This is an old revision of the document!
Table of Contents
Snake Venom
PDF Presentation: group_3_-_presentation_3_snake_venom_.pdf
PowerPoint Presentation: group_3_-_presentation_3_snake_venom_.pptx
Introduction
Snakes are limbless, flexible and elongated reptiles with 2900 different species worldwide out of which, 375 are venomous (“Basic facts about snakes”, 2017). Snakes are found worldwide except for in Antarctica, Iceland, Ireland, New Zealand and Greenland as they mostly live in tropical regions with warm climate and nest in various environments including in forests, water, and deserts (2017). Venomous snakes attack their prey by injecting venom and are known to consume their prey much larger than their own size, which is known as jaw-walking (Nielson, n.d). Snakes are also known to play a vital role in the environment by maintaining populations of rodents and allow high crop yields in farms (Nielson, n.d). Snakes normally prey small animals including fish, frogs, snails, lizards, chickens, mice, rats or sometimes, they even attack other snakes (Kang et al., 2011). Venom is their primary offensive weapon and is used to incapacitate and immobilize the prey. The secondary function is to use it in a defense mechanism against the predator and also to aid in digestion (Kang et al., 2011). A snake first accurately assesses the target on three levels before it injects venom (Young, Lee, & Daley, 2002). The situation of the encounter such as defensive or predatory, the size of the target and the type of target(Young, Lee, & Daley, 2002). A snake uses various cues to initiate its predatory behaviour such as chemosensory, visual and thermal.There are three families of venomous snakes; atractaspidids, elapids and viperids and have various fang structures (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003). Their venom can be classified into five different types of toxins with different venomous snakes having different injection tactics (Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980). Snake bites exhibit a variety of physiological effects depending on the toxicity of venom and site of the bite.
Summary Videos
How Venom Kills:
History
Snakes are historically important creatures that have strong social and cultural significance (Boquet, 1979). They feature in many different religions and cultures across the globe and are considered omens of both good and evil (Boquet, 1979). It is this dichotomy of interpretations of the snake that make it a phenomenally interesting research topic. Although the focus of this wiki page is on snake venom in particular, it is important to consider the history of snakes in general.
In historical Western culture, particularly in ancient Greece, snakes were considered divine (Boquet, 1979). Archeloos was worshipped as a river god who could transform himself, “from a snake to a bull and then from a bull to a man” (Boquet, 1979). Similarly, the snake was also associated with another god – Athena. In this case, it signified concepts such as physical and reproductive health and longevity. The snake was also important in ancient Greece for its association with medicine. Snakes were kept at the temple of Asklepios where the Greeks believed that these snakes had medicinal qualities, such as the ability to cure blindness. In contrast to these positive viewpoints on snakes, Christianity associated the snake with the Devil and heaped upon it the misfortunes of mankind (Boquet, 1979).
Similar to the negative perspective that Christianity has of the snake, the snake is also associated with evil in some Eastern cultures (Boquet, 1979). For instance, in Hinduism the god Krishna kills a snake demon in anger. However, there are also positive connotations of the snake in Eastern culture too. In Buddhism, a seven headed snake guards Buddha. In ancient Egypt, the snake symbolized the beginning and the end as it was considered to be the one to create all the other gods. Atoum was the name given to this god and he symbolized the earth itself. The Egyptian goddess Isis was also associated with a snake, a gold cobra, which symbolized knowledge among other things (Boquet, 1979). Evidently then the snake is historically vital for the role it plays in many cultures.
Evolution of Venom
There is an evolutionary relationship within the clade containing snakes, anguimorphs, iguanians, and amphisbaenians, lacertids and teiioids (Fry et al., 2012). In particular, within the clade of Toxicofera, which contains snakes, anguimorphs, and iguanians it has been demonstrated that the evolution of venom has diversified the species within this clade. In fact, 170 million years ago, there was an actual point at which venom within all these species diversified (Fry et al., 2012).
To understand the evolutionary history of venom, it is important to realize that venom proteins originated from a group of genes that encode for venom in the common ancestor of the Toxicofera clade (Fry et al., 2012). Venom evolves when the gene that encodes for a regular body protein is duplicated and expressed in the venom gland. Working within this context, snakes can be grouped with anguimorphs more so than iguanians. This is because it is believed that the, “ancestral condition [of this group] was the possession of relatively simple serous dental glands in both the mandibular and maxillary regions” (Fry et al., 2012). These glands were the precursor of the development of venom toxins. Iguanians split off from the snakes and anguimorphs group at this point and did not develop the venom system to the same degree that the other two organisms did. In contrast, the venom system of snakes and anguimorphs evolved over time and became highly specialized (Fry et al., 2012).
The diversification and evolution of the venom system in snakes was multifaceted (Fry et al., 2012). “Gland morphology, muscles, skull, dentition and biochemical diversification, and/or specialisation of the venoms” had all evolved (Fry et al., 2012). For instance, snake glands evolved with differences in structure and topography creating changes in venom delivery within different species of snakes (Fry et al., 2012).
Interesting Venom/Snake Facts
Figure 1 Source: http://www.venomoussnakes.net/
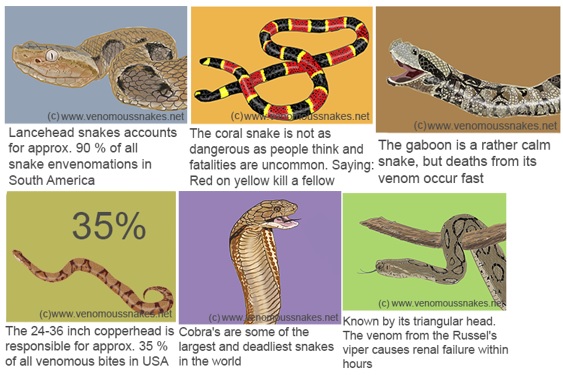
Figure 1: Interesting Snake Facts.
There are 8000 venomous snake bites are reported in USA every year, out of which, only five bites are fatal (Nielson, n.d). Copperhead snake accounts for the majority of the venomous snake bites in USA.India is the most affected by venomous snake bites and has 35000-50000 deaths every year from snake bites. Pakistan ranks second in terms of fatalities with 8200 reported annually. Snakes are known to consume their prey much larger than their own size and this is known as jaw-walking. Their jaws are only loosely attached to their skull and are unconnected, implying that they work independently. Snakes have curved teeth which allow them to pull and swallow the animal further(Nielson, n.d).
Figure 2 Source: http://www.venomoussnakes.net/
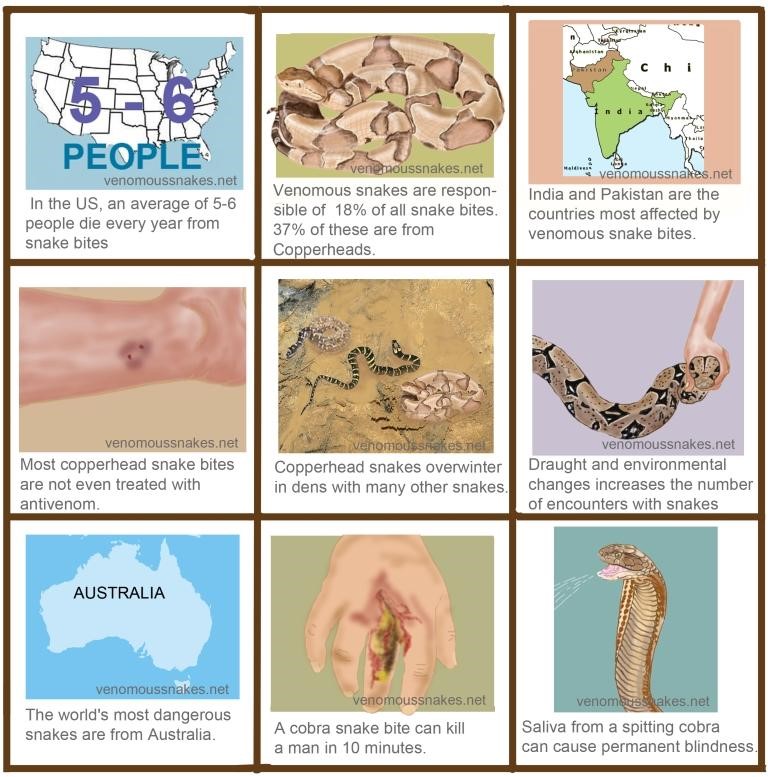
Figure 2: Interesting Snake Facts.
How To Identify Venomous Snakes
Figure 3 Source: https://1.bp.blogspot.com/-w2PccrKd2xk/WS1i_wEkBXI/AAAAAAAAPDI/_-G2buHWOxc9g0QLU6pck8bGt77LLvbFACLcB/s1600/Common%2BVenomous%2BSnakes.JPG

Figure 3: Common North American Venomous Snakes.
Venomous snakes usually have varied colors which are based on geographical location. You should pay more attention to the most colourful snakes, because they are venomous (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003). However, there are some exception, like cottonmouths. If the snakes only have a solid color, we may consider they are completely harmless (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003). On the other hand, venomous snakes normally have a triangular head because of the venomous glands, and non-venomous snakes have a spoon-shaped round head (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003). Venomous snakes also have elliptical pupils, some of them may have real rattles on the end of its tail and the nice skin pattern(Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003).
Three families of venomous snakes atractaspidids, elapids and viperids.
For atractaspidids family, also classified into the colubridae family nowadays, they may provide some lightly toxic venom, too weak to cause any harm. However, some of them can cause severe tissue necrosis(Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003). (i.e if they bite your toe, and the tip of that toe may be lost.)
For elapids family, including cobras, mambas, coral snakes (North America) and taipans, they can produce neurotoxin, which can attack the central nervous systems (or respiration) of their prey (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003).
For viperids family, including rattlesnakes, copperhead, water moccasin (North America), vipers, adders and other species, they can produce hematoxic venom which can attack the tissue and blood cells of their prey (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003).
Fang Structures
All the venomous snakes have one of three fang structures, including proteroglyphous, solenoglyphous, or opisthoglyphous. A different family of snakes have a unique fang structure (Vonk et al, 2008 and Warrell, 2010).
Figure 4 Source: https://upload.wikimedia.org/wikipedia/commons/thumb/f/ff/Crotalus_skull.jpg/220px-Crotalus_skull.jpg

Figure 4: Solenoglyphous Fang.
If snakes have solenoglyphous fang, means they belong to the viper family which includes pit vipers (i.e. rattlesnakes). Solenoglyphous fang is attached to the jaw by a hinge and can be folded up against the roof of the mouth when the snakes are not using it. Therefore, this folding action is beneficial to the vipers and that is why they have the longest fangs(Vonk et al, 2008 and Warrell, 2010).
Figure 5 Source: https://allyouneedisbiology.files.wordpress.com/2015/02/malpolon-bo.jpg?w=204&h=176
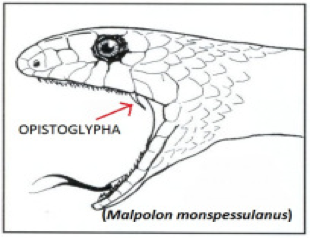
Figure 5: Opisthoglyphous Fang.
If snakes have opisthoglyphous fang, means they belong to the colubrid family. Opisthoglyphous fang is located at the back of the mouth (Vonk et al, 2008 and Warrell, 2010).
Figure 6 Source: https://upload.wikimedia.org/wikipedia/commons/thumb/8/87/Ophiophagus_hannah_skull.jpg/220px-Ophiophagus_hannah_skull.jpg

Figure 6: Proteroglyphous Fang.
If snakes have proteroglyphous fang, means they belong to the elapid family (i.e. coral snakes). Proteroglyphous fang is fixed to the jaw and cannot fold up. Therefore, the elapid fangs must be shorter than the solenoglyphous fangs (Vonk et al, 2008 and Warrell, 2010).
Classification of Snake Venom
There are five different types of snake venom:
1. Hemotoxic Venom can destroy the red blood cells and cause hemolysis, disrupts blood clotting etc. This type of venom can attacks other types of cells and tissue and cause tissue damage or sometimes organ failure (Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980). The effects of this venom may not begin for hours after a bite. The tissue damage are always permanent(Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980).
2. Myotoxic Venom can destroy the muscle tissue and affect the muscle contraction. If people is bitten, they will experience paralysis. On the other hand, kidney damage is also the outcome from this venom (Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980). However, myotoxic venom is beneficial for the snakes to eat and leave the prey flaccid (Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980).
3. Neurotoxic Venom can destroy the nerves and nervous system. If people have a progressive paralyzation of the muscles, it may cause respiratory failure as your diaphragm is not working well. Few symptoms may show up, for example vomiting, droopy eyelids etc(Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980).
4. Cytotoxic Venom can destroy and attacks the living cells of all sorts. Some symptoms may show up, for example bleeding, swelling etc(Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980).
5. Haemorrhagic Venom can cause bleeding in multiple organs including ears, nose, eyes etc(Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980).
Injection Tactics
The venomous snakes would like to bite, because they want to inject the venom into their prey (immobilize and/or kill the preys) and defend against attack by potential predators and antagonists (Malasit et al., 1986).
Fang contact plays an important role on envenomation during defensive bites(Malasit et al., 1986). For viperid snakes, they typically strike and release targets for both predatory and defensive bites (Malasit et al., 1986). For elapid snakes, they are more inclined to hold after biting, and give them more chance to delivery of more venom. Other than that, some elapids have a more sophisticated delivery system, which allows them to spit small fractions of venom for defensive purposes repeatedly(Malasit et al., 1986).
Chemistry
Venom gland is a modified version of a salivary gland and in a snake, it is located behind and under the eye (Johnson, 2012). Sea snakes, cobra and krait contain neurotoxins, which are the most powerful, resulting in muscle paralysis in victim and resulting in death (Tu, 1996). Rattlesnake venoms have tissue-damaging toxins like myonecrotic and hemorrhagic toxins. This produces muscle damage in tissues and organs along with dysfunctioning or loss of body part. Although neurotoxins are most powerful, there are other dangerous toxins such as cardiotoxins, myotoxins and cytotoxins (Tu, 1996). Different snake species have different toxins in their venom (Casewell et al., 2014). This is due to different toxin-encoding genes in the snake’s venom gland or the genome. The primary constituents of snake venom include proteins and peptides also known as toxins and they are encoded by approximately 5-10 multilocus gene families (Casewell et al., 2014). These different venoms have evolved over a long time to become a complex mix of pharmacologically active peptides, aiding them in prey capture (Kang et al., 2011). Snake venoms contain 30 to more than 100 protein toxins exhibiting enzymatic and non-enzymatic activities. Some common enzymes identified in snake venom include phospholipase A2s (PLA2s), serine proteinases, metalloproteinases, acetylcholinesterases (AChEs), l-amino acid oxidases, nucleotidases (5¢-nucleotidases, ATPases, phosphodiesterases and DNases) and hyaluronidases. AChE is largely found in venomous snakes that belong to family Elapidae. L amino acid oxidase inhibit aggregation of platelets. Some studies performed in 1990s demonstrated that snake venom induces apoptosis in endothelial cells and this is probably due to an increase in H2O2 concentrations (Kang et al., 2011). Spla2S types 1 and 2 are found in snake venoms, particularly in those of elapids and sea snakes (Harris & Scott-Davey, 2013). Alongside proteases, phospholipases, neurotoxins are shown to immobilize the prey, haemotoxins are shown to inhibit coagulation and allow circulation of incoagulable blood (Harris & Scott-Davey, 2013). Nucleotidases found in snake venoms generate purines, mostly adenosine, and liberate adenosine to aid in prey immobilization(Dhananjaya & D’Souza, 2010) . The quantity of venom in the venom gland is directly correlated to the snake’s size and increases exponentially with the snake’s size (Johnson, 2012). Venom quantity can range from 1 mg to 850 mg, with the largest amount of venom in an Eastern Diamondback rattlesnake. It is also interesting to note that the delivery of venom is a voluntary action and all venomous snakes are equally capable of delivering dry bites (Johnson, 2012). Rattlesnakes are shown to increase the quantity of venom injected based on the size of the target and inject more venom when they bite a larger target (Young, Lee, & Daley, 2002). They were shown to inject twice the amount of venom into large mice, 25g to 44g, than in medium sized mice, 7g to 11g (Young et al., 2002).
Mechanisms of Actions of Toxins
Snake venom consists of numerous combinations of different cytotoxins including several neurotoxins (O’Leary et al, 2007). Cytotoxins are substances that have toxic effects on certain cells within the body (Debinski, 2008). A cell affected by a specific cytotoxic compound can be affected in multiple ways depending on the toxin and the type of cell (Rossetto et al., 2008). The 3 most common effects include cells undergoing necrosis which is the process of losing membrane integrity and dying through cell lysis, the stoppage of active division thus decreasing cell viability or the forceful activation of controlled death though apoptosis (Debinski, 2008). The types of cytotoxins commonly found in snake venom include: neurotoxins (fasciculins, dendrotoxins and α-neurotoxins), phospholipases, cardiotoxins and hemotoxins (Rossetto et al., 2008).
Neurotoxins:
Normal Neuronal Signalling:
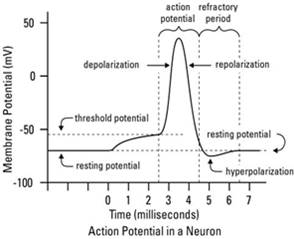
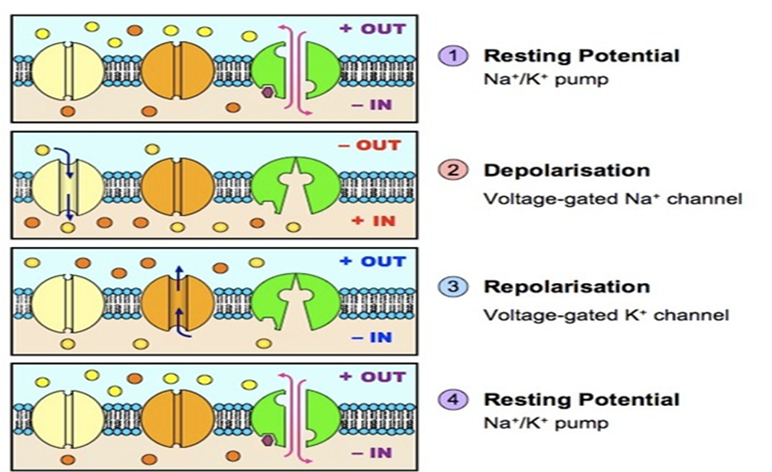
To comprehend the effects of neurotoxins a solid grasp of the basics of neuronal impulse and synapse must be had. The transfer of information from one part of the body to another occurs as neuronal cells produce electrical signals known as action potentials (Gong et al., 2008). The electrical signal is derived from ionic concentration differences across the cells plasma membrane as well as through changes with the permeability of the membrane (Gong et al., 2008). At resting potential the inside of the neuron there is an abundance of K+ ions and proteins while on the outside there are more Na+ ions and chloride ions (Gong et al., 2008). The inside of the cell is approximately -70 mv compared to the outside (Gong et al., 2008). The cell at this point is in a polarized state and this stage is known as the resting potential (Gong et al., 2008). Not only does Na+ and K+ ions pass through the cell membrane naturally there is also a Na+/K+ pump that brings in 2 K+ ions and removes 3 Na+ ions from the neuronal cell (Gong et al., 2008). When a stimulus occurs while at the resting potential state, it causes the threshold potential to be surpassed and depolarization occurs as normally gated/closed ion channels open allowing Na+ ions into the cell greatly increases the charge inside the neuron (Gong et al., 2008). The “All-or-None” phenomenon occurs and the action potential will go to completion at this point (Gong et al., 2008). When the inside of cell reaches a net charge of close to +50 mv, other K+ gated channels open allowing K+ ions to leave the cell (Gong et al., 2008). The neuron cell starts decreasing the charge and repolarization and electrical balance is occurs except this time the ion concentrations are flipped with more Na+ ions on the inside and more K+ ions on the outside (Gong et al., 2008). As the K+ channels open, the Na+ channels close (Gong et al., 2008). A period of hyperpolarization occurs when the net charge with the neuron surpasses the resting potential of -70mv (Gong et al., 2008). However this is rectified when the K+ channel closes and the pump restores resting potential balance (Gong et al., 2008). Actions potentials propagate along an axon from dendrites down to the axon terminal where the message is relayed to the adjacent neuron through chemical or electrical means (Gong et al., 2008).
Understanding Action Potentials:
Figure 6 Source: http://www.dummies.com/education/science/understanding-the-transmission-of-nerve-impulses/
Figure 6: Normal action potential.
Figure 7 Source: http://www.dummies.com/education/science/understanding-the-transmission-of-nerve-impulses/
Figure 7: Normal flow of ions in/out of a neuron throughout the different phases of an action potential.
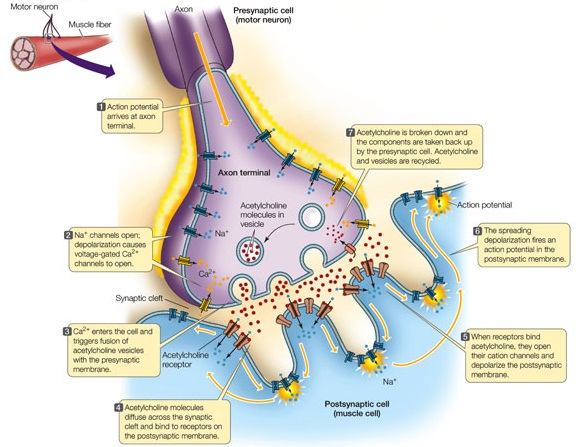
Neurotoxins specifically target synapse that transfers information by the use of neurotransmitters also known as chemical synapses (Rossetto et al., 2008). When the action potential moves along the axon in waves it eventually reaches to the presynaptic terminal at the end of the neuron, this activates the voltage-gates calcium channels which allow calcium to inter into the neuron (Veletic et al, 2016). The flow of positive charged ions causes small synaptic vesicles to fuse with the cell membrane and chemical messengers known as neurotransmitters are released into the synaptic cleft which is the small gap between the start of one neuron and the end of another (Veletic et al, 2016). The neurotransmitters diffuse across the gap and bind to their respective receptors on the postsynaptic neuron (Veletic et al, 2016). The binding to the receptors cause channels to open on the postsynaptic neuron which in turn either inhibits or excite the postsynaptic neuron/muscle (Veletic et al, 2016). The neurotransmitters are only binding for a short amount of time before they are either reuptaken by the presynaptic neuron or are broken down by enzymes in the synaptic cleft (Veletic et al, 2016). This prevents over excitation (Veletic et al, 2016).
Figure 8 Source: http://www.medicallibraryonline.com/Synaptic-Transmission
Figure 8: Neurotransmitter and normal synapse function in neuronal signaling.
Types of Neurotoxins:
Fasciculins:
Fasciculins is a neurotoxin that targets cholinergic neurons which are neurons that specifically use the neurotransmitter of Acetylcholine (Ach) (Lauridsen et al, 2016). These neurons play a role in the neuro-muscular junction which dictates muscle movement. Instead of neuron to neuron transmission it is neuron to muscle transmission (Lauridsen et al, 2016). Acetylcholinesterase (AChE) which is an enzyme that catalyzes the breakdown of Acetylcholine is the primary target of Fasciculins (Lauridsen et al, 2016). Fasciculins destroy this enzyme which now prevents the breakdown of Acetylcholine and the neurotransmitters remains locked into its receptors on the muscle wall after the message has been passed on to the muscle (Lauridsen et al, 2016). This causes tetany which is involuntary muscle contraction and can cause death (Lauridsen et al, 2016). This is mainly used to help the snake to run away from its predator (Lauridsen et al, 2016).
Dendrotoxins:
Dendrotoxins inhibit neurotrasnmitters by blocking the exchange of positive and negative ions across the neuron membrane which leads to no action potential and no nerve impulse (Laustsen et al, 2015). It specifically binds to and causes a conformational change in K+ gated channels (Laustsen et al, 2015). The conformational change prevents ion conduction and ions can no longer pass through the channel (Laustsen et al, 2015). This causes paralysis of the nerves (Laustsen et al, 2015). This may build to full body paralysis or very commonly can death mainly due to respiratory failure (Laustsen et al, 2015).
α-neurotoxins:
There are currently over 100 postsynaptic α-neurotoxins that have been identified (Lauridsen et al, 2017). These neurotoxins target Nicotinic acetylcholine receptors (nAChRs) which are receptor proteins that respond to the neurotransmitter acetylcholine (Lauridsen et al, 2017). α-neurotoxins is an antagonist of the Nicotinic acetylcholine receptors and are structurally similar to acetylcholine (Lauridsen et al, 2017). The mimicry allows it to bind and block the the Nicotinic acetylcholine receptors on post synaptic neurons or muscle fibers (Lauridsen et al, 2017). This blocks the flow of Acheylcholine and prevents the signal from being passed on (Lauridsen et al, 2017). This causes the feeling of numbness, paralysis, respiratory failure and in extreme cases death (Lauridsen et al, 2017).
Cytotoxins:
Phospholipases:
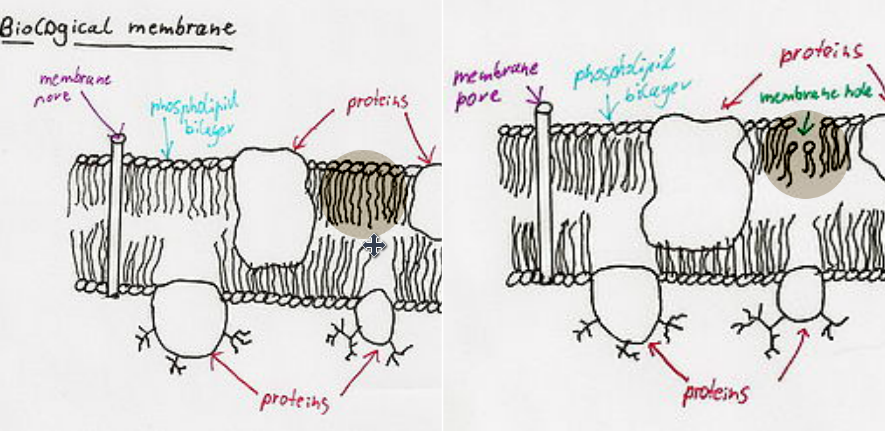
Phospholipases are enzymes that transform hydrolyze free phospholipids as well as those bound to membranes into fatty acids and lysophospholipids (Valdez-Cruz et al, 2007). The most common phospholipases found in snake venom is Phospholipase A2 (Valdez-Cruz et al, 2007). Several physiological systems will be affected depending on which phospholipids become hydrolyzed; haemostasis, neuromuscular transmission and inflammatory reactions. (Valdez-Cruz et al, 2007) Most dangerous and commonly it can cause cell membrane ruptures. The structural changes ruin the integrity of the membrane and make the membrane severally more permeable (Valdez-Cruz et al, 2007). The cell will die once integrity is lost. Cell death leads to eventual organ failure and death (Valdez-Cruz et al, 2007).
Figure 9 Source: https://en.wikipedia.org/wiki/Snake_venom#Neurotoxins
Figure 9: Phospolipases affect on destroying the cell membrane integrity.
Cardiotoxins:
The exact mechanism of cardiotoxins is not fully understood (Rong et al, 2007). What is known is that they bind to specific sites on the surface of the heart and cause depolarization which prevents muscle contraction (Rong et al, 2007). The main effect of cardiotoxins is arrhythmia which means the heart is beating too fast, too slow or irregularly (Rong et al, 2007). Depending on the size of the individual it does not take very much cardiotoxin to completely force the heart from beating and leads to immediate death (Rong et al, 2007). Some cardiotoxins have been identified that specifically alter the function of the SA node which is the hearts pacemaker (Rong et al, 2007). This causes more immediate cardiac effects (Rong et al, 2007).
Hemotoxins:
Another toxin found commonly in a variety of snake venom are Hemotoxins which cause hemolysis; the destruction of erythrocytes and/or induce blood coagulation (Tang et al, 2016). Damaging erythrocytes in the process destroys other body issue leading to organ degeneration (Tang et al, 2016). Most of the pain coming after a snake pain is due to hemotoxins (Tang et al, 2016). Even if survival occurs permanent damage to the body is still expected (Tang et al, 2016). Hemotoxins act a lot slower than neurotoxins do but are far more deadly (Tang et al, 2016). The two roles of Hemotoxins work together to kill the victim (Tang et al, 2016). Hemotoxins first destroy erythrocytes as well as vitally damage organs/blood vessel before helping with disseminated intravascular coagulation (Tang et al, 2016). The destruction of erythrocytes causes all the content to escape and makes the blood very thick. Disseminated intravascular coagulation occurs which increases clotting in the body while using up vast amounts of platelets and clotting factors (Tang et al, 2016). With less of these building blocks. serious internal bleeding will lead to death as it cannot be stopped (Tang et al, 2016). The most dangerous snakes in the world kill through this combinatory pathway(Tang et al, 2016).
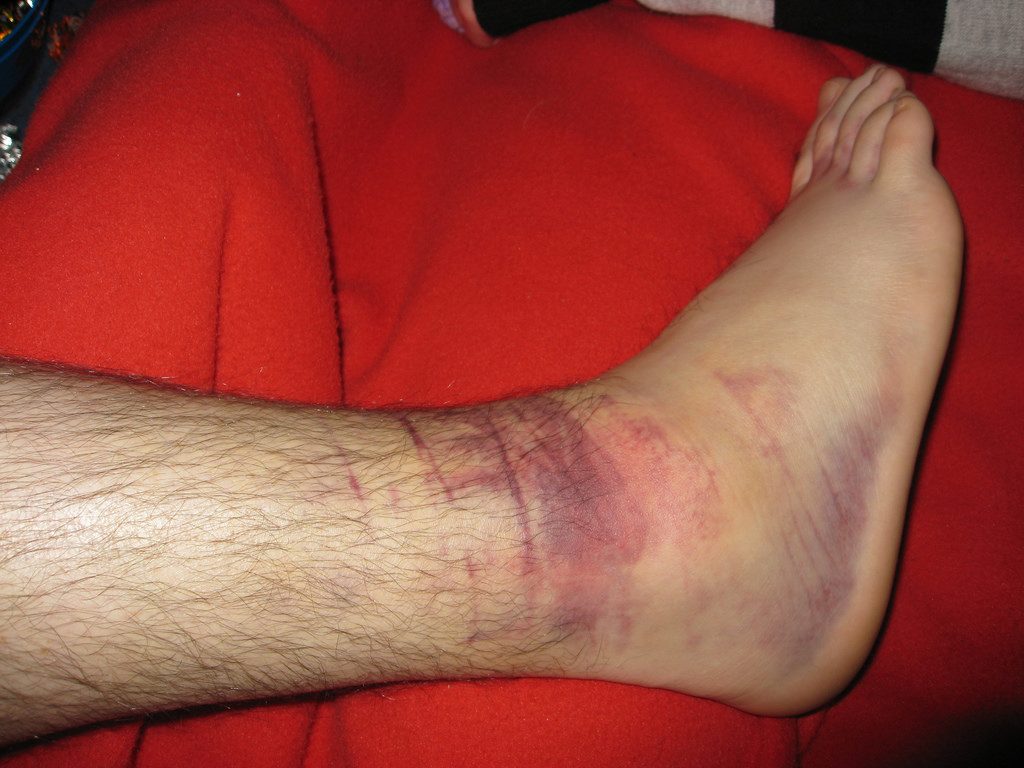
Figure 10 Source: http://howtogetridofonionbreath.com/can-i-know-symptoms-of-internal-bleeding/
Figure 10: Image of how serious internal bleeding looking from the outside.
Determining Venom Toxicity
To determine the toxicity of a specific sample of venom, a LD-50 (also known as median lethal dose) test can be done. This test was developed in 1927 originally used for biological standardization of dangerous drugs (Zbeinden and Flury-Roversi, 1981). Eventually the test was used to also test toxicological properties of other chemical compounds (Zbeinden and Flury-Roversi, 1981). This test involves finding out the required dose of a substance to kill half the members of a population of tested species over a specific duration of time. The value that is obtained from the test is considered to not be a biological constant as the results are influenced by many factors of the animals and the more animals the accurate (Zbeinden and Flury-Roversi, 1981). By performing this test, you can determine how toxic the venom from a specific species is. You can determine how much is required in order for a human or any other specie to die.
Physiological Effects
Depending on the species that bites you, you may experience different symptoms. Generally, an individual will have two puncture wounds from the fangs of the snake. Around the bite there will be some swelling and redness that begins to form (HealthLine). Around this area, pain will also be felt by the individual (HealthLine). They will experience some difficulty breathing, vomiting, nausea, blurred vision, sweating, salivating and numbness in the face and limbs (HealthLine). These are the symptoms felt from most snake bites, the common symptoms. Other various symptoms will be experienced by other species of snake, for example, rattlesnakes, coral snakes, copperheads, etc (HealthLine). Many species of snake contain venom that will interfere platelet aggregation which is an important process in thrombosis (blood coagulation) and hemostasis (a process that stops bleeding after an injury) (Kini and Evans, 1990).
Use of Snake Venom To Treat Disease
Snake venoms consist of a complex mixture of several biologically active proteins, peptides, enzymes, organic compounds and inorganic compounds (Vyas et al., 2013). One use of venom that has been studied to see for future use is in the area of cancer. It has been discovered that snake venom can inhibit cell proliferation and also promoting cell death in regards to cancer cells (Vyas et al., 2013). These processes occur due to increased calcium ion influx, decreasing or increasing the expression of proteins that control the cell cycle resulting in damage to the cell membrane, apoptosis induced cancer cells, promoting cytochrome C release, etc (Vyas et al., 2013). Certain proteins from snake venom have also been used to create drugs that help thrombin inhibition, blood thin, fibrinogen inhibition for strokes, help with high blood pressure, etc. (Koh et al., 2006).
Modern Snake Bite Treatment, Antivenom, and Immunity
Traditional Venom Treatments
Colonial remedies were quite different from the current treatment for venomous snake bites. A case in 1868 revealed some of these methods when a man was bitten by a venomous snake (Hobbins, 2017). The surgeon was tied a ligature around the patient’s arm to cut the site of bite. He then poured ammonia on the wound site in an attempt to neutralize the venom and then made the patient drink six ounces of brandy to rouse his circulation. The surgeon next proceeded by waving a powerful smelling salt under the patient’s nose and subsequently applied a mustard poultice to his abdomen, hands and feet to ease internal congestion. Another medical professor then injected ammonia into one of his veins in the bitten arm and surprisingly, saved the patient’s life. In addition to this scenario, traditional venom treatments commonly included suction or ligature to expel the venom and limit further circulation in the bloodstream (Hobbins, 2017). Furthermore, medicinal plants are also commonly used to treat venomous snake bites, as they are safe, effective, inexpensive and attainable from neighbouring forests (Gupta & Peshin, 2012). For example, Vitis vinifera L. seeds are extracted and used due to their neutralization effects of edema, from viperine snake bites (Gupta & Peshin, 2012).
Current and Future Studies
In the late twentieth century there has been significant research on understanding the biochemical and physiological mechanisms associated with the toxins found in the venom of a variety of organisms, and snakes in particular (Fox and Serrano, 2007). Even more recently, there has also been increasing interest in harvesting the toxins found in venom to try to create therapeutic agents that can be used in pharmaceutical interventions (Fox and Serrano, 2007) against disease and other conditions that adversely affects one’s health. Of the many toxins found in snake venom, snake venom metalloproteinases (SVMPs) are the most commonly found in the class viperidae (Takeda, Takeya, and Iwanaga, 2012). This toxin can cause hemorrhage and can affect the hemostatic system (Takeda, Takeya, and Iwanaga, 2012).
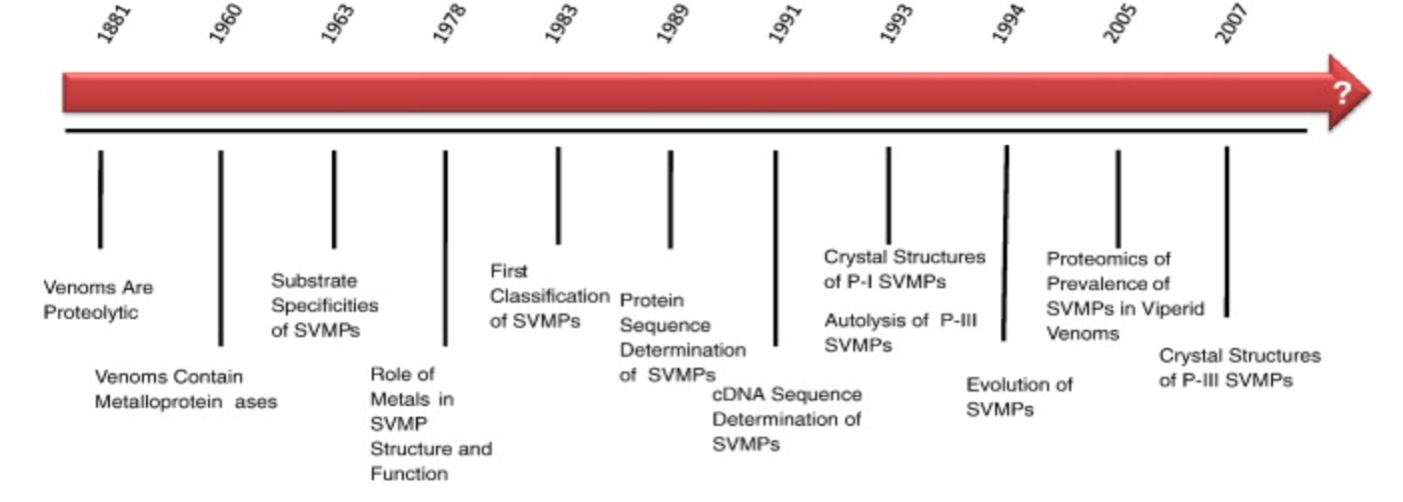
Figure 11 Source: https://www.ncbi.nlm.nih.gov/pubmed/19344655
Figure 11: Timeline of Snake Venom Metalloproteinases (SVMPs) Research.
In particular, there has been, “significant progress…regarding the function, structure and role of the snake venom metalloproteinases (SVMPs) in viperid venom pathogenesis” (Fox and Serrano, 2009). As seen in Figure 11, since 1881 to the time of Fox and Serrano’s review paper in 2007, there have been several notable discoveries (Fox and Serrano, 2009). To begin with, in 1881 it was proposed that venoms could be proteolytic and in 1990 when it was seen that venom could coagulate blood, the proteolytic enzyme activity of venom was hinted at. Then around 1960, toxins extracted from snake venom were discovered to be proteinases and were shown to have a high metal content. Thus researchers came to know of snake venom metalloproteinases. Then over the next half century numerous SVMPs have been identified and their various roles and functions have been speculated upon. One of the key discoveries made was the connection between SVMPs and the ADAMs group of proteins. This led to the creation of the protein group: reprolysins. Current research, around 2007, focused on the evolutionary basis for SVMPs and future studies may focus on elucidating a better understanding of the structure and function of different SVMP types (Fox and Serrano, 2009).
Conclusion
In conclusion, snakes inject venom to either inflict harm on a prey or predator for offensive and defensive purposes, respectively (Kang et al., 2011). India is the most affected by venomous snake bites and has 35000-50000 deaths every year from snake bites (Nielson, n.d). Through jaw-walking, they are able to prey on animals larger than them but they also prey on small animals such as fish, frogs, and mice (Kang et al., 2011). Although venomous snakes come in a variety of colors based on their geographical location, colorful snakes are most likely venomous and can cause the most harm (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003). The three families of venomous snakes are atractaspidids, elapids, and viperids with atractaspidids snakes having a less toxic venom, elapids producing neurotoxins and viperids producing hemotoxic venom (Underwood, 1979; Savage & Slowinski, 1992 and Reed, 2003). All the venomous snakes have one of three fang structures, including proteroglyphous, solenoglyphous, or opisthoglyphous (Vonk et al, 2008 and Warrell, 2010) through which they inject the five different types of venom including hemotoxic, myotoxic, neurotoxic, cytotoxic and haemorrhagic (Chang, 1979; Karlssoni, 1979; Ohsaka, 1979 and Russell, 1980). Fang contact plays an important role on envenomation during defensive bites with viperids striking and releasing the target and elapid snakes holding onto the target for more venom delivery (Malasit et al., 1986). Different toxins target different body components and process including action potentials and red blood cells and have various physiological effects including difficulty breathing, vomiting, interference with platelet aggregation (Kini and Evans, 1990). The toxins found in snake venoms consist of a complex mixture of several biologically active proteins, peptides, enzymes, organic compounds and inorganic compounds and one use of venom that has been studied to see for future use is in the area of cancer where venom can inhibit cell proliferation and also promote cell death (Vyas et al., 2013).
References
Basic facts about snakes. (2017). Defenders of wildlife. Retrieved 1 December 2017, from https://defenders.org/snakes/basic-facts
Casewell, N. R., Wagstaff, S. C., Wüster, W., Cook, D. A. N., Bolton, F. M. S., King, S. I., … Harrison, R. A. (2014). Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proceedings of the National Academy of Sciences, 111(25), 9205–9210. https://doi.org/10.1073/pnas.1405484111
Chang, C. C. (1979). The action of snake venoms on nerve and muscle. In Snake venoms (pp. 309-376). Springer Berlin Heidelberg.
Debinski, W. (2008). Molecular targeting with recombinant cytotoxins for the treatment of brain tumors. Drug Development Research, 69(7), 407-414. https://doi.org/10.1002/ddr.20272
Dhananjaya, B. L., & D’Souza, C. J. M. (2010). The pharmacological role of nucleotidases in snake venoms. Cell Biochemistry and Function, 28(3), 171–177. https://doi.org/10.1002/cbf.1637
Elli, M. (2017a). Snake Bites. Healthline. Retrieved 17 November 2017, from https://www.healthline.com/health/snake-bites#coral-snakes6
Ellis, M. (2017b). Snake Bites. Healthline. Retrieved 17 November 2017, from https://www.healthline.com/health/snake-bites#overview1
Gong, B., Liu, M., & Qi, Z. (2008). Membrane potential dependent duration of action potentials in cultured rat hippocampal neurons. Cellular and Molecular Neurobiology, 28(1), 49-56. https://doi.org/10.1007/s10571-007-9230-5
Gupta, Y. K., & Peshin, S. S. (2012). Do herbal medicines have potential for managing snake bite envenomation? Toxicology International, 19(2), 89–99. https://doi.org/10.4103/0971-6580.97194
Harris, J. B., & Scott-Davey, T. (2013). Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins, 5(12), 2533–2571. https://doi.org/10.3390/toxins5122533
Hobbins, P. (n.d.). Hissstory: how the science of snake bite treatments has changed. Retrieved November 25, 2017, from http://theconversation.com/hissstory-how-the-science-of-snake-bite-treatments-has-changed-71408
Johnson, s. (2012). Venomous Snake FAQs. Ufwildlife.ifas.ufl.edu. Retrieved 25 November 2017, from http://ufwildlife.ifas.ufl.edu/venomous_snake_faqs.shtml
Kang, T. S., Georgieva, D., Genov, N., Murakami, M. T., Sinha, M., Kumar, R. P., … Kini, R. M. (2011). Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. FEBS Journal, 278(23), 4544–4576. https://doi.org/10.1111/j.1742-4658.2011.08115.x
Karlsson, E. (1979). Chemistry of protein toxins in snake venoms. In Snake venoms (pp. 159-212). Springer Berlin Heidelberg.
Kini, R., & Evans, H. (1990). Effects of snake venom proteins on blood platelets. Toxicon, 28(12), 1387-1422. http://dx.doi.org/10.1016/0041-0101(90)90155-z
Koh, D., Armugam, A., & Jeyaseelan, K. (2006). Snake venom components and their applications in biomedicine. Cellular And Molecular Life Sciences, 63(24), 3030-3041. http://dx.doi.org/10.1007/s00018-006-6315-0
Lauridsen, L. P., Laustsen, A. H., Lomonte, B., & Gutiérrez, J. M. (2017). Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of naja melanoleuca. Journal of Proteomics, 150, 98-108. https://doi.org/10.1016/j.jprot.2016.08.024
Lauridsen, L. P., Laustsen, A. H., Lomonte, B., & Gutiérrez, J. M. (2016). Toxicovenomics and antivenom profiling of the eastern green mamba snake (dendroaspis angusticeps). Journal of Proteomics, 136, 248-261. https://doi.org/10.1016/j.jprot.2016.02.003
Laustsen, A. H., Lomonte, B., Lohse, B., Fernández, J., & Gutiérrez, J. M. (2015). Unveiling the nature of black mamba (dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. Journal of Proteomics, 119,126-142. https://doi.org/10.1016/j.jprot.2015.02.002
Malasit, P., Warrell, D. A., Chanthavanich, P.,Viravan, C., Mongkolsapaya, J., Singhthong, B., & Supich, C. (1986).Prediction, prevention, and mechanism of early (anaphylactic) antivenom reactions in victims of snake bites. Br Med J (Clin Res Ed), 292(6512), 17-20.
Nielsen, A. Venomous Snakes - facts, species, bites and natural history. Venomoussnakes.net. Retrieved 24 November 2017, from http://www.venomoussnakes.net/
Ohsaka, A. (1979). Hemorrhagic, necrotizing and edema-forming effects of snake venoms. In Snake venoms (pp. 480-546). Springer Berlin Heidelberg.
O'Leary, M. A., Schneider, J. J., Krishnan, B. P., Lavis, C., McKendry, A., Ong, L. K., & Isbister, G. K. (2007). Cross-neutralisation of australian brown and tiger snake venoms with commercial antivenoms: Cross-reactivity or antivenom mixtures? Toxicon, 50(2), 206-213. https://doi.org/10.1016/j.toxicon.2007.03.014
Reed, R. N. (2003). Interspecific patterns of species richness, geographic range size, and body size among New World venomous snakes. Ecography, 26(1), 107-117.
Russell, F. E.(1980). Snake venom poisoning. Philadelphia, 18, 139-234.
Rong, H., Li, Y., Lou, X., Zhang, X., Gao, Y., Teng, M., & Niu, L. (2007). Purification, partial characterization, crystallization and preliminary X‐ray diffraction of a novel cardiotoxin‐like basic protein from naja naja atra (south anhui) venom. Acta Crystallographica Section F, 63(2), 130-134. https://doi.org/10.1107/S1744309107002564
Rossetto, O., Morbiato, L., Caccin, P., Rigoni, M., Carli, L., Paoli, M., . . . Montecucco, C. (2008). Tetanus, botulinum and snake presynaptic neurotoxins. RENDICONTI LINCEI, 19(2), 173-188. https://doi.org/10.1007/s12210-008-0010-z
Savage, J. M.,& Slowinski, J. B. (1992). The colouration of the venomous coral snakes (family Elapidae) and their mimics (families Aniliidae and Colubridae). Biological Journal of the Linnean Society, 45(3), 235-254.
Tang, E. L. H., Tan, C. H., Fung, S. Y., & Tan, N. H. (2016). Venomics of calloselasma rhodostoma, the malayan pit viper: A complex toxin arsenal unraveled. Journal of Proteomics, 148, 44-56. https://doi.org/10.1016/j.jprot.2016.07.006
Tu, A. T. (1996). Overview of snake venom chemistry. Advances in Experimental Medicine and Biology, 391, 37–62. https://doi.org/10.1007/978-1-4613-0361-9
Underwood, G.(1979). Classification and distribution of venomous snakes in the world. In Snake venoms (pp. 15-40). Springer Berlin Heidelberg.
Valdez-Cruz, N. A., Segovia, L., Corona, M., & Possani, L. D. (2007). Sequence analysis and phylogenetic relationship of genes encoding heterodimeric phospholipases A2 from the venom of the scorpion anuroctonus phaiodactylus. Gene, 396(1), 149-158. https://doi.org/10.1016/j.gene.2007.03.007
Veletic, M., Floor, P. A., Chahibi, Y., & Balasingham, I. (2016). On the upper bound of the information capacity in neuronal synapses. IEEE Transactions on Communications, 64(12), 5025-5036. https://doi.org/10.1109/TCOMM.2016.2613970
Vonk, F. J., Admiraal, J. F., Jackson, K., Reshef, R., de Bakker, M. A., Vanderschoot, K.,… & Mirtschin, P. J. (2008). Evolutionary origin and development of snake fangs. Nature, 454(7204), 630-633.
Vyas, V., Brahmbhatt, K., Bhatt, H., & Parmar, U. (2013). Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pacific Journal Of Tropical Biomedicine, 3(2), 156-162. http://dx.doi.org/10.1016/s2221-1691(13)60042-8
Warrell, D. A.(2010). Snake bite. The Lancet, 375(9708), 77-88.
Young, B. A., Lee, C. E., & Daley, K. M. (2002). Do Snakes Meter Venom? BioScience, 52(12), 1121–1126. https://doi.org/10.1641/0006-3568(2002)052[1121:DSMV]2.0.CO;2
Zbinden, G., & Flury-Roversi, M. (1981). Significance of the LD50-test for the toxicological evaluation of chemical substances. Archives Of Toxicology, 47(2), 77-99. http://dx.doi.org/10.1007/bf00332351