This is an old revision of the document!
Table of Contents
Breast Cancer Powerpoint
Breast Cancer
INTRODUCTION
The breast is an organ that is primarily composed of adipose tissue that is found on the chest region, situated right in front of the pectoralis major muscle. The organ is found in both males and females, but have no functional purpose in males. The breast in females has a specialized function which is the production of milk for lactation. The breast anatomy is composed of 2 components the epithelial component, the lobules where milk is made, and the duct component, which connects the lobules to the external area of the nipple. In male breast anatomy, the ductal component is shunted creating blind ducts, which differentiates them from female breasts.
Breast cancer is the result of the abnormal division of breast cells within the organ. Breast cancer can be an inherited or sporadic cancer, but the most common type of genes involved are the BRCA1 and BRCA2 genes. The cells within the breast can create a tumor at the primary site in the breast. Breast cancer normally develops from the ductal tissues which is known as ductal carcinomas or lobular tissues which is known as lobular carcinomas. Breast cancer has a high potency of metastasizing to other nearby tissues.
Within Canada, 24,400 women and 210 men are diagnosed per year, with over 5,000 women and 60 men dying of disease. Globally, breast cancer in women accounts to 25% of all cancer cases, making it one of the largest sector of cancer in women (Boyle & Levin, 2008). Moreover, breast cancer is more common in developed countries compared to developing countries. Most cases of breast cancer are not reported because males or females refuse to report it to doctors, feminizing syndrome, or it could be simply caught too late (da Silva, 2015).
SIGNS & SYMPTOMS
The five most common signs of breast cancer are as follows;
1. A new lump
- A new lump or thickening in the breast or armpit area
2. Nipple Change
- A newly inverted [pulled in] or retracted nipple
3. Skin Change
- A change in the skin of the breast, areola or nipple e.g. color, dimpling, puckering, or reddening
4. Shape Change
- A change in the breast shape or size
5. Nipple Discharge
- A discharge from the nipple that occurs without squeezing
EPIDEMIOLOGY
Breast cancer is the most common malignant cancer in females, comprising of 18% of all female cancers. An estimated 1.4 million cases of breast cancer related incidents are recorded per year globally. Out of these 1.4 million cases, 460, 000 are confirmed deaths (Tao, 2015). Projecting these incident rates to 2050, it is estimated that the cases will increase to 3.2 million cases recorded per year, more than double of the current incident rates (Tao, 2015). Incident rates are determined to continue growing throughout the oncoming years (McPherson, 2000).
However, in parallel to the rising incidence rates, mortality rates have declined since the 1990’s (Li, 2010). This is merely due to the cancer detecting technology that has been introduced, such as screening mammography. Screen tests has allowed doctor’s to detect cancer at early stages, increasing incident rates, and treat them before the aggressive stages, declining mortality rates (Li, 2010).
Growing research in the cancer field has brought among greater technology and therapies to target and destroy the cancer, but unfortunately this is also leading to a discrepancy in some geographical distributed regions in reference to global incidence and mortality numbers (DeSantis, 2015). Particularly, low income countries have been continuing to show an increase in incidence and mortality rates. These countries do not have the means to employ screening technologies and therapies due to the high costs. Once primarily known as a disease of western women, now more than half of breast cancer incident cases and mortality cases are recorded in economically developing countries. (DeSantis, 2015)
In terms of age, studies in the United Kingdom have shown highest incident rates are recorded among females within the age of 50-64 years (McPherson 2000). This may be due to standardized annual screening tests implemented by the government which are required to be completed. Additionally, breast cancer does cause the greatest deaths in the ages of 40-50 years, this may be due to gene reactivity or changes in female systems occurring during these middle ages (McPherson, 2000)
Some related factors to breast cancer incidences are genetic predisposition of the cancer gene, mutations in the DNA leading to the cancer cell production and proliferation from radiation, geographical variation which ties into the differences in genetic makeup of females living in particular regions or are exposed to carcinogens in their environments, lifestyle, and female bodily cycles such as menopause which causes significant changes in the body (McPherson, 2000). Some preventative measures may be taken such as dietary intervention. These changes may be difficult for some to adapt due to cultural bringing up of an individual. One particular key point in terms of dietary factors includes a study which proposes if a female gains 10-20 pounds more from her weight at the age of 18, this may be associated with an increase of risk to breast cancer (McPherson, 2000). Other preventative measures include hormonal control which tackles the naturally occurring cycles in the female body from getting out of control (McPherson, 2000).
RISK FACTORS
DIAGNOSIS
Prior to being diagnosed with breast cancer there are ways to test for an individual’s genetic risk factors for developing the disease. Majority of inherited cases of breast cancer is a result of mutations within the BRCA1 or BRCA2 genes. BRCA1 is found on chromosome 17, whereas BRCA2 is found on chromosome 13 (Radford & Zehnbauer, 1996). This is an important gene to consider as an individual only needs one mutated copy in order to possess increased chances of developing breast cancer. If one parent has this mutation, their offspring will automatically have a 50% chance of inheriting it and developing specific cancers (Radford & Zehnbauer, 1996). Un-mutated BRCA genes produce tumor suppressor proteins that aid in the repair of damaged DNA (Radford & Zehnbauer, 1996). As a result, when mutations occur within either BRCA gene, the damaged DNA is left un-repaired, resulting in higher chances of future cancer development.
Though majority of breast cancer cases are sporadic, approximately ten percent of cases result from an inherited autosomal dominant mutation within the BRCA genes (Radford & Zehnbauer, 1996). However, once a mutation is inherited, there is a 65-85% chance that an individual will develop breast cancer by the time they are 70 years old (Radford & Zehnbauer, 1996). Genetic screening options are available with the purpose of identifying individuals at risk of developing certain cancers within their lifetime. There are two main methods used when screening for inherited breast cancer-causing mutations. The first form of genetic screening searches for every known mutation that is linked to cancer. This is done by collecting a sample of DNA, typically a buccal sample, and examining it for cellular mutations. The second method collects a DNA sample in a similar way, however it also requires the collection of DNA from at least four family members who are known to have a BRCA mutation (Radford & Zehnbauer, 1996). By doing this, the test screens for those particular mutations that are currently present within the family. Once the tests have been completed an individual is then identified as being positive or negative. A positive test implies that they possess a known BRCA mutation (Radford & Zehnbauer, 1996). However, it is important to understand that carrying a mutation does not necessarily mean an individual will develop the disease, it just informs them that they have an increased chance of future development.
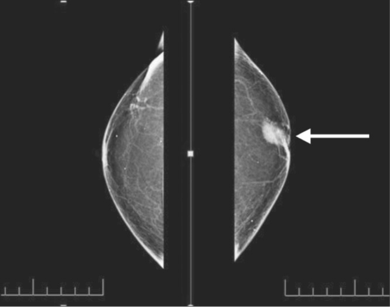
There are also a variety of indirect examinations to test for the presence of breast cancer without collecting DNA samples. One such test is a mammography. This type of screening makes use of breast imaging via low-dose X-rays (Canadian Cancer Society, 2017). Using this technique it is possible to detect the presence of breast cancer at its earliest stages, when it is most treatable and mortality rates are low. During this procedure, each breast is individually compressed by parallel plates. In doing so, the breast thickness is reduced, making it easier to recognize abnormal masses. X-rays are then used to create images for examination (Canadian Cancer Society, 2017). Additionally, in the province of Ontario it is recommended that females between the ages of 50-74 get mammograms done every two years, in order to detect early development of breast cancer.
A second procedure is a breast biopsy which is used to remove patches of tissue or fluid from abnormal lumps within the breast. There are three types of breast biopsies when trying to discover the presence or absence of breast cancer. The first is fine needle aspiration. This is the least commonly used method out of the three with regards to breast cancer. However, this method is used when a lump is easily reachable and expected to be fluid-filled (Ballo & Sneige, 1996). The extremely thin needle allows for extraction of the fluid to be used for examination. The second method is core needle biopsy. This method makes use of a slightly larger, hollow needle. Using this technique technicians are able to remove layers of tissue from suspicious areas within the breast in order to determine how far cancer has penetrated (Ballo & Sneige, 1996). The final method is called surgical biopsy. This is done by making an incision, ranging from one to two inches on the breast (Ballo & Sneige, 1996). Following this, part or all of the abnormal mass is removed, along with a small amount of surrounding tissue. All three methods are used to determine if cancer cells are present in abnormal masses within the breast.
PATHOPHYSIOLOGY
Breast Cancer is similar to other cancers in which abnormal cell growth can potentially invade and spread to other areas of the body. In normal cell growth, cells can divide as necessary and have functioning signals that can stop cell division. These cells stay attached and localized in their respective sites and do not spread or invade other areas. Normal cells can also undergo programmed cell death, which keeps a balance in the cell population and discards unnecessary cells (Hartwell & Kastan, 1994).
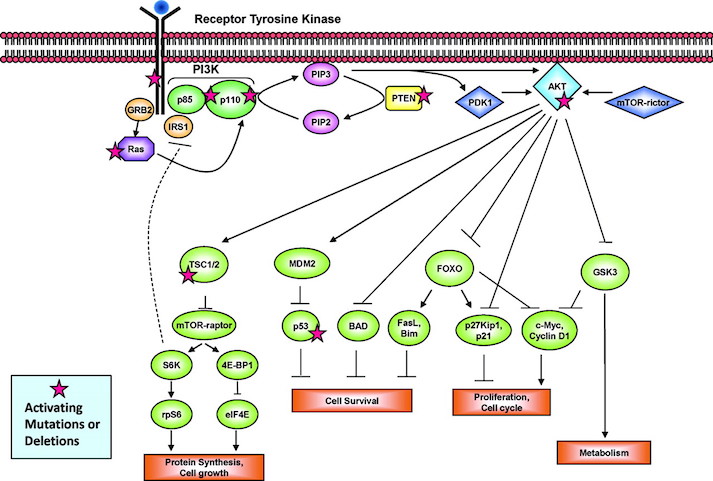
The P13K Pathway and HER2 Gene
The P13K pathway is an example of one of the most important pathways that protect cells by controlling functions such as cell growth, differentiation, motility, survival, and proliferation. P13Ks are lipid kinases that phosphorylate phosphoinositides. The P13Ks involved in breast cancer are Class 1A, which are comprised of a regulatory subunit (p85) and a catalytic subunit (p110). The activation of the P13K is initiated from growth factor or ligand binding to its respective receptor tyrosine kinase (RTK). These receptors contain two important growth factor receptors; the human epidermal growth factor receptor (HER) family and the insulin-like growth factor 1 receptor (IGF-1R). Once activated by a receptor, the P13K heterodimer interacts with a portion of the p85. This binding removes the inhibitory effect of p85 on p1110 rendering the P13K pathway active. The activation leads to a cascade of events downstream of the pathway, resulting in diverse functions such as cellular metabolism, proliferation, differentiation and survival (Liang & Slingerland, 2003).
In breast cancer this pathway is aberrantly hyperactive, as shown in Figure __. This results from mutations in various parts of the pathway but most predominantly in PIK3CA, which encodes for the catalytic p110a subunit. Mutations in these promote downstream signaling elements such as Akt that increase enzymatic function. The overexpression of p110a subunit, therefore leads to robust activation of the pathway (Berns et al., 2007). P13K pathway also involves tumor suppressors such as PTEN, which turn off the pathway and induce cell death. However, in cases such as breast cancer, the PTEN protein may be mutated and unable to turn off the pathway. Therefore, there is excessive cell proliferation and the cell does not undergo programmed cell death. In this case, low expression of PTEN leads to excessive cell growth (Depowski, Rosenthal, & Ross, 2001).
The HER2 Gene
The ErbB is a family of receptor tyrosine kinases, which include the Her2 (ErbB2) receptor-tyrosine-protein kinase. Both the P13K/AKT pathway and Ras-Raf-MAPK pathway are important signalling routes for the ErbB protein. In normal cell activity, the ErbB protein forms homodimers and heterodimers upon activation by growth factor ligands. Upon dimerization these proteins become autophosphorylated and act as binding sites for intracellular signal activators. This triggers a signal cascade leading to increased cell proliferation and inhibition of apoptosis (cell death). Mutation in the HER2 gene leads to amplification of this oncogene and excessive ErbB signalling. Therefore, this increased signalling leads to aberrant cell proliferation and increased inhibition of cell apoptosis (Mitri, Constantine, & O’Regan, 2012).
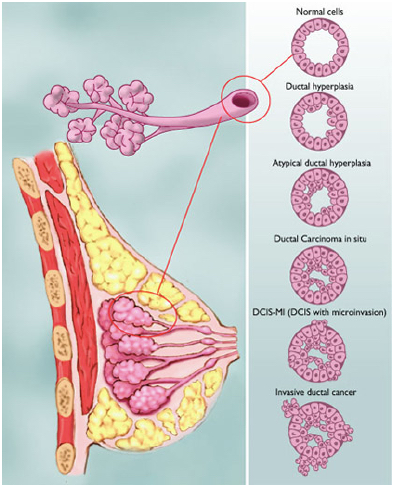
Types of Abnormalities
Figure shows the various types of abnormalities that can result from abnormal cell growth. Ductal hyperplasia contains lesions in the breast that are not cancerous but have active growth and division in the breast tissue cells. Atypical Ductal Hyperplasia and Ductal Carcinoma In Situ (DCIS) occurs when breast tissue is beginning to take an abnormal appearance, however the abnormal cells are still localized in within the tissue. In DCIS-microinvasion and Invasive ductal cancer, the excessive cell growth spreads outside of the tissue and invades other areas of the body (Hüsemann et al., 2008).
Spread of Malignant Tumours
The spread of the tumour varies with different stages in breast cancer. Usually, the least progressive is when atypical cells are in the breast tissue and there are no signs of spread to lymph nodes. As the tumour size increases it begins to spread to lymph nodes and there is metastases in axillary lymph nodes. From here the tumours of any size may spread into the skin, chest wall, and eventually the cancer spreads to other organs and tissues (Hüsemann et al., 2008).
The cancer cells are able to spread to various areas of the body by penetrating the extracellular matrix and blood vessels. Cells are constrained in their respective organs by cell-cell adhesion and basement membrane. However, cancer cells can penetrate the basement membrane by using invadopodia. Invadopodia is the process in which cells form actin-rich protrusions in the plasma membrane and subsequently degrade the extracellular matrix. Genes involved in these protrusions and invasions are upregulated in malignant cancer cells, allowing increased cell invasion and motility. This way, some cells contained in the tumour can separate from their parent mass and enter vascular and lymphatic circulation (Linder, 2007).
CURRENT TREATMENTS
There are many procedures to treat breast cancer ranging from invasive to non-invasive treatments. One of the least invasive treatments is a lumpectomy, which is considered breast conserving surgery. This form of treatment aims to remove any abnormal masses within the breast, without removing all breast tissue (Fisher et al, 1993). Along with the mass itself, a layer of surrounding tissue, specifically regions near the axillary nodes, are also extracted to ensure cancer has not spread to neighboring regions (Fisher et al, 1993). However, due to the conservation of most breast tissue, this method is most effectively used at the earliest stages of breast cancer, before it has spread to multiple layers of tissue.
One of the most commonly used treatments is a mastectomy. This is because a lot of breast cancer cases are reported late, after cancer has already spread to multiple layers of tissue. Using this method all of the breast tissue, including the nipple and areola is removed on the affected breast(s) (Canadian Cancer Society, 2017). Once removed, the skin is stitched closed with a temporary tube attached to an opening in the wound, allowing for any fluid post-surgery to be removed safely (Canadian Cancer Society, 2017). After surgery, a pathologist examines the removed tissue to determine the extent of cancer penetration, and detect whether it has spread to neighboring regions. Since the axillary nodes have the highest likelihood for cancer to spread from the breasts, a few lymph nodes are also removed during surgery for examination (Canadian Cancer Society, 2017). If cancer has appeared to spread to other regions, then other treatments may be used in conjunction with a mastectomy, such as radiation therapy, in order to kill all remaining cancer cells.
Radiation therapy is a form of treatment that targets specific suspected regions. This type of therapy uses high-energy X-rays to assist in targeting and killing cancer cells (Canadian Cancer Society, 2017). Due to its ability to target cells, this form of treatment is typically used post-surgery in order to kill any remaining cancer cells that were missed during surgical procedures. This form of treatment used alone is an option for those experiencing early stage breast cancer, when all the cancer cells are contained within a specific region. However, radiation therapy is also an option for individuals with more advanced breast cancer if used in combination with other forms of treatment such as surgery or chemotherapy (Canadian Cancer Society, 2017).
Majority of lumpectomy procedures are followed by radiation therapy to ensure the cancer is killed from remaining breast tissue (Fisher et al, 1993). By doing this it will lower the chance of breast cancer recurrence by 50 percent, along with death due to breast cancer events by approximately 20 percent (Fisher et al, 1993). In terms of a mastectomy, radiation therapy has not shown to be a beneficial post-procedure (Canadian Cancer Society, 2017). However, in rare cases when radiation therapy is used after mastectomy, its purpose is to treat neighboring regions that are highly susceptible to the spread of cancer, such as the chest wall and axillary nodes (Canadian Cancer Society, 2017).
One of the most common forms of treatment for general cancer cases is chemotherapy. During this form of treatment tumors are treated with high levels of radiation to kill the rapidly diving cells (Canadian Cancer Society, 2017). This form of treatment targets cells non-specifically, and is therefore highly invasive as it kills both healthy and cancerous cells within the body. The radiation from chemotherapy causes the cells within the body to remain in the metaphase-anaphase stage during cell division, leading to apoptosis instead of replication (Canadian Cancer Society, 2017). Due to the killing of these cells there are high toxicity levels associated with this form of treatment which cause side effects such as hair loss, fatigue and nausea (Canadian Cancer Society, 2017).
Chemotherapy is divided into two categories depending on when it is used. Chemotherapy done prior to surgery is referred to as Neoadjuvant therapy. This method is used to shrink large tumors before attempting surgery to increase the chances of successful lump extraction, preventing breast removal (Kuerer et al, 1999). Neoadjuvant therapy can also be used in those with larger tumors in need of a mastectomy. In this case it would be used to increase the chances of tumor shrinkage, and promote a lumpectomy breast conserving procedure instead. Treatment this way also monitors how the tumor responds to treatment, making it safer for tumor removal (Kuerer et al, 1999). Chemotherapy used post surgery is referred to as Adjuvant therapy. This method is given to those who have no current evidence of containing cancer cells in their body (Kuerer et al, 1999). The purpose of adjuvant therapy is to kill the cancer cells that may potentially have been left behind or have metastasized, to reduce the risk of recurrence.
Patients who have been diagnosed with estrogen-receptor positive tumors often experience beneficial results from undergoing endocrine therapy (Lumachi et al, 2011). This type of therapy is usually done through oral contraceptives containing specific estrogen-receptor modulators (SERMs) (Lumachi et al, 2011). A few of the most commonly used SERMs are aromatase inhibitors, GnRH agonists and tamoxifen. Aromatase inhibitors decrease estrogen levels, whereas tamoxifen blocks estrogen receptors, both of which aim to either decrease the tumor’s size or delay its growth (Lumachi et al, 2011). Since endocrine therapy is used amongst those with estrogen-receptor positive tumors, women are classified as premenopausal or postmenopausal in order to determine which method of treatment to undergo. Women in the premenopausal stage who have the estrogen/progesterone receptor are normally treated with tamoxifen for a length of five years (Lumachi et al, 2011). This drug’s ability to block estrogen receptors lowers the risk of breast cancer recurrence by approximately 50 percent (Lumachi et al, 2011). Alternatively, women who are postmenopausal and estrogen-receptor positive have higher beneficial rates from adjuvant therapy, using an aromatase inhibitor (Lumachi et al, 2011). Since postmenopausal women still produce low levels of estrogen in specific fatty tissues, such as the breast tissue, this estrogen-lowering drug reduces the amounts of hormones which contribute to breast cancer growth.
References
Bacharier, L. B., Boner, A., Carlsen, K. H., Eigenmann, P. A., Frischer, T., Götz, M., … & Platts‐Mills, T. (2008). Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy, 63(1), 5-34.
Bryan, S. A., Leckie, M. J., Hansel, T. T., & Barnes, P. J. (2000). Novel therapy for asthma. Expert opinion on investigational drugs, 9(1), 25-42.
Chiang, L. C., Ma, W. F., Huang, J. L., Tseng, L. F., & Hsueh, K. C. (2009). Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial.International Journal of Nursing Studies, 46(8), 1061-1070.
Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004; 22: 789-815. Review
Duffy DL. (1997). Genetic epidemiology of asthma. Epidemiol Rev, 19, 129–143.
Edfors-Lubs M. (1971). Allergy in 7000 twin pairs. Acta Allergol, 26:249–285
Gold DR & Wright R. (2005). Population disparities in asthma. Annu Rev Public Health 26: 89–113.
Keller, M., Lowenstein, S. (2002). Epidemiology of Asthma. Seminars in Respiratory and Critical Care Medicine, 23(4). 317-329.
Kuna, P., & Kuprys, I. (2002). Symbicort Turbuhaler: a new concept in asthma management. International journal of clinical practice, 56(10), 797-803.
Lambrecht, B., Hammad, H. (2015). The Immunology of Asthma. Nature Immunology, 16(1), 45-56.
Pauwels, R. A., Löfdahl, C. G., Postma, D. S., Tattersfield, A. E., O'Byrne, P., Barnes, P. J., & Ullman, A. (1997). Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine, 337(20), 1405-1411.
Pawankar. (2014). Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J., 7(1), 12.
Potter, P. C. (2010). Current guidelines for the management of asthma in young children. Allergy, asthma & immunology research, 2(1), 1-13.
Reed, C. (2006). The natural history of asthma. Journal of Allergy and Clinical Immunology, 118( 3), 549-550.
Ronmark E, Lundback B, Jonsson EA, et al. (1997). Incidence of asthma in adults—report from the Obstructive Lung Disease in Northern Sweden study. Allergy, 52:1071–1081.
Sears, M. (2014). Trends in the Prevalence of Asthma. Chest, 145(2), 219-225.
Skloot G, Permutt S,Togins A. (1995). Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest, 96, 2393–2403.
Warner JA, Jones CA, Jones AC, et al. (2000). Prenatal origins of allergic disease. J Allergy Clin Immuno. 2(2), 493–498.
Weiss ST. Diet as a risk factor for asthma. In: Chadwick D, Cardew G, eds. The Rising Trends in Asthma. New York: Wiley; 1997:244–253