This is an old revision of the document!
Table of Contents
Breast Cancer Powerpoint
Breast Cancer
INTRODUCTION
The breast is an organ that is primarily composed of adipose tissue that is found on the chest region, situated right in front of the pectoralis major muscle. The organ is found in both males and females, but have no functional purpose in males. The breast in females has a specialized function which is the production of milk for lactation. The breast anatomy is composed of 2 components the epithelial component, the lobules where milk is made, and the duct component, which connects the lobules to the external area of the nipple. In male breast anatomy, the ductal component is shunted creating blind ducts, which differentiates them from female breasts.
Breast cancer is the result of the abnormal division of breast cells within the organ. Breast cancer can be an inherited or sporadic cancer, but the most common type of genes involved are the BRCA1 and BRCA2 genes. The cells within the breast can create a tumor at the primary site in the breast. Breast cancer normally develops from the ductal tissues which is known as ductal carcinomas or lobular tissues which is known as lobular carcinomas. Breast cancer has a high potency of metastasizing to other nearby tissues.
Within Canada, 24,400 women and 210 men are diagnosed per year, with over 5,000 women and 60 men dying of disease. Globally, breast cancer in women accounts to 25% of all cancer cases, making it one of the largest sector of cancer in women (Boyle & Levin, 2008). Moreover, breast cancer is more common in developed countries compared to developing countries. Most cases of breast cancer are not reported because males or females refuse to report it to doctors, feminizing syndrome, or it could be simply caught too late (da Silva, 2015).
SIGNS & SYMPTOMS
The five most common signs of breast cancer are as follows;
1. A new lump
- A new lump or thickening in the breast or armpit area
2. Nipple Change
- A newly inverted [pulled in] or retracted nipple
3. Skin Change
- A change in the skin of the breast, areola or nipple e.g. color, dimpling, puckering, or reddening
4. Shape Change
- A change in the breast shape or size
5. Nipple Discharge
- A discharge from the nipple that occurs without squeezing
EPIDEMIOLOGY
Blah
RISK FACTORS
DIAGNOSIS
Blah
PATHOGENESIS
Blah
PATHOPHYSIOLOGY
Breast Cancer is similar to other cancers in which abnormal cell growth can potentially invade and spread to other areas of the body. In normal cell growth, cells can divide as necessary and have functioning signals that can stop cell division. These cells stay attached and localized in their respective sites and do not spread or invade other areas. Normal cells can also undergo programmed cell death, which keeps a balance in the cell population and discards unnecessary cells (Hartwell & Kastan, 1994).
The P13K Pathway and HER2 Gene
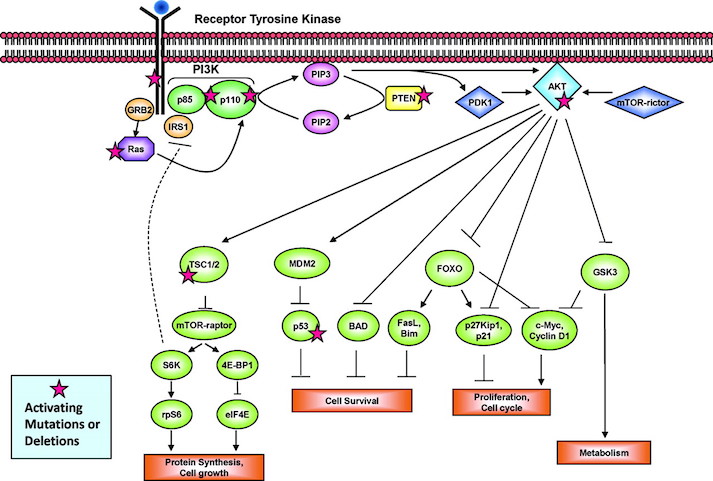
The P13K pathway is an example of one of the most important pathways that protect cells by controlling functions such as cell growth, differentiation, motility, survival, and proliferation. P13Ks are lipid kinases that phosphorylate phosphoinositides. The P13Ks involved in breast cancer are Class 1A, which are comprised of a regulatory subunit (p85) and a catalytic subunit (p110). The activation of the P13K is initiated from growth factor or ligand binding to its respective receptor tyrosine kinase (RTK). These receptors contain two important growth factor receptors; the human epidermal growth factor receptor (HER) family and the insulin-like growth factor 1 receptor (IGF-1R). Once activated by a receptor, the P13K heterodimer interacts with a portion of the p85. This binding removes the inhibitory effect of p85 on p1110 rendering the P13K pathway active. The activation leads to a cascade of events downstream of the pathway, resulting in diverse functions such as cellular metabolism, proliferation, differentiation and survival (Liang & Slingerland, 2003).
In breast cancer this pathway is aberrantly hyperactive, as shown in Figure __. This results from mutations in various parts of the pathway but most predominantly in PIK3CA, which encodes for the catalytic p110a subunit. Mutations in these promote downstream signaling elements such as Akt that increase enzymatic function. The overexpression of p110a subunit, therefore leads to robust activation of the pathway (Berns et al., 2007). P13K pathway also involves tumor suppressors such as PTEN, which turn off the pathway and induce cell death. However, in cases such as breast cancer, the PTEN protein may be mutated and unable to turn off the pathway. Therefore, there is excessive cell proliferation and the cell does not undergo programmed cell death. In this case, low expression of PTEN leads to excessive cell growth (Depowski, Rosenthal, & Ross, 2001).
The HER2 Gene
The ErbB is a family of receptor tyrosine kinases, which include the Her2 (ErbB2) receptor-tyrosine-protein kinase. Both the P13K/AKT pathway and Ras-Raf-MAPK pathway are important signalling routes for the ErbB protein. In normal cell activity, the ErbB protein forms homodimers and heterodimers upon activation by growth factor ligands. Upon dimerization these proteins become autophosphorylated and act as binding sites for intracellular signal activators. This triggers a signal cascade leading to increased cell proliferation and inhibition of apoptosis (cell death). Mutation in the HER2 gene leads to amplification of this oncogene and excessive ErbB signalling. Therefore, this increased signalling leads to aberrant cell proliferation and increased inhibition of cell apoptosis (Mitri, Constantine, & O’Regan, 2012).
Types of Abnormalities
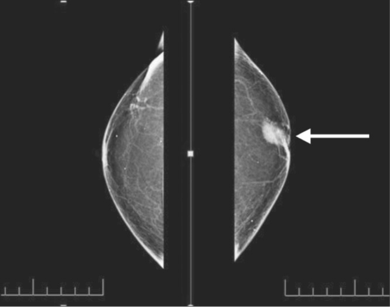
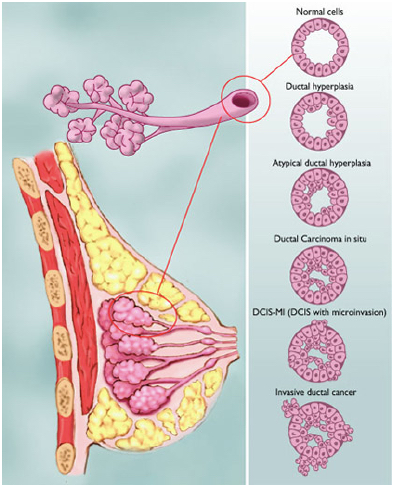
Figure shows the various types of abnormalities that can result from abnormal cell growth. Ductal hyperplasia contains lesions in the breast that are not cancerous but have active growth and division in the breast tissue cells. Atypical Ductal Hyperplasia and Ductal Carcinoma In Situ (DCIS) occurs when breast tissue is beginning to take an abnormal appearance, however the abnormal cells are still localized in within the tissue. In DCIS-microinvasion and Invasive ductal cancer, the excessive cell growth spreads outside of the tissue and invades other areas of the body (Hüsemann et al., 2008).
Spread of Malignant Tumours
The spread of the tumour varies with different stages in breast cancer. Usually, the least progressive is when atypical cells are in the breast tissue and there are no signs of spread to lymph nodes. As the tumour size increases it begins to spread to lymph nodes and there is metastases in axillary lymph nodes. From here the tumours of any size may spread into the skin, chest wall, and eventually the cancer spreads to other organs and tissues (Hüsemann et al., 2008).
The cancer cells are able to spread to various areas of the body by penetrating the extracellular matrix and blood vessels. Cells are constrained in their respective organs by cell-cell adhesion and basement membrane. However, cancer cells can penetrate the basement membrane by using invadopodia. Invadopodia is the process in which cells form actin-rich protrusions in the plasma membrane and subsequently degrade the extracellular matrix. Genes involved in these protrusions and invasions are upregulated in malignant cancer cells, allowing increased cell invasion and motility. This way, some cells contained in the tumour can separate from their parent mass and enter vascular and lymphatic circulation (Linder, 2007).
TREATMENTS
At its current state, there has yet to be an established permanent cure to individuals suffering from asthma. Fortunately, various management techniques are available and can be implemented in order to help alleviate the various symptoms associated with the disease. The primary suggestion asthmatic patients are recommended to do is to avoid any of the mentioned irritants involved in triggering an inflammatory response of the airway. In addition to avoiding any potential allergens, medication is available for both short-term and long-term relief. While short-term medication can provide immediate relief to counteract the constriction of the airway, long-term medication can be taken in the form of a daily supplement to aid in controlling against any future aggravation (Kuna & Kuprys, 2002).
A common short-term therapeutic can be found in the form of bronchodilators such as Ventolin. Bronchodilators are classified as beta-agonists, which seek target beta-adrenergic G-protein coupled receptors (GPCRs) of the bronchial smooth muscle (Kuna & Kuprys, 2002). Upon binding to these membrane-bound receptors, surrounding adenyl cyclase and ATP interact, leading to the production of cyclic-AMP (cAMP) and a subsequent cascading release into the cell. With the function of cAMP being a secondary messenger for enzyme-catalyzed processes within the cell, the relaxation of the smooth muscle cells acts further downstream in this reaction pathway (Kuna & Kuprys, 2002). Normally requiring epinephrine to produce smooth muscle relaxation, Ventolin acts as mimic to adrenaline due to both its conformation and structure, allowing it to produce the same intended result when taken by an asthmatic patient (Kuna & Kuprys, 2002). Another alternative to Ventolin is another inhaler known as Formoterol, which is better known for its more long-term viability. Taking advantage of a mechanism similar to Ventolin, the active ingredients in Formoterol allow the drug to remain at the level of the cell-membrane for an extended period of time (Pauwels et al., 1997). In addition to allowing the bronchodilation of the patient, Formoterol is also able to bind to beta2-adrenergic receptors located on the membrane of mast cells. With the binding of Formoterol to beta2-adrenergic receptors, the release of histamine, an inflammatory agent released by cells in response to any allergic response, is negatively inhibited (Pauwels et al., 1997). With the inhalation of Formoterol on a regular basis, individuals suffering from asthma will be able to relieve themselves of both bronchoconstriction and a hypersensitive response due to any irritants over a long term period.
Figure 5: Ventolin, a bronchodilator used in providing short-term relief from symptoms of asthma
A different type of medication used to treat asthma is anti-inflammatory medication. These anti-inflammatory drugs are corticosteroids such as Budesonide and alike bronchodilators, this form of drug is taken by inhalation. Budesonide reaches the target cells within one minute of inhalation and closes vascular micro leaks via vasoconstriction of the bronchi. Relaxation of the bronchial smooth muscles is experienced after 2-3 hours due to the activation of transcription factors, which increases the number of accessible beta-2 receptors (Pauwels et al., 1997). A new type of therapeutic being tested is a combination therapy involving both bronchodilators and anti-inflammatory pathways. Symbicort is a prime example of this combination medication and its use displayed that the two mechanisms have synergistic effects. Since budesonide leads to an increased number of expressed beta-2 adrenergic receptors, there are more total receptors for formoterol to bind to. In addition to this, the binding of formoterol to these beta-2 receptors increases the activity of budesonide, leading to an increased amount of anti-inflammation (Bryan et al., 2000).
Figure 6: Symbicort, used in combination drug therapy, is efficient due to the synergistic effects provided when using both bronchodilators and anti-inflammatory medication in tandem.
References
Bacharier, L. B., Boner, A., Carlsen, K. H., Eigenmann, P. A., Frischer, T., Götz, M., … & Platts‐Mills, T. (2008). Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy, 63(1), 5-34.
Bryan, S. A., Leckie, M. J., Hansel, T. T., & Barnes, P. J. (2000). Novel therapy for asthma. Expert opinion on investigational drugs, 9(1), 25-42.
Chiang, L. C., Ma, W. F., Huang, J. L., Tseng, L. F., & Hsueh, K. C. (2009). Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial.International Journal of Nursing Studies, 46(8), 1061-1070.
Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004; 22: 789-815. Review
Duffy DL. (1997). Genetic epidemiology of asthma. Epidemiol Rev, 19, 129–143.
Edfors-Lubs M. (1971). Allergy in 7000 twin pairs. Acta Allergol, 26:249–285
Gold DR & Wright R. (2005). Population disparities in asthma. Annu Rev Public Health 26: 89–113.
Keller, M., Lowenstein, S. (2002). Epidemiology of Asthma. Seminars in Respiratory and Critical Care Medicine, 23(4). 317-329.
Kuna, P., & Kuprys, I. (2002). Symbicort Turbuhaler: a new concept in asthma management. International journal of clinical practice, 56(10), 797-803.
Lambrecht, B., Hammad, H. (2015). The Immunology of Asthma. Nature Immunology, 16(1), 45-56.
Pauwels, R. A., Löfdahl, C. G., Postma, D. S., Tattersfield, A. E., O'Byrne, P., Barnes, P. J., & Ullman, A. (1997). Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine, 337(20), 1405-1411.
Pawankar. (2014). Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J., 7(1), 12.
Potter, P. C. (2010). Current guidelines for the management of asthma in young children. Allergy, asthma & immunology research, 2(1), 1-13.
Reed, C. (2006). The natural history of asthma. Journal of Allergy and Clinical Immunology, 118( 3), 549-550.
Ronmark E, Lundback B, Jonsson EA, et al. (1997). Incidence of asthma in adults—report from the Obstructive Lung Disease in Northern Sweden study. Allergy, 52:1071–1081.
Sears, M. (2014). Trends in the Prevalence of Asthma. Chest, 145(2), 219-225.
Skloot G, Permutt S,Togins A. (1995). Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest, 96, 2393–2403.
Warner JA, Jones CA, Jones AC, et al. (2000). Prenatal origins of allergic disease. J Allergy Clin Immuno. 2(2), 493–498.
Weiss ST. Diet as a risk factor for asthma. In: Chadwick D, Cardew G, eds. The Rising Trends in Asthma. New York: Wiley; 1997:244–253