitated bioprocess consolidation (Chen & Dixon, 2007; Verma & Dwivedi, 2014).
- Increasing the thermochemical deconstruction susceptibility of biomass through lignin modification (Ciesielski et al., 2014).
- Incorporation of phenolic molecules into lignin polymer to improve its degradation (Verma & Dwivedi, 2014).
Environmental Effects: Benefits
Reduced Pesticides and Insecticides usage
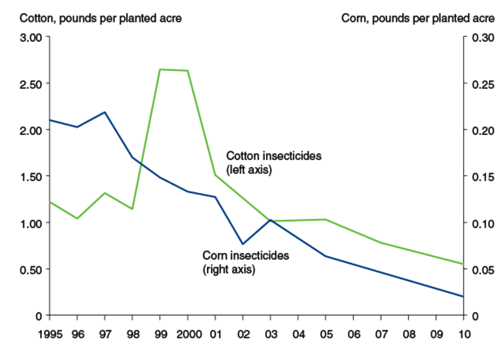
Genetically modified insect-resistant plants have the potential for reducing usage of pesticides (Wolfenbarger et al, 2000). After the adoption of genetically modified crops in the 1990s, there was a reduction in approximate 8.22 million pounds of pesticides used within the first year of usage (United States Department of Agriculture, 2000). Reducing amounts of pesticides in the environment can benefit the environment in multiple ways. There has been increasing amounts of evidence regarding the hazards that pesticides pose to the environment. Specifically, pesticides have the ability to contaminate soil, ground water and other vegetation present (Aktar et al, 2009). A study done by the U.S Geological Survey across multiple river basins in the United States found that greater than 90% of water and fish samples tested from these bodies of water contained one or more pesticides (Kole et al, 2001). Furthermore, this study revealed that levels of pesticides present in most urban streams exceeded the recommended guidelines that were created to protect aquatic wildlife from harm (U.S. Geologic Survey, 1999). The trend noticed within the first few years after the adoption of genetically modified crops has been encouraging as it has shown an overall reduction of pesticides used (Wolfenbarger et al., 2000).
Increased Crop Yield
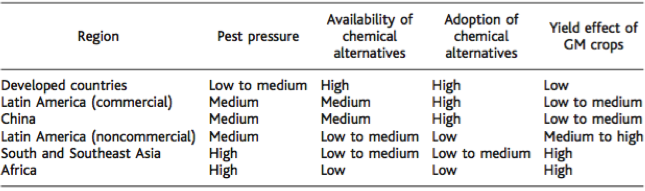
Genetically modified crops have the potential to improve crop yield in developing countries, such as Southeast Asia and Africa, which stand to most to gain since agricultural output is limited (Wolfenbarger et al., 2000). The current majority of genetically modified crops have traits that infer resistance of insects and herbicides (James, 2001). Bt corn, which is a genetically modified crop, which produces the toxin bacillus thuringiensis, has been widely used since its approval in 2002 (Qaim and Zilberman, 2003). Data obtained from 2001 trials of 157 farms using Bt and non-Bt corn in India showed average yields of Bt- hybrids was 80-87% higher. These genetically modified crops in developing areas have a great potential. Farmers in these areas have limited accessibility to insecticides and other chemical alternatives. Additionally, due to tropical and subtropical weather climates, these areas experience the most pest pressure. (Oerke et al., 1994). The hypothesis that these crops can improve crop yield was tested by field-trial results from India, which experience conditions similar to Sub-Saharan Africa and Southeast Asia (Ismael et al., 2002). Overall, an increase in crop yield has the potential for preservation of natural habitats since less land will need to be converted into agricultural use in the future, thus promoting land and habitat conservation (Wolfenbarger et al., 2000).
Promoting Soil Integrity and Conversation
Two main ways genetically modified crops can improve soil integrity and conservation is through phytoremediation and promoting no-till weed management.
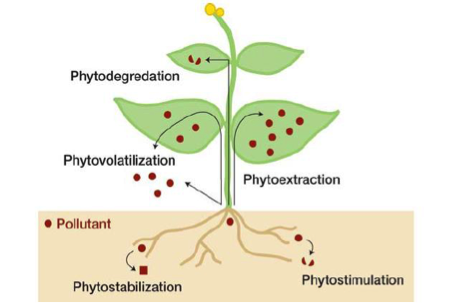
Some genetically modified plants have the ability to provide in situ remediation of polluted soil, sediments and surface waters (Wolfenbarger, 2000). These plants overexpress metabolism enzymes and are able to remove organic pollutants, included herbicides and insecticides commonly found in soils. An enzyme that is regularly selected for is Chytochrome P450, which is typically involved in the metabolism of herbicides in mammalian livers. (Inui & Ohkawa, 2005). Through phytoremediation, absorbed pollutants are able to be converted into non-toxic metabolites and accumulate in the plants or be released back into the soil (Kawahigashi, 2009). This has been proven to both a sustainable and effective technology (Salt et al, 1998). Plants that have this ability must themselves be resistant to the pollutants being removed as well as have a large biomass so they can remediate large amounts of chemicals (Kawahigashi, 2009). Positive implications for the environment include decreased levels of pollutants in soil and less need for harmful and costly physical or chemical remediation processes (Wolfenbarger, 2000).
Increased usage of genetically modified herbicide-tolerant and resistant crops can lead to environmental benefits by facilitating a shift towards conservation tillage practices. These crops allow farmers to apply herbicides to crops after they emerge, since now these genetically modified crops can tolerate them (Wolfenbarger, 2000). Post-emergent weed control encourages the use of soil-friendly weed control mechanisms that promote conservation of the land. Now farmers will not need to rely on tilling, which is a mechanism of mechanical weed control. Due to the rough nature of this process, tilling causes degradation of topsoil and overall soil moisture as well as leads to soil erosion (Snow et al., 2005). By using herbicide-resistant plants, farmers will now benefit from improved soil quality due to the change from tilling to light-chemical and no-till weed control mechanisms (Cannell & Hawes, 1994).
Environmental Effects: Risks
Cellulosic ethanol does not compete with production in the food industry. An alternative fuel, such as corn ethanol, interferes with the food industry greatly. This is because it requires croplands, which may normally be used for food growth. However, humans are unable to digest the polysaccharide cellulose because the necessary enzymes required to break beta acetal links are not present (Oyetunji, 2009). Thus, cellulose production does not interfere with food production.
The entire plant can also be harvested. Because plants are primarily made up of cellulose, a large portion of the plant can actually be harvested to produce ethanol for fuel. Since more of the plant content is being used, it produces much greater yields. Approximately 10 tons of ethanol is produced per acre using the cellulose portion of plants, compared to 4 tons of ethanol produced per acre using grain crops.
Feedstock to make cellulosic ethanol is abundant. Ethanol production using cellulosic biomass is extremely sustainable because it can be produced from a large variety of resources. For example, urban wastes such as wood, and agricultural crops such as switchgrass and corn stovers are large feedstocks of cellulosic ethanol (Nelson, 2007). In the United States alone, approximately 323-million tons of material, which is made up of cellulose, is thrown away as waste. Converting these wastes into ethanol itself will produce 30% of the present fuel usage (Nelson, 2007).
The expense to produce of cellulosic biomass is lower than the expense to produce corn. Expense of course plays a large factor in determining whether something is sustainable. The expense is lower because less inputs, such as fertilizers, herbicides and energy, are required to produce large yields. Switchgrass, a perennial plant, is a major livestock for cellulosic ethanol. It produces double the amount of ethanol/acre compared to corn, which means it requires less land for production (Montenegro, 2006). This translates to reduced habitat destruction and not endangering existing wildlife. Switchgrass also has long widespread roots, which aid in improving the quality of soil, either by reducing soil erosion and/or augmenting nutrient capture (Nelson, 2007). A 5-year study conducted by Schmer and colleagues determined that using switchgrass as the primary crop to produce ethanol produces 500% more energy than what is consumed for production itself (Schmer et al., 2008).
The usage of cellulosic ethanol as fuel accounts for reduced greenhouse gas emissions. It has been found that the burning of ethanol produced from cellulose discharges less sulphur and carbon monoxide into the atmosphere, compared to ethanol produced by corn and other fossil fuels (Huanga et al., 2009).
Enzyme Cost as a Limitation
Cellulase and hemicellulase are enzymes that break down the cellulose content in plants, and play an important role in the production of cellulosic ethanol. However, these enzymes are more expensive than the enzymes used in ethanol production with grain. The greater cost is attributed to needing a larger quantity of cellulase enzymes. A greater amount of cellulase enzymes is required because they are a few orders of magnitude lower in efficiency (Yang & Wyman, 2007). Thus, 40-100x more cellulase enzyme is required relative to the amount of enzyme required to make other forms of ethanol (Yang & Wyman, 2007).
Conclusion
Based on the available research findings, it could be prospected that biofuels will form a large share of the fuel industry in the near future. With scientific and technological innovations, more selective methods of extraction, production and refinement could become a reality which will greatly improve its cost efficiency without compromising quality. In addition, strategies to genetically engineer raw materials could also contribute to a large scale production and use of biofuels. Currently, biofuels are sought of as a viable substitute for non-renewable resources such as coal, petroleum and natural gas, and thus serving as part of the solution for the world’s energy crisis (Bhattarai, Stalick, Mckay, Geme, & Bhattarai 2011).
References
Bayer, E. A., Chanzy, H., Lamed, R., & Shoham, Y. (1998). Cellulose, cellulases and cellulosomes. Current opinion in structural biology, 8(5), 548-557.
Bhat, M. K., & Bhat, S. (1997). Cellulose degrading enzymes and their potential industrial applications. Biotechnology advances, 15(3), 583-620.
Bhattarai, K., Stalick, W. M., Mckay, S., Geme, G., & Bhattarai, N. (2011). Biofuel: An alternative to fossil fuel for alleviating world energy and economic crises. Journal of Environmental Science and Health Part A-Toxic/hazardous Substances & Environmental Engineering, 46(12), 1424-1442. doi:10.1080/10934529.2011.607042
Chen, F., & Dixon, R. A. (2007). Lignin modification improves fermentable sugar yields for biofuel production. Nature Biotechnology, 25(7), 759-761. doi:10.1038/nbt1316
Ciesielski, P. N., Resch, M. G., Hewetson, B., Killgore, J. P., Curtin, A., Anderson, N., Donohoe, B. S. (2014). Engineering plant cell walls: Tuning lignin monomer composition for deconstructable biofuel feedstocks or resilient biomaterials. Green Chemistry, 16(5), 2627-2635. doi:10.1039/c3gc42422g
Fu, C., Mielenz, J. R., Xiao, X., Ge, Y., Hamilton, C. Y., Rodriguez, M., … & Wang, Z. Y. (2011). Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proceedings of the National Academy of Sciences, 108(9), 3803-3808.
Himmel, M. E. (2007). Biomass recalcitrance: Engineering plants and enzymes for biofuels production (vol 315, pg 804, 2007). Science, 316(5827), 982-982.
Himmel, M. E., Ruth, M. F., & Wyman, C. E. (1999). Cellulase for commodity products from cellulosic biomass. Current Opinion in Biotechnology, 10(4), 358-364.
Huanga, H., Ramaswamya, S., Al-Dajania, W., Tschirnera, U., & Cairncrossb, R. A. (2009). Effect of biomass species and plant size on cellulosic ethanol: A comparative process and economic analysis. Biomass Bioenergy, 33, 234-246.
Linder, M., & Teeri, T. T. (1997). The roles and function of cellulose-binding domains. Journal of Biotechnology, 57(1), 15-28.
Ma, F., Yang, X, Yu, H., Zhang, X., & Chen, S. (2011). Effects of biopretreatment of corn stover with white-rot fungus on low-temperature pyrolysis products. Bioresource technology, 102(3), 3498-3503.
Montenegro, M. (2006). The numbers behind ethanol, cellulosic ethanol, and biodiesel in the U.S. Grist.
Morris, D. (2006). Putting the pieces together: Commercializing ethanol from cellulose. US Department of Energy. Retrieved 2014-09-29.
Nelson, R. (2007). Cellulosic ethanol: bioethanol in Kansas. Kansas State University Engineering Extension. Retrieved 2014-09- 29.
Oyetunji, R. (2009). Enzymatic hydrolysis Of cellulose in a NMMO/H2O solution. Electronic Theses, Treatises and Dissertations, Paper 2435.
Rabinovich, M. L., Melnick, M. S., & Bolobova, A. V. (2002). The structure and mechanism of action of cellulolytic enzymes. Biochemistry (Moscow), 67(8), 850-871.
Ragauskas, A. J., Williams, C. K., Davison, B. H., Britovsek, G., Cairney, J., Eckert, C. A., … & Tschaplinski, T. (2006). The path forward for biofuels and biomaterials. science, 311(5760), 484-489.
Schmer, M. R., Vogel, K. P., Mitchell, R. B. & Perrin, R. K. (2008). Net energy of cellulosic ethanol from switchgrass. Proc. Nat. Acad. Sci., 105(2), 464-469. Yang, B., & Wyman, C. E. (2007). Pretreatment: the key to unlocking low‐cost cellulosic ethanol. Biofuels, Bioproducts and Biorefining, 2(1), 26-40.
Sukumaran, R. K., Singhania, R. R., Mathew, G. M., & Pandey, A. (2009). Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renewable Energy, 34(2), 421-424.
Van Zessen, E., Reith, J. H., Den Uil, H., Weismann, M., Bakker, R. R., & Elbersen, H. W. (2003). Ligno Cellulosic-Ethanol. A second opinion. Netherlands Agency for Energy and the Environment Novem, Utrecht (Netherlands).
Verma, S. R., & Dwivedi, U. N. (2014). Lignin genetic engineering for improvement of wood quality: Applications in paper and textile industries, fodder and bioenergy production. South African Journal of Botany, 91(0), 107-125. doi:http://dx.doi.org.libaccess.lib.mcmaster.ca/10.1016/j.sajb.2014.01.002
Walker, L. P., & Wilson, D. B. (1991). Enzymatic hydrolysis of cellulose: an overview. Bioresource Technology, 36(1), 3-14.
Yang, B., & Wyman, C. E. (2007). Pretreatment: the key to unlocking low‐cost cellulosic ethanol. Biofuels, Bioproducts and Biorefining, 2(1), 26-40.
Zeng, Y., Zhao, S., Yang, S., & Ding, S. (2014). Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Current Opinion in Biotechnology, 27, 38-45. doi:10.1016/j.copbio.2013.09.008