Table of Contents
Genetically Modified Organisms
Introduction
A Genetically Modified Organism is any organism created by gene splicing techniques and often involves merging DNA from different species (31). Scientists directly manipulate an organism’s genome to isolate traits or characteristics deemed of value. Currently, the safety of GMOs is under research as they are relatively recent developments (22).
History
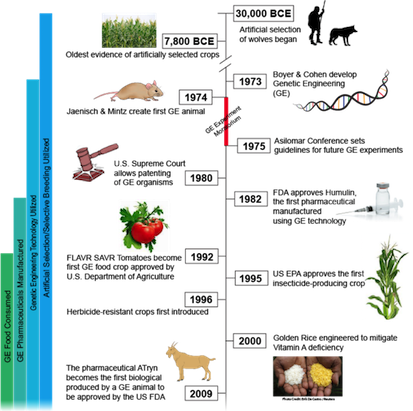
Humans have been domesticating and improving plants and animals since 30,000 BCE (25). Initially, ancestors began genetically modifying dogs through selective breeding of traits (25). This is achieved by only allowing dogs with desirable characteristics to reproduce until such trait is amplified. Modern genetic modifications did not begin until 1973 when two scientists, Herbert Boyer and Stanley Cohen, designed a method to splice a gene from one organism and insert it into the genome of another (25). Bacteria were the initial organisms manipulated to have antibiotic resistance, followed by inserting foreign DNA into mice a year later (11). The field continued to grow exponentially with many foreseeable possibilities.
As GMOs began to be used in industry by farmers and countries they were thought of as the answer to world hunger. GMOs were used to achieve many different results; a more visually attractive organism, one that is easier to cultivate and breed, pest resistance, drought resistance and an increase in nutritional value were all goals of the developing GMO market. Agencies such as the government, natural resources, environmental groups, and the media worried about the effect of GMOs on the environment and the food chain (25). There have also been changes in public opinion as many of the long-term side effects have yet to be determined both from an environmental and biological health perspective.
Procedure
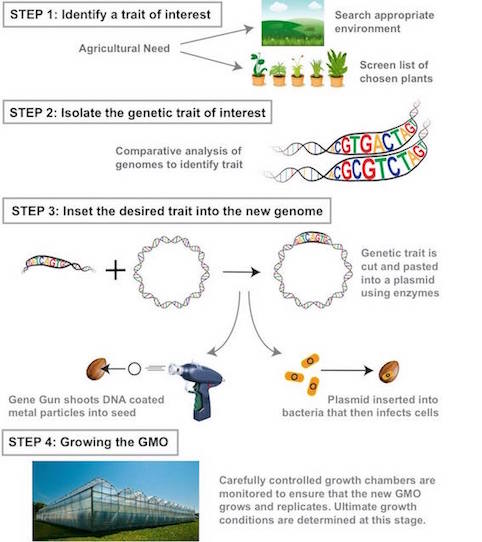
Step 1: Identifying the trait of interest. Scientists identify the trait they wish to include. For example, increased resistance in an environment and look for an organism that is naturally resistant/ has that trait (22). Then they must identify that gene sequence responsible.
Step 2: Isolate the genetic trait of interest Comparative analysis is used to decode what part of the genetic makeup contains the gene (22). Then genomes of the same species with and without the trait are compared to identify varying regions containing the gene of interest (5). If no database exists scientists will knock out parts of the genome until the gene of interest is lost. Seed chipping is a method where a piece of the seed is shaved off before it is planted so that its DNA can be studied. Then plants are allowed to grow and the one with the desired traits are traced back to initial DNA harvested (5).
Step 3: Insert the desired genetic trait into a new genome Gene guns are the most common mechanisms of gene transfer (22). A metal particle coated with DNA is inserted into a plant (22). Enzymes insert DNA of interest into a plasmid, then shuck the plasmid to guarantee replication and incorporation of the gene into the organism's genome.
Step 4: Growing the GMO Must monitor the organism to ensure proper growing conditions with the correctly modified genome. A huge effort is made to keep desired plants alive and reproducing once working to monitor optimal growing conditions for seeds (22).
Biological Effects: Benefits
Consumerism
Efficiency is determined by the optimal yield of a product, considering the costs associated with producing this yield. Genetic modification of organisms increases the efficiency of products, rendering beneficial factors for both producer and consumer. The use of bovine somatotropin (bST)/ recombinant bovine growth hormone (rbGH) in lactating dairy cows demonstrates such efficiency.
The anterior pituitary gland naturally secretes somatotropin, a peptide hormone which stimulates growth, cell regeneration, and reproduction in human and animals (3). Bovine somatotropin (bST), otherwise referred to as recombinant bovine growth hormone (rbGH), is produced through recombinant DNA techniques (3). The direct effects of bSt/ rbGH result in alterations to numerous tissues, metabolic processes involving all nutrient classes, and cellular mechanisms such as intracellular signal transduction systems and response to homeostatic signals. Indirect effects are suspected to be mediated by the insulin-like growth factor (IGF) and have been found to impact mammary glands. The environment and management factors, such as nutritional status, play regulatory roles in such effects and responses (3).
Bovine somatotropin (bST)/ recombinant bovine growth hormone (rbGH) is utilized within cows to increase milk production (WHO). Milk response in these cows is greatest during the declining phase of lactation and milk quality is reportedly unaltered (3).
Pharming
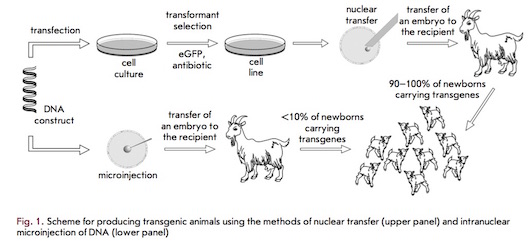
Advances in molecular and reproductive technology have driven the practice of commercial animal pharming over the past twenty years (14). Pharming is a branch of biotechnology which bridges together the contrasting fields of pharmaceuticals and farming. Transgenic plants or animals are utilized for producing pharmaceuticals synthesized for human or animal consumption (7). Animal pharming is promoted as a cost-effective method of biopharmaceutical production (14) and has the potential to become a highly lucrative industry (7).
The need for pharmaceutical intervention is evident amongst patients possessing hereditary antithrombin deficiencies. In 2006, an anticoagulant called Antithrombin became the first recombinant protein to be approved for commercialization by the United States’ Food and Drug Administration (FDA) (18). Antithrombin is extracted and purified from the milk of transgenic dairy goats (16). Transgenic animals, such as these goats, are produced via one of two contemporary methods: intra-pronuclear zygotic DNA microinjection (MI) or somatic cell nuclear transfer (NT). As linear DNA enters the nucleus, it is capable of integrating into the genome of cell lines or living organisms (18).
The most promising site for production of recombinant proteins is the mammary gland due to the large quantities of protein that can be produced (16). Since milk is often produced in abundance, deriving and producing pharmaceuticals in this manner is determined to be cost efficient (14).
Gene Therapy
Gene therapy involves transplanting healthy genes into cells of defective areas in an attempt to correct and/ or reverse adverse effects. Gene therapy holds the greatest promise for the localized, sustained, and regulatable delivery of proteins to the Central Nervous System (21). Ex vivo gene therapy is specific to the delivery of secreted proteins, whereas in vivo gene therapy can potentially deliver intracellular proteins in addition to the secreted proteins (21).
In ex vivo gene therapy, host cells are genetically modified in vitro (or ex vivo) to produce a desired material. These host cells are then harvested and put back into the host and the newly altered cells act as a pump for local delivery of the materials (21).
In vitro gene therapy requires a simpler approach and does not require as many steps as ex vivo therapy. Rather than removing, and re-introducing, viral vectors carrying specific genetic material are injected directly into host cells (21).
Prior to human trials, Tuszynski (2002) demonstrated NGF (nerve growth factor) gene therapy in primates and the implications which provided valuable insight in combatting human CNS diseases such as Parkinson’s Disease (PD) and Alzheimer’s Disease (AD).
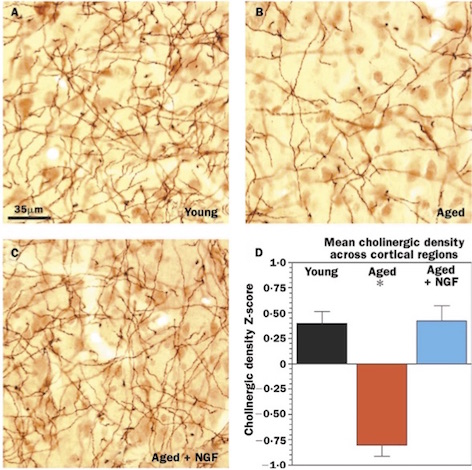
Tuszynski and his colleagues studied lesions to the fornix of rhesus monkeys, and also the effects of aging on the monkeys’ cholinergic innervation (32). In the lesion model, a unilateral lesion to the fornix was immediately grafted with either A) primary fibroblasts genetically modified to produce/ secrete human NGF, or B) unmodified primary fibroblasts (32). A month after the procedure, the team found that the monkeys that received the NGF-secreting grafts had significantly less cholinergic neuronal degeneration than the monkeys that received the unmodified grafts. In the unmodified group, only approximately 25% of neurons were detectable after the lesion; in contrast, up to 90% of the neurons were protected upon examining the NGF group (32).
In his aging model, Tuszynski (2002) investigated whether the atrophy of cholinergic neurons in the basal forebrain of monkeys could be reversed by NGF gene delivery. NGF-secreting cells were grafted into the cholinergic basal forebrain of aged monkeys. After 3 months, the research team found substantial reversal of age-related neuronal atrophy in aged monkeys that received the NGF-secreting grafts (32).
Biological Effects: Risks
Multiple Toxins From GMOs Detected In Maternal and Fetal Blood
A first of its kind, research from Canada was successfully able to identify the presence of pesticides that were associated with genetically modified foods in maternal, fetal and non-pregnant women’s blood (2). Moreover, the presence of Monsanto’s Bt toxin was detected as well. The study was published in the Journal Reproductive Toxicology in 2011.
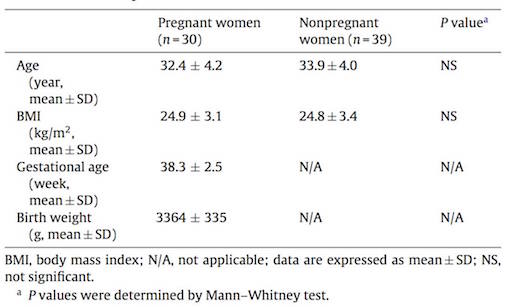
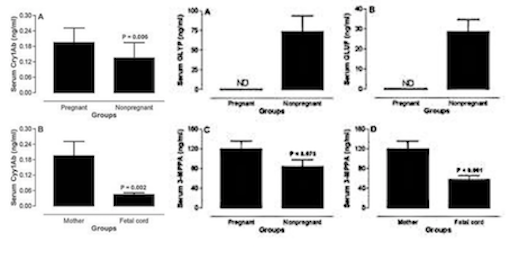
Subjects consisted of pregnant and non-pregnant women living in Sherbrooke, an urban area of Eastern Townships of Quebec, Canada (2). None of the subjects had worked or lived with a spouse that were working in contact with pesticides. In order to determine the presence of herbicide and metabolites, levels of glyphosate (GLYP) and its metabolite aminomethyl phosphoric acid (AMPA), gluphosinate ammonium (GLUF) and its metabolite 3-methylphosphinicopropionic acid (3-MMPA) were measured using gas chromatography-mass spectrometry (2).
Results showed that GLYP was not detected in maternal and fetal blood, but present in the blood of some non-pregnant women, whereas its metabolite AMPA was not detected in any of the samples (2). However, GLUF was also only detected in non-pregnant women, its metabolite 3-MPPA was detected in 100% of maternal and umbilical cord blood samples, and in 67% of the non-pregnant women's blood samples. This implies that the metabolite is more detectable than its precursor GLUF and therefore seems to easily cross the placenta to reach the fetus (2). GLUF in general has demonstrated in mouse embryos to cause growth retardation, increased death or hypoplasia.
Furthermore, Cry1Ab toxin was detected in 93% and 80% of maternal and fetal blood samples, respectively and in 69% of tested blood samples from non-pregnant women (2).
Given the potential toxicity of these environmental pollutants and the fragility of the fetus, more studies are needed, particularly those using the placental transfer approach. Researchers state that these results will provide a baseline data for future studies in the area related to nutrition, toxicology and reproduction in women.
New Study Links GMOs To Gluten Disorders That Affect 18 Million Americans
This study was recently released by the Institute for Responsible Technology (IRT), and uses data from the US department of Agriculture, US Environmental Protection Agency, medical journal reviews as well as other independent research.
“Gluten sensitivity can range in severity from mild discomfort, such as gas and bloating, to celiac disease, a serious autoimmune condition that can, if undiagnosed, result in a 4-fold increase in death,” said Jeffrey M. Smith, executive director of IRT.
The Institute for Responsible technology is a world leader in educating policy makers and the public about GMO foods and crops. The institute reports and investigates the impact GM foods can have on health, environment, agriculture and more (26). In soy, corn, canola oil, zucchini, yellow squash, Hawaiian papaya, and alfalfa, “Bt-toxin, glyphosate, and other components of GMOs, are linked to five conditions that may either initiate or exacerbate gluten-related disorders,” according to Smith.
The genetically modified (GM)-related damage was linked to five different areas of symptoms:
- Intestinal Permeability
- Imbalanced Gut Bacteria
- Immune Activation and Allergic Response
- Impaired Digestion
- Damage to the Intestinal Wall
IRT has also released a statement that glyphosate, a pesticide was found to have a negative effect on intestinal bacteria. Unfortunately, GMO crops contain high concentrations of this toxin during harvest and consumption by humans (26).
With these symptoms, Dr. Tom O'Bryan, with his expertise on gluten sensitivity and Celiac Disease stated that “the introduction of GMOs is highly suspect as a candidate to explain the rapid rise in gluten-related disorders over the last 17 years” (26).
DNA From Genetically Modified Crops Can Be Transferred Into Humans Who Eat Them
In a study published and peer reviewed Public Library of Science (PLOS) in 2013, researchers emphasize that there is sufficient evidence that meal-derived DNA fragments carry complete genes that can enter into the human circulation system through an unknown mechanism (30).
In one of the blood samples taken from the subjects, relative concentrations of plant DNA was significantly higher than human DNA. Sample size was based on the analysis of over 1000 human samples from four different independent studies (30).
“Our bloodstream is considered to be an environment well separated from the outside world and the digestive tract. According to the standard paradigm large macromolecules consumed with food cannot pass directly to the circulatory system“ (30).
As the process of digestion occurs, proteins and DNA are recognized to be degraded into smaller counterparts, which are amino acids and nucleic acids respectively. This is then absorbed through an active process and subsequently distributed to various regions of the body using the circulatory system. Results from the study have shown that the plant DNA concentration displayed a precise log-normal distribution in the plasma samples while non-plasma control sample was found to be free of it. According to the demonstration of the study's results, this implies that these meal-derived DNA fragments are large enough to carry complete genes that can avoid degradation through an unknown mechanism to enter the human circulatory system.
Although this study was not conclusive as pure evidence that GMOs are able to enter into our cells, it is an assumption that can be made into a possibility. Results from this study demonstrate another cause for concern and reason to research further into the safety of GMO consumption.
Environmental Effects: Benefits
Reduced Pesticides and Insecticides usage
Genetically modified insect-resistant plants have the potential for reducing usage of pesticides (37). After the adoption of genetically modified crops in the 1990s, there was a reduction in approximate 8.22 million pounds of pesticides used within the first year of usage (33). Reducing amounts of pesticides in the environment can benefit the environment in multiple ways. There has been increasing amounts of evidence regarding the hazards that pesticides pose to the environment. Specifically, pesticides have the ability to contaminate soil, ground water and other vegetation present (1). A study done by the U.S Geological Survey across multiple river basins in the United States found that greater than 90% of water and fish samples tested from these bodies of water contained one or more pesticides (15). Furthermore, this study revealed that levels of pesticides present in most urban streams exceeded the recommended guidelines that were created to protect aquatic wildlife from harm (35). The trend noticed within the first few years after the adoption of genetically modified crops has been encouraging as it has shown an overall reduction of pesticides used (37).
Increased Crop Yield
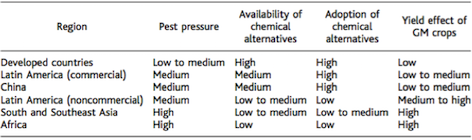
Genetically modified crops have the potential to improve crop yield in developing countries, such as Southeast Asia and Africa, which stand to most to gain since agricultural output is limited (37). The current majority of genetically modified crops have traits that infer resistance of insects and herbicides (12). Bt corn, which is a genetically modified crop, which produces the toxin bacillus thuringiensis, has been widely used since its approval in 2002 (24). Data obtained from 2001 trials of 157 farms using Bt and non-Bt corn in India showed average yields of Bt- hybrids was 80-87% higher. These genetically modified crops in developing areas have a great potential. Farmers in these areas have limited accessibility to insecticides and other chemical alternatives. Additionally, due to tropical and subtropical weather climates, these areas experience the most pest pressure (20). The hypothesis that these crops can improve crop yield was tested by field-trial results from India, which experience conditions similar to Sub-Saharan Africa and Southeast Asia (10). Overall, an increase in crop yield has the potential for preservation of natural habitats since less land will need to be converted into agricultural use in the future, thus promoting land and habitat conservation (37).
Promoting Soil Integrity and Conversation
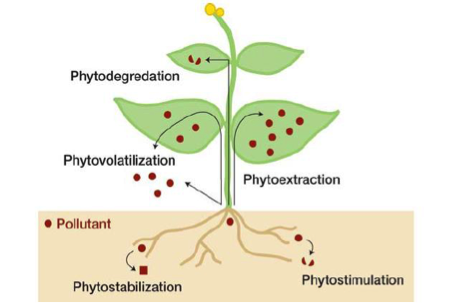
Two main ways genetically modified crops can improve soil integrity and conservation is through phytoremediation and promoting no-till weed management.
Some genetically modified plants have the ability to provide in situ remediation of polluted soil, sediments and surface waters (37). These plants overexpress metabolism enzymes and are able to remove organic pollutants, included herbicides and insecticides commonly found in soils. An enzyme that is regularly selected for is Chytochrome P450, which is typically involved in the metabolism of herbicides in mammalian livers (9). Through phytoremediation, absorbed pollutants are able to be converted into non-toxic metabolites and accumulate in the plants or be released back into the soil. This has been proven to both a sustainable and effective technology (27). Plants that have this ability must themselves be resistant to the pollutants being removed as well as have a large biomass so they can remediate large amounts of chemicals. Positive implications for the environment include decreased levels of pollutants in soil and less need for harmful and costly physical or chemical remediation processes (37).
Increased usage of genetically modified herbicide-tolerant and resistant crops can lead to environmental benefits by facilitating a shift towards conservation tillage practices. These crops allow farmers to apply herbicides to crops after they emerge, since now these genetically modified crops can tolerate them (37). Post-emergent weed control encourages the use of soil-friendly weed control mechanisms that promote conservation of the land. Now farmers will not need to rely on tilling, which is a mechanism of mechanical weed control. Due to the rough nature of this process, tilling causes degradation of topsoil and overall soil moisture as well as leads to soil erosion (28). By using herbicide-resistant plants, farmers will now benefit from improved soil quality due to the change from tilling to light-chemical and no-till weed control mechanisms (6).
Environmental Effects: Risks
Invasion into ecosystems
There are many concerns about the release of genetically modified organisms into the wild. The major fear is that they would become invasive species. An invasive species is a non-native species that was introduced into an ecosystem, and causes (directly or indirectly) the degradation of the natural function and structure of the ecosystem. The modifications to these organisms, whether they are crops or animals, often give them an advantage in their environment; allowing them to succeed when the native species may not (37).
For example, GM Altantic salmon are made to be bigger at a younger age, which could give them a significant advantage if they escaped to the wild. Since they are larger, the transgenic fish feed longer than the smaller wild salmon, and ultimately can outcompete for the food supply (4).
Gene Transfer
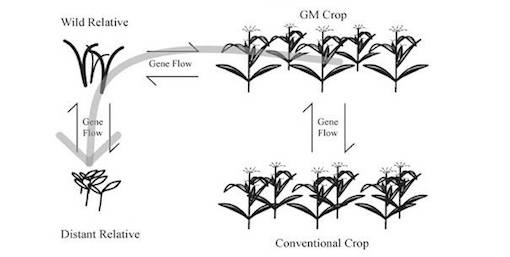
Gene flow is a great environmental risk when it comes to GMOs. This is where transgenes in the modified organism could transfer to wild species, soil bacteria (horizontal gene flow) or even humans. Gene flow occurs naturally within different species of plants, but there is a threat of transfer of undesirable genes into the pool, and populations could permanently change (23).
It was shown in a Canadian study that GM Atlantic salmon were able to breed with natural-born salmon, and with closely related brown trout. They observed that the offspring were viable, which means they transferred their manipulated genes into the wild populations (29). We can assume that gene flow will occur between GMOs and native species, but what is more concerning is the extent at which this will affect the native ecosystem (23). Johnson (2000) said, ‘To add genes from other plants unwittingly and randomly to native gene pools may result in phenotypic effects which could change the way entire genomes relate to their physical and biotic environments’. The release or escape of genetically modified plants and animals should be avoided since it is difficult to predict the effect they may have on native species and ecosystems (4).
Impacting non-target insects
There are several different ways in which GM crops can impact non-target insects. A prevalent example in the public has been the monarch butterfly and is considered a species of conservational value. In a lab study, Monarch larvae on milkweed leaves were dusted with Bt maize pollen and left to grow. It was found that the larvae with BT pollen ate less, grew slower and experienced higher mortality compared to the larvae cultivated with non-GM pollen. It is still difficult to measure the effect of Bt pollen on monarchs in the wild due to many natural factors, including; the required dosage of pollen, the likelihood of exposure to pollen, and the effect of degradation of Bt from rain (37).
Conclusion
Public Opinion
According to Langer, G., 2016, over 90% of Americans, want to know if their food is a GMO . Over half of this population would avoid the food because they are either unsure about it or believe it to be unsafe (17). Many cultures benefit from GMO because for example they now have the ability to farm in environments that were previously too volatile. Parents, consisting largely of mothers, are against GMOs due to worry of genetic transfer and fetal harm (17). Scientists largely remain supportive and say that the public do not have all of the information. Scientists say that many have minimal understanding about what these products actually are, and their effects on the body or environment (11). There are also organisations like Chipotle, a popular American burrito restaurant, that influences public opinion. In 2013 Chipotle chose to disclose which menu items were genetically modified, and later took them off their menu entirely (11). This fuelled the public’s wariness of GMOs. Part of the problem with the lack of GMO awareness is due to Monsanto, a leader in GMO development and biotechnology, who failed to educate the public on the costs and benefits of GMOs from the early stages of production (11). This resulted in a lot of fear and speculation and now Monsanto is stuck trying to quell this fear instead of educating consumers. Recently they have started attending public events to speak about GMO safety and help improve the public’s opinion. They had over 40 planned events in 2015 alone (11).
Future Directions
Scientists can continue to develop ways to make GMOs safer for the ecosystem and people. The ultimate goal is to make the technology more accepted by the public, something that could be obtained from education initiatives. A large setback to GM technology is its environmental effects; therefore addressing this problem will solve many issues. There should be efforts made for example, to refine what a plant resistant to pests secretes so that it targets only the pest and not other organisms (see details on monarch butterfly). Companies can also aim to increase types of pharmaceuticals they are pharming in genetically modified organisms to decrease the need to produce it by other means. Overall the process can be streamlined to allow for easier and more effective relocation of genes. Finally, if these things can be implemented successfully this will allow more food to reach different areas. Perhaps countries that must import a variety of food items could begin to grow them on their own instead. This would allow them to be more self-sufficient and cut down on the environmental strain of transporting food from other countries.
Controversies
Controversy is mainly surrounding health and environmental risk factors of GMOs. This includes factors that are known and unknown due to lack of research. Rumours and stigmas about GMOs that are portrayed by the media are often results from scientific papers that have been exaggerated. The USA does not currently have labelling laws for GM food (25). It remains to be decided if labelling GM products is good or bad. On one hand, labelling something as GMO makes the consumer more aware but means that less will buy the product (as mentioned in Public Opinions). This results in increased food market prices and resource strain as companies attempt to satisfy the demand for non-GMOs. By 2050 the UN predicts that humans will need to produce 70% more food than we currently do now in 2016 (19). This increase in food production alone will strain resources, and GMOs have the potential to help by providing food with more nutrients, the ability to grow in harsh climates and many other altercations that could be a solution to our growing population’s food demands. The question remains, is a world without GMOs sustainable?
References
1. Aktar, M. W., Sengupta, D., & Chowdhury, A. (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary Toxicology, 2(1), 1–12. http://doi.org/10.2478/v10102-009-0001-7
2. Aris A., Leblanc S. (2011, May). Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Reproductive Toxicology 31(4): 528-33. Doi:10.1016/j.reprotox
3. Bauman, D. (1999, February 5). Bovine somatotropin and lactation: From basic science to commercial application. Domestic Animal Endocrinology, 17(2-3), 101-116. doi:10.1016/s0739-7240(99)00028-4
4. Benfey, T. (2014). Opinion: Sizing up GM Salmon; On the Potential Benefits and Risks of Genetically Modified Fish Entering the Marketplace. The Scientist. Retrieved 23 September from http://www.the-scientist.com/?articles.view/articleNo/40086/title/Opinion--Sizing-Up-GM-Salmon/
5. Boyle, R. (2011). How to Genetically Modify a Seed, Step by Step. Popular Science. Retrieved from http://www.popsci.com/science/article/2011-01/life-cycle-genetically-modified-seed
6. Cannell, R.Q & Hawes, J.D. (1994). Soil Till Res. 30. 245.
7. Engelhard, M., Hagen, K., & Thiele, F. (2007, November). Pharming A New Branch of Biotechnology. Europäische Akademie, 43, 7-12.
8. Institute for Green Energy and Clean Environment. (2009). Phytoremediation and Phytosensing Technologies. Retrieved from: http://systemsbiology.usm.edu/BrachyWRKY/WRKY/Phytoremediation.html
9. Inui, H. & Ohkawa, H. (2005). Herbicide resistance in transgenic plants with mammalian P450 monooxygenases genes. Pest Management Science. 61:286-291.
10. Ismael, Y., Bennett, R., Morse, S. (2002). Agricultural Biology Forum. 5 (4).
11. Jaenisch, R. Mintz, B. (1974). Simian Virus 40 DNA Sequences in DNA of Healthy Adult Mice Derived from Preimplantation Blastocysts Injected with Viral DNA. NCBI, 71(4). Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC388203
12. James, C. (2002). Global review of commercialized transgenic crops: (Feature: Bt Cotton), International Service for the Acquisition of Agri-biotech Applications. 26. 12.
13. Johnson, B. (2003). Problems of plant conservation in agricultural landscapes: can biotechnology help or hinder? Methods for Risk Assessment of Transgenic Plants. (109-120). Peterborough, UK: English Nature
14. Kind, A., & Schnieke, A. (2008, July 29). Animal pharming, two decades on. Transgenic Res Transgenic Research, 17(6), 1025-1033. doi:10.1007/s11248-008-9206-3
15. Kole, R.K., Banerjee, H., Bhattacharyya, A. (2001). Monitoring of market fish samples for endosulfan and hexachlorocyclohexane residues in and around Calcutta. Bull Environ Contam Toxicol. 67(4). 554-9.
16. Kues, W. (2004). The contribution of farm animals to human health. Trends in Biotechnology, 22(6), 286-294. doi:10.1016/j.tibtech.2004.04.003
17. Langer, G. (2016). Skepticism of Genetically Modified Foods. ABC News. Retrieved from http://abcnews.go.com/Technology/story?id=97567&page=1
18. Maksimenko, O. G., Deykin, A. V., Khodarovich, Y. M., & Georgiev, P. G. (2013). Use of Transgenic Animals in Biotechnology: Prospect and Problems. Acta Naturae, 5(16), 1st ser., 33-46.
19. Northoff, E. (2016). 2050 A third more mouths to feed. Food and Agriculture Organization of the United Nations. Retrieved from http://www.fao.org/news/story/en/item/35571/icode/
20. Oerke, E.C., Dehen, H.W., Schonbeck F. & Weber, A. (1994). Crop Production and Crop Protection: Estimated Losses in Major Food and Cash Crops. Elsevier.
21. P. A., & J. R. (2001, September). Recombinant proteins for neurodegenerative diseases: The delivery issue. Trends in Neurosciences, 24(9).
22. Powell, C. (2015). How to Make a GMO. Science in the News, Harvard University. Retrieved from http://sitn.hms.harvard.edu/flash/2015/how-to-make-a-gmo/
23. Pretty, J. (2001). The rapid emergence of genetic modification in world agriculture: Contested risks and benefits. Environmental Conservation, 28(3), 248-262. doi:10.1017/S0376892901000261
24. Qaim, M., & Zilberman, D. (2003). Yield effects of genetically modified crops in developing countries. Science, 299(5608), 900-902.
25. Rangel, G. (2015). Form Corgis to Corn. Science in the News, Harvard University. Retrieved from http://sitn.hms.harvard.edu/flash/2015/from-corgis-to-corn-a-brief-look-at-the-long-history-of-gmo-technology/
26. RT Autonomous Nonprofit Organization “TV-Novosti” (2013, November 26) GMOs linked to gluten disorders plaguing 18 million Americans . https://www.rt.com/usa/gmo-gluten-sensitivity-trigger-343
27. Salt, D.E., Smith, R.D., Raskin, I. (1998). Phytoremediation. Annual Rev Plant Physiology Plant Mol Biol. 49: 643-668.
28. Snow, A. A., Andow, D. A., Gepts, P., Hallerman, E. M., Power, A., Tiedje, J. M., & Wolfenbarger, L. L. (2005). Genetically engineered organisms and the environment: current status and recommendations. Ecological Applications,15(2), 377-404.
29. Späth, A. (2013). Unleashing the Frankenfish. News24. Retrieved 23 September from http://www.news24.com/Columnists/AndreasSpath/Unleashing-the-Frankenfish-20130603
30. Spisak S., Solymosi N., Ittzes P., Bodor A., Kondor D., Vattay G., et al. (2013, July 30). Complete Genes May Pass from Food to Human Blood. PLoS ONE 8(7): e69805. Doi: 10.1371/journal.pone.0069805
31. Suzuki, D. (2014). Understanding GMO. The David Suzuki Foundation. Retrieved from http://www.davidsuzuki.org/what-you-can-do/queen-of-green/faqs/food/understanding-gmo/
32. Tuszynski, M. H. (2002, May). Growth-factor gene therapy for neurodegenerative disorders. The Lancet Neurology, 1(1), 51-57. doi:10.1016/s1474-4422(02)00006-6
33. United States Department of Agriculture (2000). Genetically engineered crops: has the adoption reduced pesticide use? Retrieved from: www.ers.usda.gov/epubs/pdf/agout/aug2000/ao273f.pdf
34. United States Department of Agriculture (2014). Adoption of Genetically Engineered Crops by US Farmers has Increased Steadily for Over 15 years. Retrieved from: http://www.ers.usda.gov/amber-waves/2014-march/adoption-of-genetically-engineered-crops-by-us-farmers-has-increased-steadily-for-over-15-years.aspx#.V-wfE5MrKt9.
35. U.S. Geological Survey. (1999). The quality of our nation's waters – nutrients and pesticides. Retreieved from:http://water.usgs.gov/pubs/circ/circ1225/
36. World Health Organization (WHO). (2013, November). Evaluation of certain veterinary drug residues in food. Joint FAO/WHO Expert Committee on Food Additives, (78), 988th ser. doi:10.1002/food.19880320917
37. Wolfenbarger, L. L., & Phifer, P. R. (2000). The ecological risks and benefits of genetically engineered plants. Science, 290(5499), 2088-2093.