Table of Contents
HIV Powerpoint
Introduction
The human immunodeficiency virus (HIV) is a virus that can negatively impact the host once transmitted. Although concentrated in some regions of the globe, it can be found all around. HIV has been studied in the past however, many questions still remain which continue to fuel the need for further research.
History
HIV is classified into two different groups, HIV-1 and HIV-2 [1]. HIV-1 is most commonly found around the world, while HIV-2 is less severe and targets a smaller population [1]. HIV-1 is found mostly in Africa, but it is present is almost every country (Sharp). HIV-1 is thought to be transmitted through a Simian immunodeficiency virus (SIV) found in chimpanzees [1]. While the exact origins of HIV are still unknown, one widely accepted theory is that it was passed to humans through hunting [2]. When hunters killed an animal with SIV, it was transmitted to humans through the blood of the chimpanzee [2]. SIV was then mutated to form HIV through multiple transmissions [2]. Patient zero is believed to have lived in Western Africa in southeast Cameroon [3]. HIV-1 is classified into three groups, M, N, and O, with group M being found all across the globe [1]. The oldest case of HIV is from 1959, a blood sample was found to have HIV-1 group M [2]. Recently, most countries have been seeing a decline in HIV rates [4]. Most people with HIV belong low or middle-income families, with lack of access to treatment or are unable to afford it [4]. There is now a World AIDS day on December 1st, created by WHO in 1988, to help raise awareness for HIV/AIDS [4].
Epidemiology
The first case of AIDS was in the US in 1981, found in five gay men in Los Angeles [5]. In Canada, AIDS was first reported in 1982 [6]. Initially, HIV was associated with gay men and drug users, however, it is spread through unprotected hetereosexual intercourse in many countries. Through international travel and intercourse, HIV has spread all across the globe. HIV was first named “HIV” in 1986, and in 1987, FDA approved of the first antiretroviral therapy, zidovudine (AZT), for HIV [5]. In that same year, WHO confirmed mother-to-infant transmission for HIV and a blood test to detect for HIV was also introduced [5]. In 1994, the first oral test for HIV was introduced [5]. As of 2017, 59% of people with HIV are now on antiretroviral therapy [4]. Approximately 37 million people across the globe have HIV - of them, 9 million don’t know that they are infected [4]. HIV has killed around 35 million people globally, and 1.8 million people become newly infected by HIV in 2017 [4].
Causes
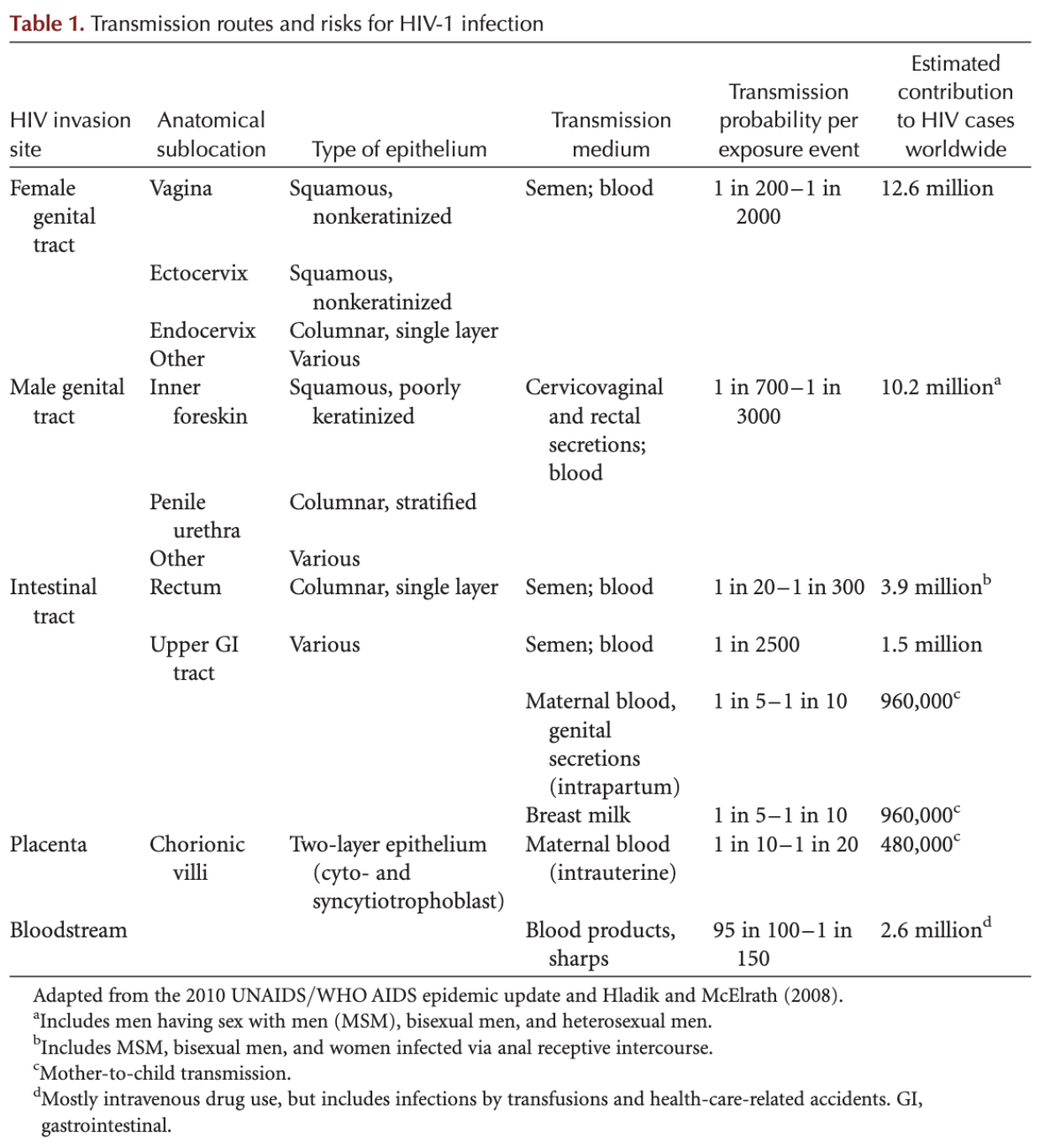
HIV is a sexually transmitted infection that is caused by unprotected sex [7] [8]. It can also be passed on to babies from their mothers before or during birth with rates ranging from 14-42% depending on various settings [7] [8] [9]. Moreover, it may also be transmitted during breastfeeding [7]. Although less common, HIV can also be transmitted by sharing needles either syringes or other injecting equipment [7]. Unlike other viruses which spread through the air, HIV is not easily passed from one person to the next [7]. Instead, for HIV transmission there needs to be a fluid exchange between someone who has HIV and another person who does not have it [7].
There are a few targeted ways that the virus can enter the bloodstream. Initially, the virus can enter if it is injected into the bloodstream with needles or other equipment that has been used by other people who have been infected by HIV [7]. Additionally, it can enter through the lining on or inside the anus, vagina, and/or genitals [7]. It is also possible for the virus to enter through the lining of the mouth and eyes and from cuts or sores in the skin [7].
Pathophysiology
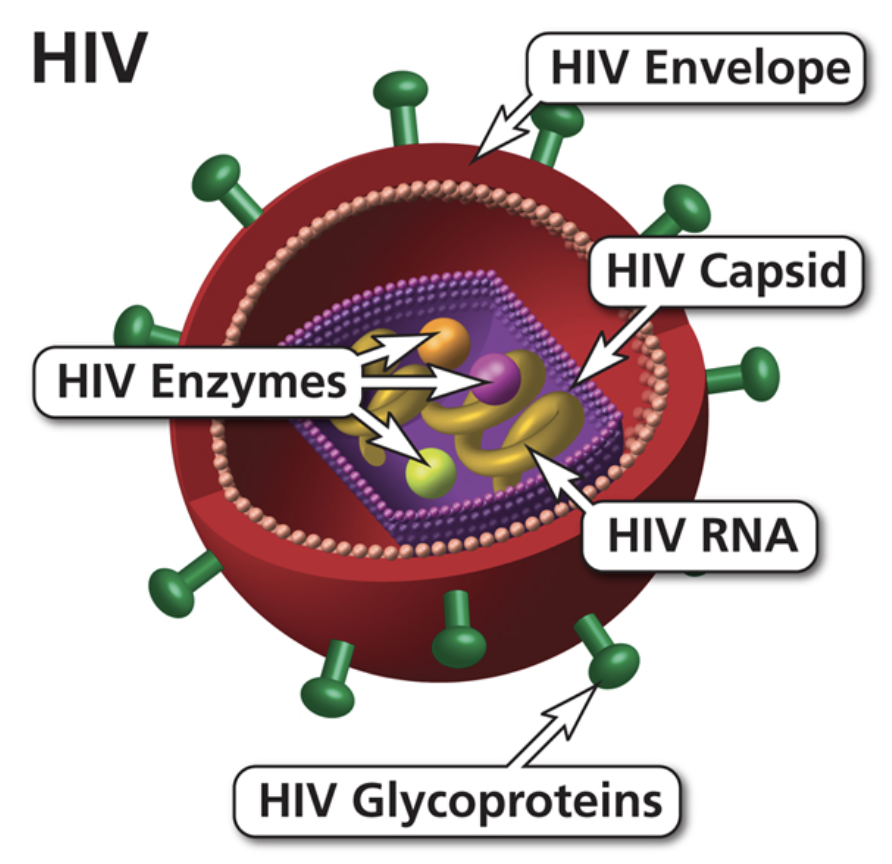
HIV has a cylindrical center that is surrounded by a lipid bilayer envelope. In this bilayer envelope, there are 2 primary proteins, which are gp120 and gp41 [10]. These are used to help with the recognition of the CD4+ T helper cells allowing the virus to attach and invade [10]. These CD4+ cells are used in the body's natural immune response to help activate the active B cells, killer cells, and macrophages. When the HIV virus binds to these cells it will have copies of genomic material, RNA, multiple proteins and enzymes all of which are crucial for invasion [10]. In order to alter the genetic makeup of the immune cell the HIV virus will undergo reverse transcription [10]. Through this process, it will code for 3 principal genes, which are gag, pol, and env [11]. The gag gene will help to encode the core proteins in the cell [11]. The pol gene is what will code the reverse transcriptase, which is the protein that is necessary when undergoing this process [11]. Finally, the env gene is used for the structural components of HIV [11]. This can include glycoproteins, which will help to provide support for the cells.
6 phases of binding [12]
- Binding and Entry: The gp120 and gp41 will bind to the CD4+cell receptors and co-receptors that are outside the cells. The CCR5 and CXCR4 will help to further facilitate the insertion into the cell. The joining proteins and receptors will help the contents of the HIV membrane enter the cell. These will then begin integrating with the enzymes in the cell.
- Reverse Transcription: The HIV RNA must now be converted to DNA so that they begin being incorporated in the DNA of the CD4+ cells. The HIV virus will bring in the reverse transcriptase enzyme to help expedite this process. From this, a single strand of DNA is produced, which will then be duplicated into a double-stranded HIV DNA.
- Integration: After the reverse transcription, the DNA can enter the nucleus of the CD4+ cell now. This allows the cell to begin producing the necessary proteins for replication
- Replication: The host cell will then produce messenger DNA for the synthesis of HIV proteins
- Budding: There is now an accumulation of proteins developing inside the cell. These proteins will congregate together to form the CD4+ new membrane. These proteins will begin to push through the walls of the cell. This will now have components to infect other CD4+cells.
- Maturation: The newly formed cell will now have the necessary components to infect, but still must mature. This includes the HIV protease enzyme cutting the long HIV protein into the smaller functional units. These will then reassemble to form the mature virus.
So, when the HIV cell has infected the cell it will begin producing multiple copies of itself. This can help with further infiltration in other cells around the body. In fact, there are between 10 million to 10 billion virions produced each day [13]. Within the first 24 hours, the HIV virus will capture the dendritic cells in the mucous membrane and skin [13]. After 5 days they will infiltrate the lymph nodes and the peripheral blood. This is where viral replication will become much faster. This is because at the lymph nodes there is an increased recruitment of CD4+ cells [13]. This will cause more binding sites for the HIV virus allowing it to proliferate even faster. There are two different forms of the HIV virus. For the most part these two viruses are very similar. They have the same mode of transmission and the same likelihood of infection [14]. The primary difference is that HIV 2 is shown to be a slower progression [14].
Symptoms
The symptoms of HIV vary, depending on the phase of infection.
Primary infection (Acute HIV)
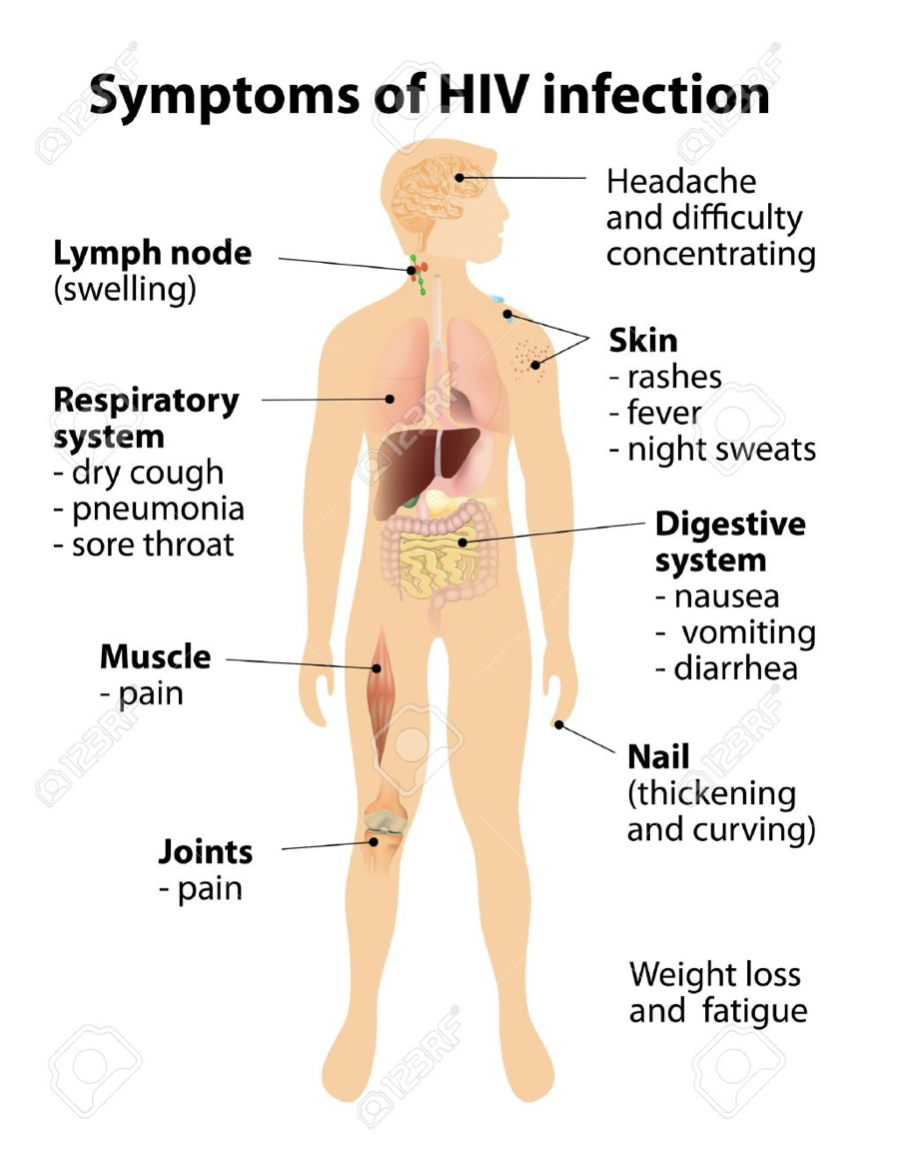
Most people infected by HIV develop flu-like symptoms within a month or two after the virus enters the body [15]. This is known as primary or acute HIV infection, which can last for a few weeks. Possible signs and symptoms include:
- Fever
- Headache
- Nausea and Vomiting
- Aching muscles
- Sore throat and painful mouth sores
- Swollen lymph glands, mainly on the neck
These symptoms can be so mild that you might not even notice them. However, the amount of virus in your bloodstream is quite high at this time [15]. As a result, the infection spreads more easily during primary infection than during the next stage.
Chronic HIV
During this stage, some people develop persistent swelling of lymph nodes. After your immune system is unable to battle with HIV, the flu-like symptoms disappear, therefore there are no specific signs and symptoms at this stage. This is referred to the clinical latent period. HIV remains in the body and in infected white blood cells [15]. During this time, untreated HIV will be killing CD4 T-cells and destroying the immune system. This stage of HIV infection generally lasts around 10 years if you're not receiving antiretroviral therapy [15]. But sometimes, even with this treatment, it lasts for decades. Some people develop more severe disease much sooner.
Symptomatic HIV infection
As the virus continues to multiply and destroy immune cells, the cells in your body that help fight off germs may develop mild infections or chronic signs and symptoms such as:
- Fever
- Fatigue
- Swollen lymph nodes
- Diarrhea
- Weight loss
- Oral yeast infection
- Shingles
Diagnosis
HIV diagnosis is based on the detection of specific antibodies, antigens, or both [16]. The sensitivity and specificity of different assays vary, and test devices or equipment can be made by one company, but distributed and sold under various brand names [17]. The WHO Prequalification of Diagnostics Programme, evaluates the performance of commercially available technologies such as various serological assays (EIAs and rapid tests).
Serological tests
Antibodies to HIV can be measured by a variety of techniques. None of the techniques detect HIV itself, but rather detect an immune response to the virus, and therefore take time to develop and become reactive after HIV infection has been acquired [17]. Antibodies to HIV are detected by EIA, also known as enzyme-linked immunosorbent assay (ELISA), rapid test devices, and western blot (WB) tests.
Enzyme immunoassay (EIA)
Enzyme immunoassay is a common immunological technique that has been adapted for the detection of HIV antibodies. EIAs have a high sensitivity and specificity and are able to detect all subtypes of HIV [17]. EIAs screen large numbers of specimens (about 90 or more at a time). An EIA procedure takes around two hours, hence some large-scale labs are able to report the EIA results the same day. EIAs require sophisticated equipment, and are technically demanding; automatic pipettes, incubators, washers, readers and constant electricity supply must be available. The validity of the test results depends on the skills of technicians, who prepare the necessary reagents and operate the equipment [17].
Rapid Test Devices
Rapid antibody tests are qualitative immunoassays that should be used in combination with the clinical status, history, and risk factors of the person being tested. Rapid test devices with diagnostic performance comparable to that of traditional EIA methods are currently commercially available [17]. These assays are helpful to use in resource-limited settings since they can be performed in clinic or community settings and little equipment is required. Most rapid tests are presented as a kit incorporating the reagents and do not usually require additional equipment [17]. Rapid HIV assays are quick and easy to perform, making them more cost-effective than EIAs. As the procedures are simpler and involve a limited number of steps, there is a lesser chance of error and they can be carried out by health-care workers who have received appropriate training [17]. Test results become available within 10–30 minutes.
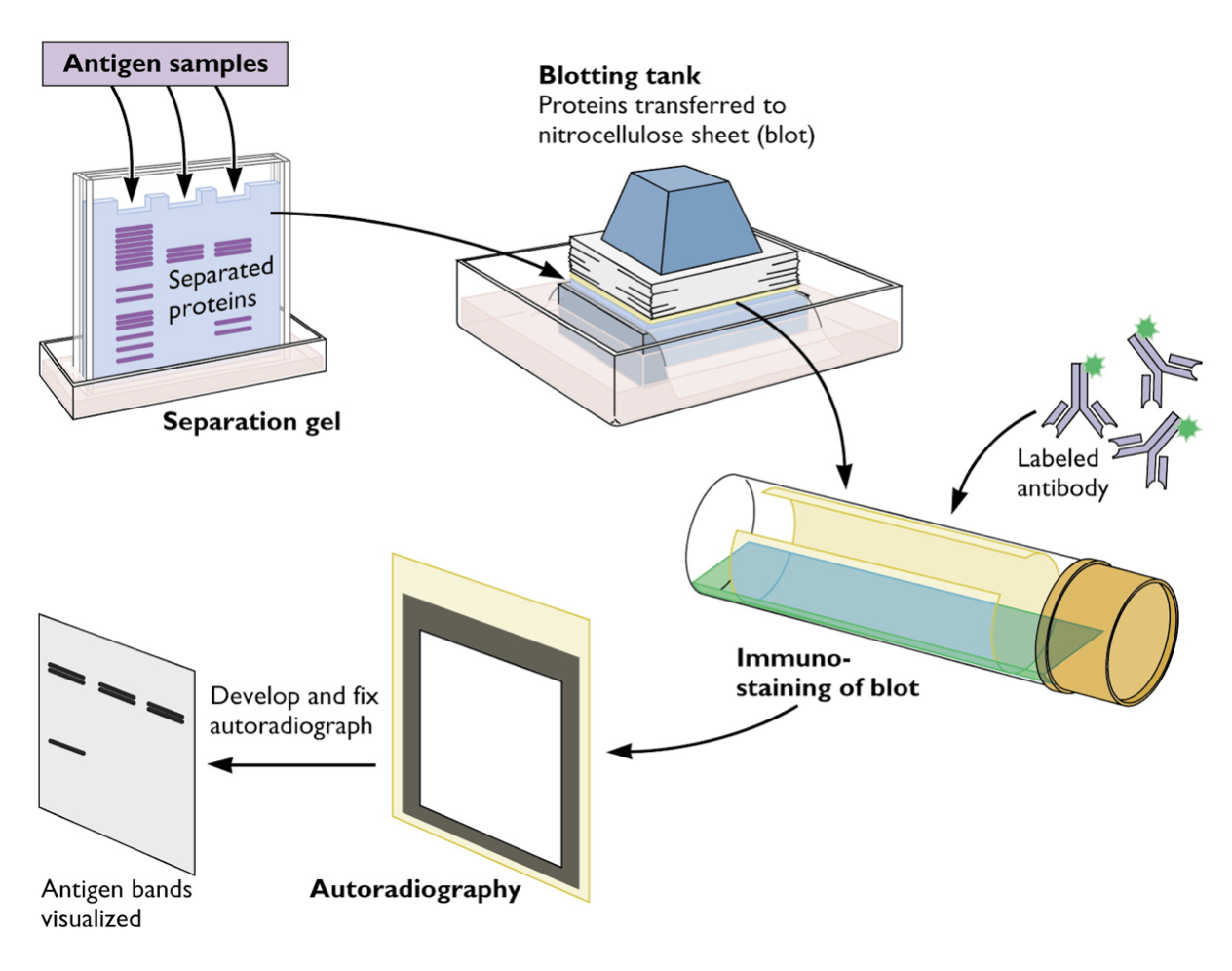
Western Blotting
The WB assay consists of a multilayer process similar to an EIA [17]. HIV antigens are laid out on a strip from the highest in molecular weight to the lowest. When a specimen is incubated with the strip, any existing HIV antibodies bind to these HIV antigens [17]. An antibody-enzyme complex is formed with the addition of the enzyme. Then a chemical is added that changes colour when it comes into contact with the protein–antibody–enzyme layers [17].
Drugs and Medication
There is no cure available for HIV, however the current drugs and medications have been very effective at counteracting the damaging consequences of the infection. The World Health Organization recommends Antiretroviral therapy (ART) to fight against HIV. ART consists of a combination antiretroviral drugs to significantly suppress symptoms of the virus and prevent the transmission of the virus to others. It is recommended that ART is used as soon as possible, as the efficacy of it is most potent in the early stages of the diseases [18]. There are over 40 antivirals that have been approved for treatments and most patients on ART will take two or more everyday for the rest of their lives to maintain suppression of the symptoms. ART can reduce the amount of HIV present in the blood to undetectable amounts, reducing their chances of transmitting the disease onto others to very minimal [19]. There are side effects to the drugs, most of which are minor, like occasional headaches and dizziness, however more serious complications may arise such as tongue and throat swelling or liver damage, which may be life threatening [20]. As the HIV virus continues to spread through the body, there is a chance that the virus mutates becoming resistant to the current medication that the patient is using. This may require the patient to use a different set of drug(s), as the virus has become resistant to the previous one.
There are 8 different classes of HIV medicine, some play a role in preventing the virus from multiplying, others prevent HIV from entering immune cells:
- Nucleoside Reverse Transcriptase Inhibitors (NRTI): blocks the reverse transcriptase of the HIV molecule, not allowing the virus to multiply [19].
- Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI): binds and then Alters the reverse transcriptase of HIV, preventing the spread of the virus [19].
- Protease Inhibitors (PI): Blocks the protease of the HIV molecule, preventing the disease to make copies of itself [19].
- Fusion inhibitors: prevents HIV from infecting CD4 cells of the immune system [19].
- CCR5 antagonists: block the receptors of the immune system that HIV needs to bind to in order to infect the immune cells [19].
- Post attachment inhibitors: blocks CD4 receptors on the surface of certain immune cells stopping HIV from entering the cell [19].
- Integrase Inhibitors: Block the Integrase enzyme needed to replicate the HIV molecule [19].
- Pharmacokinetic enhancers: Increases the efficacy of the HIV drugs that are part of ART [19].
These drugs are often taken in combination to fight against HIV.
Future Therapeutics
With the current therapies, there are a few new therapeutics that are also being explored. There is the “shock-and-kill” strategy, gene therapy which is also being researched, and broadly neutralizing antibodies which have been analyzed.
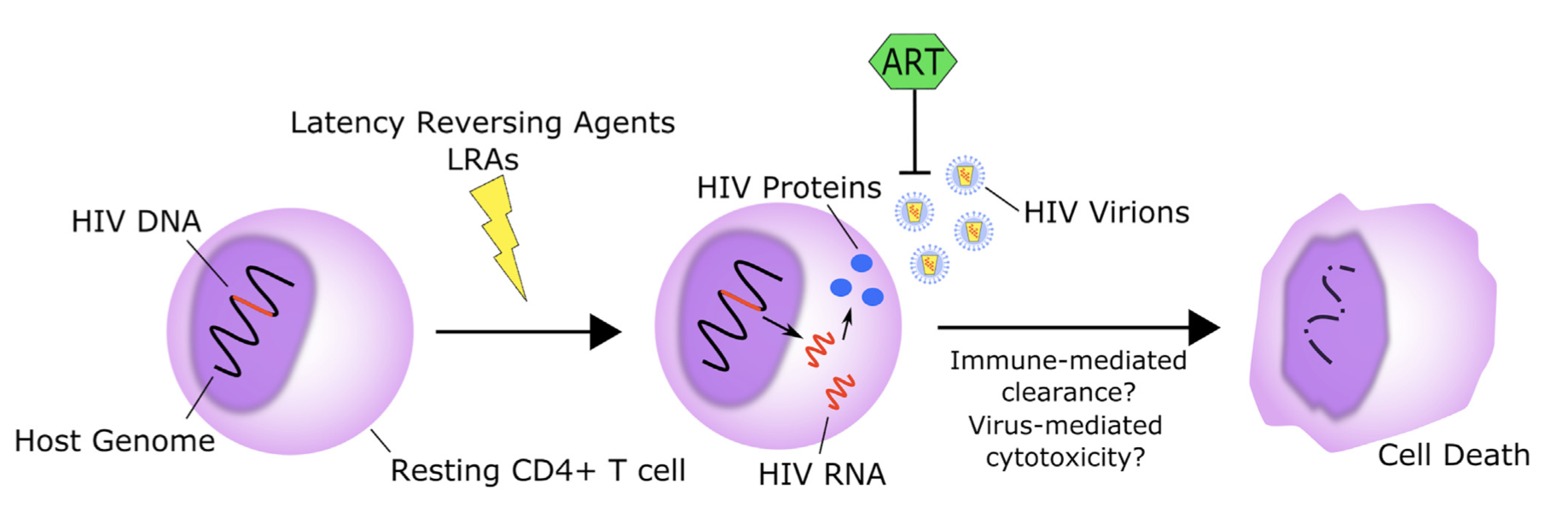
Shock-and-Kill Approach
This approach reactivates the latent virus is using latency reversing agents [21]. The aim of this approach is to activate cell death through virus-induced cytolysis or immune-mediated clearance [21]. This has only however, been observed through a limited number of agents that have been used in clinical trials [21]. These agents included: HDACis vorinostat, panobinostat, the disulfiram drug which has been observed to affect PI3K/Akt, and the TLR9 agonist MGN1703 [21-30]. With this therapy, various studies have noted that the activation of viral transcription on its own is insufficient to induce cell death and some latency reversing agents may infect promote cell survival [21].
Moreover, this therapy has been found to be cost effective to administer to individuals who have low-incomes [21]. While there are potential bandits of this therapy, more research needs to be conducted to further gain a better understanding of key components (e.g. effects it can have) of the “shock and kill” approach [21].
Gene therapy
Another therapy is gene therapy through three different strategies [31]. First, it targets the proviral genome directly through a gene editing technology which could include clustered regularly interspaced short palindromic repeats (CRISPR) or through transcriptional silencing [31-32] . The second step includes targeting future HIV infections by blocking HIV from entering by releasing certain proteins or build an HIV resistance by modifying HIV receptors through gene editing [31][33]. Lastly, this approach uses immunotherapy-based approaches that are currently being used in clinical trials for cancer. The aim of immunotherapy-based approached is to genetically modify T cells or natural killer cells to identify and bind to target cell types and eliminate them [31].
Broadly Neutralizing Antibodies
Finally, another approach which suggests potential agents that lead to prevention, treatment, and elimination of the HIV-1 infection involves broadly neutralizing antibodies [34]. This is achieved by maintaining viral suppression or removing the cells which are latently infected [34]. This can be done wither through antibody induced activation or without it [34]. Compared to previous models, this antibody has increased potency, breadth, plasma half-life, and effector function [34].
Conclusion
HIV is a public health issue that affects men and women. HIV is classified into two different groups, HIV-1 and HIV-2. HIV-1 is more common and severe compared to HIV-2. HIV is caused by three different ways: through unprotected sex, passed on to babies by mothers or sharing needles/other injecting equipment. To diagnose patients with HIV various test can be conducted. A variety of immunoassays are available to provide accurate results.
Since there is no cure for HIV currently, drugs and medication are available to help suppress symptoms as well as prevent transmission of this virus. Modern medical science has developed various types of drugs to assist people suffering from HIV. Antiretroviral therapy (ART) is the most common therapy and over 40 antivirals have been approved as treatment options. There is research continually collected and tested to advance our knowledge in this field. With the knowledge we know and continue to gain, researchers are looking for ways to prevent and cure HIV.
References
[1] Sharp, P. M., & Hahn, B. H. (2011). Origins of HIV and the AIDS pandemic. Cold Spring Harbor perspectives in medicine, 1(1), a006841. doi:10.1101/cshperspect.a006841
[2] Watanabe, M. E. (2006). Origins of HIV: The Interrelationship between Nonhuman Primates and the Virus. BioScience, 54(9), 810. https://doi.org/10.1641/0006-3568(2004)054[0810:oohtib]2.0.co;2
[3] Pépin, J. (2013). The origins of AIDS: from patient zero to ground zero. Journal of Epidemiology and Community Health, 67(6), 473–475. https://doi.org/10.1136/jech-2012-201423
[4] World Health Organization. (2019). Data and Statistics. Retrieved from https://www.who.int/hiv/data/en
[5] Office of the Commissioner. (2018, May 1). HIV/AIDS History of Approvals - HIV/AIDS Historical TimeLine 1995-1999. Retrieved from https://www.fda.gov/ForPatients/Illness/HIVAIDS/History/ucm151079.htm
[6] HIV and AIDS History. (n.d.). Retrieved from https://canfar.com/hiv-and-aids/history-of-hiv/
[7] Causes HIV and AIDS [Internet]. NHS Choices. NHS; 2018 [cited 2019Mar24]. Available from: https://www.nhs.uk/conditions/hiv-and-aids/causes/
[8] Shaw GM, Hunter E. HIV transmission. Cold Spring Harbor perspectives in medicine. 2012 Nov 1;2(11):a006965.
[9] Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the timing of HIV transmission from mother to infant. Jama. 2001 Feb 14;285(6):709-12.
[10] Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, Nayfield SG. Workshop on HIV infection and aging: what is known and future research directions. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008 Aug 15;47(4):542.
[11] Seibert SA, Howell CY, Hughes MK, Hughes AL. Natural selection on the gag, pol, and env genes of human immunodeficiency virus 1 (HIV-1). Molecular biology and evolution. 1995 Sep 1;12(5):803-13.
[12] Turville SG, Cameron PU, Handley A, Lin G, Pöhlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nature immunology. 2002 Oct;3(10):975.
[13] Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan;373(6510):123.
[14] Loussert-Ajaka I, Brun-Vézinet F, Simon F, Ly TD, Chaix ML, Saragosti S, Couroucé AM, Ingrand D. HIV-1/HIV-2 seronegativity in HIV-1 subtype 0 infected patients. The Lancet. 1994 Jun 4;343(8910):1393-4.
[15] HIV/AIDS [Internet]. Mayo Clinic. Mayo Foundation for Medical Education and Research; 2018 [cited 2019Mar30]. Available from: https://www.mayoclinic.org/diseases-conditions/hiv-aids/symptoms-causes/syc-20373524
[16] Simon V, Ho DD, Karim QA. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. The Lancet. 2006 Aug 5;368(9534):489-504.
[17] World Health Organization. WHO recommendations on the diagnosis of HIV infection in infants and children.
[18] World Health Organization. (2019, March 18). Treatment and care. Retrieved March 30, 2019, from https://www.who.int/hiv/topics/treatment/en/
[19] U.S. National Library of Medicine. (2019, March 04). FDA-Approved HIV Medicines Understanding HIV/AIDS. Retrieved March 30, 2019, from https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-approved-hiv-medicines
[20] WebMD. (2019, February 2). HIV: Antiretroviral Therapy (ART) - Types, Brand Names, How They Work. Retrieved March 30, 2019, from https://www.webmd.com/hiv-aids/aids-hiv-medication#1
[21] Kim Y, Anderson JL, Lewin SR. Getting the “kill” into “shock and kill”: strategies to eliminate latent HIV. Cell host & microbe. 2018 Jan 10;23(1):14-26.
[22] Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012 Jul;487(7408):482.
[23] Archin NM, Kirchherr JL, Sung JA, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, Eron J. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. The Journal of clinical investigation. 2017 Aug 1;127(8):3126-35.
[24] Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS pathogens. 2014 Nov 13;10(11):e1004473.
[25] Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. The lancet HIV. 2014 Oct 1;1(1):e13-21.
[26] Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS pathogens. 2015 Sep 17;11(9):e1005142.
[27] Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, Savic R, Roney J, Hoh R, Solomon A, Piatak M. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. The lancet HIV. 2015 Dec 1;2(12):e520-9.
[28] Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, Emad F, Buckheit III R, McCance-Katz EF, Lai J, Kennedy M. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1–infected adults on antiretroviral therapy. Clinical infectious diseases. 2013 Dec 12;58(6):883-90.
[29] Xing S, Bullen CK, Shroff NS, Shan L, Yang HC, Manucci JL, Bhat S, Zhang H, Margolick JB, Quinn TC, Margolis DM. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. Journal of virology. 2011 Jun 15;85(12):6060-4.
[30] Vibholm L, Schleimann MH, Højen JF, Benfield T, Offersen R, Rasmussen K, Olesen R, Dige A, Agnholt J, Grau J, Buzon M. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clinical Infectious Diseases. 2017 Jun 15;64(12):1686-95.
[31] Ahlenstiel CL, Turville SG. Delivery of gene therapy to resting immune cells for an HIV cure. Current Opinion in HIV and AIDS. 2019 Mar 1;14(2):129-36.
[32] Suzuki K, Hattori S, Marks K, Ahlenstiel C, Maeda Y, Ishida T, Millington M, Boyd M, Symonds G, Cooper DA, Okada S. Promoter targeting shRNA suppresses HIV-1 infection in vivo through transcriptional gene silencing. Molecular Therapy-Nucleic Acids. 2013 Jan 1;2:e137.
[33] Burke BP, Levin BR, Zhang J, Sahakyan A, Boyer J, Carroll MV, Colón JC, Keech N, Rezek V, Bristol G, Eggers E. Engineering cellular resistance to HIV-1 infection in vivo using a dual therapeutic lentiviral vector. Molecular Therapy-Nucleic Acids. 2015 Jan 1;4:e236.
[34] Gama L, Koup RA. New-generation high-potency and designer antibodies: role in HIV-1 treatment. Annual review of medicine. 2018 Jan 29;69:409-19.