Table of Contents
Biological Basis of Sexuality
Biological Basis of Sexuality PowerPoint
Overview of Human Sexuality
Human Sexuality is the way in which we experience and express ourselves as sexual beings. Your awareness of yourself as a female or male is a part of sexuality as well as the capacity to have erotic experiences and responses. Knowledge of gender roles in culture also influences sexuality. Human sexuality is studied by biologists, medical researchers, sociologists and psychologists (Pearson Education, n.d.). All fields make a contribution because sexuality is influenced by the interaction of biological, psychological, social, economic, political, cultural, legal, historical, religious and spiritual factors. Human sexuality is a central aspect of being human throughout life and encompasses sex, gender identities, gender roles, sexual orientation, eroticism, pleasure, intimacy and reproduction. Sexuality is both experienced and expressed in thoughts, fantasies, desires, beliefs, attitudes, values, behaviours, practices, roles and relationships. Sexuality can include all of these dimensions, not all of them are always experienced or expressed (World Health Organization, 2018).
Key Terms
Sexual Orientation: Term describing a person’s sexual, emotional, or romantic attraction as well as the gender of people they are attracted to.
Gender Identity: Person’s psychological identification with a particular gender, rather than their attraction to people.
Monosexual: Attracted to a single gender.
Heterosexual: One who is physically, emotionally and/or romantically attracted to individuals of a gender other than their own.
Homosexual: One who is physically, romantically and/or emotionally attracted to individuals of the same gender with which they identify.
Plurisexual: Attracted to multiple genders.
Pansexual: Attracted to others regardless of sex or gender. This includes attraction to people who identify as male, female, transgender, intersex, third gender, genderqueer, or anything in between.
Queer: Term for all non-heterosexual, non cis-gender identities. For example, a lesbian woman who does not wish to identify as lesbian, can identify as queer.
LGBTQIAP: Lesbian, Gay, Bi, Transgender, Queer/Questioning, Intersex, Asexual, Pansexual. Used to describe the community of people who identify as something other than heterosexual and/or cisgender. More inclusive term than LGBTQ. It is possible to identify with multiple orientations and gender identities.
Androsexuality/Androphilia: Attraction to men or masculinity. Includes heterosexual women and homosexual men.
Aromanticism: People who do not feel a romantic desire. Not the same as asexual, as asexual identify does not rule out possible relationships. Not interested in pursuing either sexual or romantic relationships with others.
Asexual: Does not experience sexual attraction or has little to no desire to engage in sexual activity. Derive no physical pleasure or emotional attachment with a sexual act. Still desire romantic relationships with people of same or other genders.
Demisexuality: Encompasses those who do not feel sexual attraction to others unless they feel emotionally connected to them. Relates most to the formation of committed romantic relationships, but does allow for other types of relationships like sexual relationships to form as well.
Genes and Sexuality
There has been extensive research done to determine if there are genes that lead to different phenotypic traits of sexuality. Most studies have focused on homosexuality in males, mainly because sexuality in females is typically more fluid, and thus males are easier to research. In addition, many researchers have concluded that male homosexuality is genetic and likely passed on from the maternal chromosomes. These conclusions have led to the following two main hypotheses that look into genes for homosexuality in males.
Man-Loving Genes Hypothesis
This hypothesis was first conceived by an Italian researcher named Andrea Camperio-Ciani. He and his colleagues found that there is “evidence for maternally inherited factors favouring male homosexuality and promoting female fecundity” (Camperio-Ciani, Corna, & Capiluppi, 2004). In other words, they hypothesized that women who are very sexually active have a man-loving gene which makes them more sexually attracted to men and results in more offspring. This “Man-loving gene” is inherited by the son through the X chromosome of the mother, and is found to code for attractiveness to males. They believe this gene to be X-linked because of the observation that homosexual sons had more homosexual male relatives on their mother's side of the family compared to their father's. The gene survives as a result of increasing female's fitness, as she is more attracted to males, and therefore creates more offspring (Camperio-Ciani, Corna, & Capiluppi, 2004). In some cases, this gene is passed onto her son and leads to homosexuality. Other supporting evidence for this hypothesis shows that homosexual men have more relatives in general on the mother's side, suggesting her increased fitness again (Camperio-Ciani, Corna, & Capiluppi, 2004). The “Man-loving gene” would be a disadvantage in terms of reproductive fitness for the son, since he will not be passing on his genes to his offspring. However, the researchers believe that the benefit to the mother's fitness outweighs the cost to the son's fitness, so the gene is able to persist.
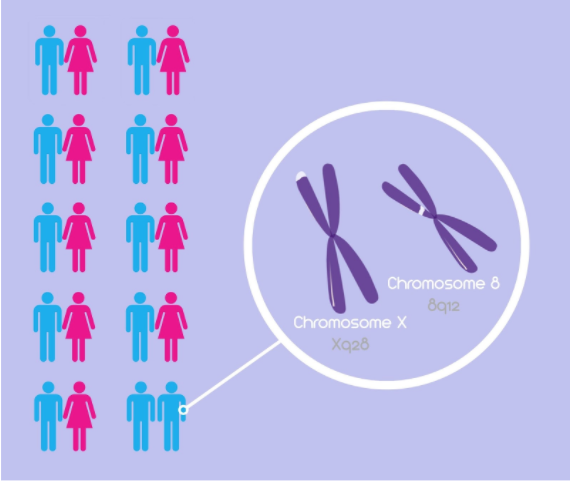
Chromosomes Xq28 and 8
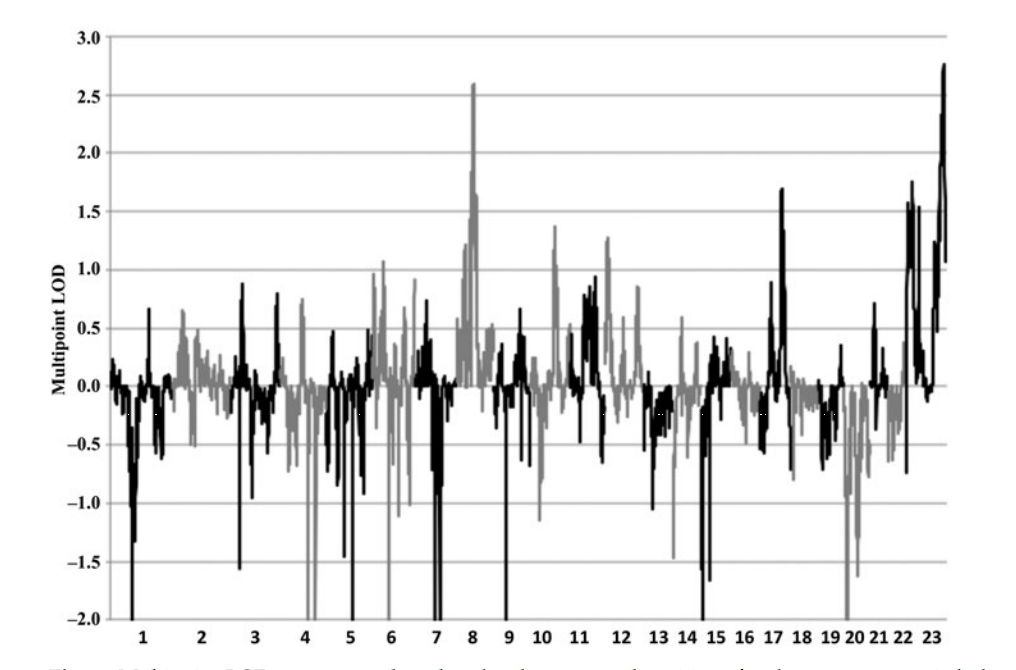
A 2015 study by Sanders et al. looked at chromosomes X and 8 and whether or not they may influence the male phenotypic trait of homosexuality. The study consisted of 351 families, specifically 908 genotyped family members. Of these 908 members, 793 were homosexual brothers, 33 were heterosexual brothers, 49 were mothers and 33 were fathers. Among these individuals, Sanders and colleagues calculated allele frequency estimates using a deCODE genetic mapping system to discover common SNPs among these individuals (Sanders et al., 2015). It was discovered that common SNPs throughout homosexual brothers were found on chromosomes X and 8, specifically regions Xq28 and 8q12 respectively (Figure 1). Therefore, “it is likely that genes contributing to variation in male sexual orientation reside in these regions” (Sanders et al., 2015). Figure 2 illustrates the logarithm of odds (LODs), which shows the likelihood of SNPs on a chromosome being inherited together, and the corresponding chromosome. The two chromosomes of interest - chromosomes 8 and 23 (or chromosome X) - show distinct peaks, indicating that the SNPs on the respective chromosomes will be inherited together. It was important in the study to look at the genotype of mothers and fathers, as it was noted that the Xq28 SNPs were more common among mothers than fathers, suggesting a correlation with the Man-loving genes hypothesis and strengthening the association between homosexuality and the X chromosome (Sanders et al., 2015).
Sexuality and the Environment
Another contributor to human sexuality may be external, environmental factors. While certain environmental theories underlying human sexuality are greatly debated, there is scientific research supporting the notion that environmental factors may play a role in the formation of sexual orientation and preference by altering “normal” patterns of development. Quotations are placed around the word “normal” to emphasize that while every sexual orientation and preference should be accepted and treated equal, the scientific literature often evaluates environmental factors underlying different sexual orientations (I.e. Homosexual, asexual, etc.) in comparison to heterosexuality.
Fraternal Birth Order
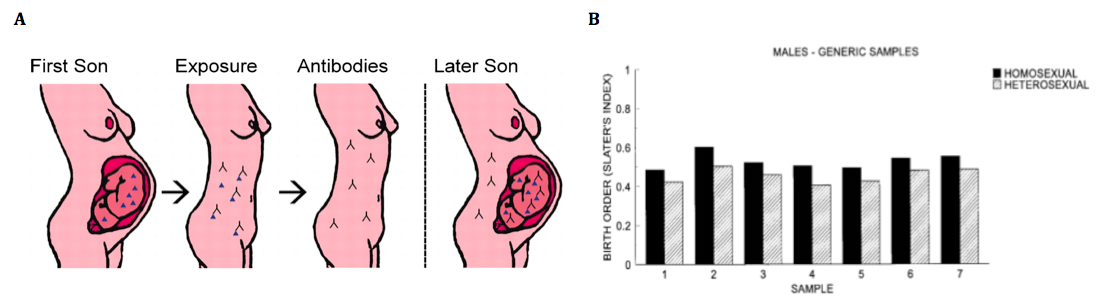
Fraternal birth order, or the order in which male offspring are born to the same mother, has been studied for decades as a possible theory underlying differences in male sexuality. Blanchard (2011) describes that the fraternal birth order hypothesis is based upon the mother’s in-utero immune response changing during each subsequent pregnancy in response to presence of the fetus, specifically the Y chromosome. The Y chromosome in the male fetus produces Y-linked minor histocompatibility antigens (H-Y antigens). H-Y antigens are involved in sexual differentiation in vertebrates by aiding in the masculinization of certain brain structures and ultimately, sex-typical traits. During pregnancy with a male fetus, the mother’s immune system mounts a defense against the H-Y antigens because they are seen as foreign invaders, and therefore produces anti-H-Y antibodies. With each subsequent male pregnancy, the mother’s body will create increasing amounts of anti-H-Y antibodies. A visual representation of this process can be seen in Figure 3A. The effects H-Y antigens normally have on masculinization of male brain structures will be diminished, having an effect on sexual differentiation in the fetal brain and potentially, playing a role in sexual orientation. Based on his review of the literature, Blanchard (2011) summarizes that each older brother increases the odds of homosexuality by close to 30% (Figure 3B). Conversely, having three older sisters does not correlate with increasing probability of homosexuality.
Figure 3: A. After a pregnancy with a male fetus, the mother's body will produce anti-H-Y antigens as an immune response to the H-Y antigens produced by the "invading" male fetus. These anti-H-Y antigens will affect development of a subsequent male fetus. B. Male offspring with younger male siblings are more likely to be homosexual.
Neurohormonal or Neuroendocrine Theory
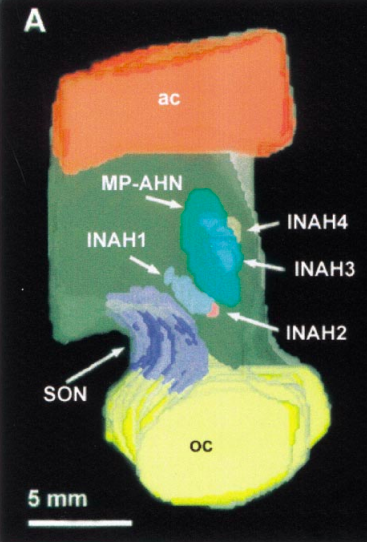
The fraternal birth order phenomenon is evidence for neurohormonal or neuroendocrine theory, which examines the possibility that homosexual individuals may have been exposed to atypical levels of hormones during fetal development. Additionally, this atypical hormone exposure may in turn result in atypical neural differentiation Byne et al. (2001) provided evidence for this theory in their study examining the brain tissue of 34 presumed heterosexual men, 34 heterosexual women, and 14 homosexual men. This study found that the interstitial nucleus of the anterior hypothalamus (INAH-3; Figure 4) occupied a smaller volume in the homosexual versus heterosexual men in their sample. Byne et al. (2001) also found that the INAH-3 contained more neurons and occupied a greater volume in heterosexual males versus heterosexual females, suggesting that the size of INAH-3 in homosexual males showed greater resemblance to that of heterosexual females.
The data presented by Byne et al. (2001) points to a possible relationship between neural development and sexual orientation. However, while the results of this study are significant, it is important to note that the homosexual men in this study were HIV positive, which may have affected brain tissue development and the validity of their conclusions. The authors identified this limitation in the discussion section of their paper. In addition, because the brain tissue was examined post-mortem, the sexual orientation of the subjects was presumed and could not be confirmed.
Maternal Stress Theory
Another external factor that may contribute to differences in human sexuality is stress experiened by a mother while she is pregnant. In one of the first studies exploring maternal stress theory, Bailey, Willerman, and Parks (1991) collected stress-proneness data and retrospective reports of stress during pregnancy from mothers of male and female heterosexuals and homosexuals. Each mother also rated their pregnancy stress for a heterosexual sibling of the subject in order to provide comparison data. The researchers found that mothers of homosexual sons reported no more stress during their pregnancies than mothers of heterosexual sons, indiciating an insignficant correlation between sexual orientation and maternal stress.
Research on maternal stress theory in male offspring is ongoing and the literature contains contradictory data on whether or not maternal stress during pregnancy is significantly correlated with sexual oritentation, particularly male homosexuality. Research on external environmental factors that may influence female development and sexual orientation is exremely limited, with most studies focusing on male instead of female homosexuality.
Sexuality and The Brain
Research has shown that the fetal brain during the intrauterine period initially develops towards the male direction. This direction of development in directly influenced by testosterone on the developing nerve cells. According to this evidence, our gender identity (belonging to the male or female gender) and sexual orientation should be programmed into our brain while still within the womb (Savic, Garcia-Falgueras & Swaab, 2010). However other research have shown that sexual differentiation of genitals does not take place until the first and second months of pregnancy and that the sexual differentiation of the brain does not start until the second half of pregnancy. This means that both of these events can be influenced independently resulting in transsexuality. This also means that in the event of ambiguous sex at birth, the degree of masculinisation by the brain may not be reflected by the genitals (Hughes et al., 2006; Swaab, 2004).
Programmed Gender Identity is Irreversible
There is irrevocable evidence of the irreversibility of programmed gender identity as illustrated by the sad story of John-Joan-John case, or more commonly known as David Reimer. In the 1970s it was believed that a child is born as a clean slate but is subsequently forces into either a male or female identity by society. Even with undeniable evidence that children as young as 2 can accurately identify themselves and others according to their gender, J. Money argued otherwise (Ahmed et al., 2004). With the lack of evidence suggesting that society or external event can affect this process of identifying gender, J. Money postulated argued that Gender identity is incompletely differentiated at birth so as to allow successful assignment of a genetic male as a girl (Savic, Garcia-Falgueras & Swaab, 2010). He further postulated that the assigned gender identity will then differentiates in keeping with the experiences of rearing. He refused to acknowledge that gender imprinting occurs well before birth, and this view led to the decision to reassign an 8 month old Canadian boy into a girl after he lost his penis due to a botched circumcision (Money and Erhardt, 1972). Subsequently the testicles of this child was surgically removed before the age of 17 months, so as to enable biological feminisation. The child was dressed as a girl, and reared as a girl including psychological counselling and administration of oestrogen during puberty. Money believed that the child had developed as a normal female, however it was made clear that later the child reverted back to a male in adulthood (Diamond and Sigmundson, 1997). This supports the existence of early permanent gender printing of brain sex prior to birth.
Sexual Orientation and the Brain
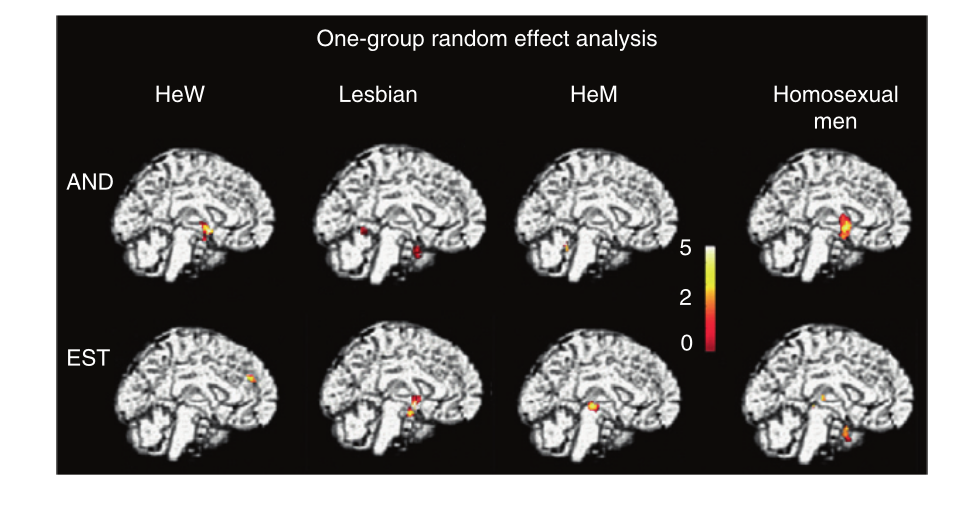
Scientific evidence has shown that sex differences in the brain already exists in the brain by the age of 2. Behavioural differences such as sexual orientation and gender identity are manifestations of this sex differences in the brain. A number of these brain structures have been clinically implicated in sexual orientation. A change of sexual orientation from heterosexual to homosexual have been reported in some patients with Kluver-Bucy syndrome characterised by the lesions of the temporal lobe. This same shift in sexual orientation have been reported in patients with tumours in the hypothalamus and temporal lobe and some of these patients became pedophilic in nature. In an animal study lesions in the pre optic area of the hypothalamus in male rodents induced a shift in sexual orientation (Swaab, 2003). Conversely lesions in the same regions in the female counterparts do not provide a shift in sexual orientation, instead it resulted in increased aggression towards the males. However lesions in the ventromedial hypothalamic nuclei of female rodents resulted in these females approaching other females (Savic, Garcia-Falgueras & Swaab, 2010). Additionally a study by (Zufall, 2005) showed that male rat knockouts lacking Ca-TRP channels, a necessary component of pheromone signal transduction avoid fertile females but approach and mount male rats. There are two implications to this data: first is the importance of intact pheromone signal detection, together with intact hypothalamic transduction in heterosexual behaviour. Second is that the data suggests there exists a sex difference in the mediation of sexual behaviour by the hypothalamic nuclei. The exact functions of these nuclei remain unknown, but appears to be crucial in approaching sexual partners (Schooner & Pfaff, 2007). Another structural and functional difference in the brain related to sexual orientation is the SCN or brain clock. Swaab, found that homosexual men possessed SCN twice as large as heterosexual men. This differences can be experimentally induced in rodents by pharmacologically disturbing the interaction between testosterone and the brain around the time of birth. This is done using dramatise inhibitor 1,4,6-androstatrien-3,17-dione (ATD) during the first month following birth. Another study by LeVay reported smaller volume of hypothalamic nucleus (INAH-3) in homosexual men and heterosexual women alike (Savic, Garcia-Falgueras & Swaab, 2010). Recent studies using functional imaging have showed differences in the activation of the hypothalamus in relation to sexual orientation. It was showed that the hypothalamus of homosexual men as not as responsive as heterosexual men. Through the use of the several pheromones e.g. androstadienone (AND) a similar activation of the hypothalamus was observed in homosexual men and heterosexual women but no hypothalamic response was illicited from heterosexual men (Figure 5) (Savic, Garcia-Falgueras & Swaab, 2010). These observations show that sexual orientation are affected by brain circuitry such as the hypothalamic circuits.
Sexuality and Evolution
There is considerable evidence that human sexual orientation is genetically influenced. So, with as many as 4% of the population identifying as homosexual according to a 2016 poll (Gates, 2017), this begs the questions of question of how homosexuality, a trait that reduces an individual’s reproductive fitness, is maintained at a relatively high frequency. According to Darwin, alleles that are relatively common in a given population that also reduce an individual’s fitness should typically be eliminated from the population through selection. That said, this does not occur with the phenotype of homosexuality and has been a topic of research and debate for several decades.
Kin Selection
It has been previously proposed that homosexuality could be maintained through kin selection. Instead of reproducing directly, homosexual individuals would invest in the children of close relatives through resource provision and child care, thereby increase their kin's chance of survival and reproduction as a result. This would enable them to indirectly pass on their genes through their kin. However, when the kin selection hypothesis of homosexuality was tested by David Bobrow and Michael Bailey (2001) and once again by Rahman and Hull (2005), it was not supported. Among the different measures of kin altruism, Bobrow and Bailey (2001) and Rahman and Hull (2005) used similar variables, such as: familial affinity, willingness to provide financial and emotional resources, and benevolent tendencies (such as the willingness to babysit).
Instead of the anticipated findings of homosexual male relatives providing significantly more support to their closely-related relatives kin, they instead found no significant differences between heterosexual and homosexual men (Bobrow & Bailey, 2001; Rahman & Hull, 2005). These findings contradict the theory of homosexuality being maintained through kin selection.
However, researchers have identified real-world evidence of kin selection within the Samoan population. Researchers have demonstrated that Samoan homosexual men, known locally as fa'afafine (Figure 6), exhibit significantly higher altruistic tendencies toward nieces and nephews compared to Samoan women and heterosexual men. Vasey & VanderLaan (2010) used money given to, and received from, oldest and youngest siblings' sons and daughters as a behavioural assay of kin altruism. This enabled them to measure the altruistic behaviour directed to nieces and nephews from their respective relatives. Once the data had been collected, they compared the results to female relatives and heterosexual male relatives. The results showed that the fa'afafine relatives gave significantly more money, particularly to their youngest sibling’s daughters (Vasey & VanderLaan, 2010). These finds thereby support the kin selection hypothesis for contributing to the maintenance of the phenotype of homosexuality. These three studies also illustrate the ongoing debate with regards to the validity of the kin selection hypothesis with regards to its role in the maintenance of homosexuality.
Pleiotropic Genes
Another explanation for this Darwinian paradox has been proposed by Miller (2000). He proposed the evolutionary explanation for the maintenance of homosexuality is the presence of a compensatory mechanism to counteract the decrease in reproductive success, such as increased fitness in heterosexuals carrying the allele. Miller argues that a gene which produces only homosexuality would be strongly selected against and could not survive; however, a pleiotropic gene for which homosexuality was one of only several mechanisms could potentially survive selection (Miller, 2000). His hypothesis states that the homosexual trait is influenced by a number of pleiotropic genes (genes that have more than one effect): one effect would contribute to homosexuality—resulting in a disadvantage with regards to reproductive success; the other effects could, contrarily, contribute to reproductive success in heterosexuals (Miller, 2000). For example, an increase in the prevalence or intensity of desired traits in sexual partners. Miller postulates that the pleiotropic genes affecting homosexuality would alter male development, resulting in a female-driven development and its associated traits (Miller, 2000).
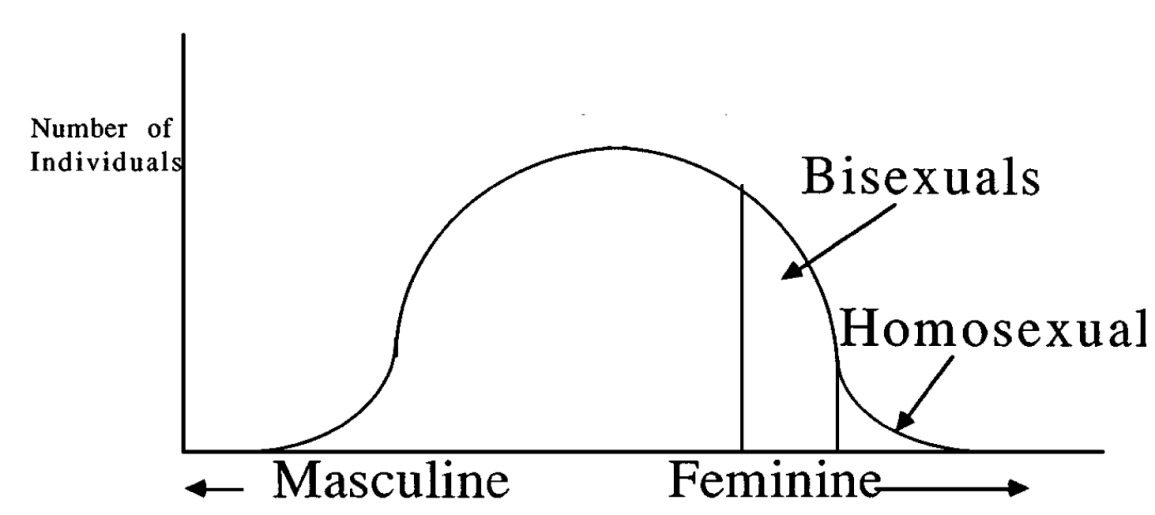
To illustrate this, Miller created a normal curve with masculine and feminine traits on the x-axis and number of individuals on the y-axis (Figure 7). If a male inherits multiple female-driven alleles, he then becomes either homosexual or bi-sexual, depending on the number of alleles inherited. However, these alleles are most often inherited along with other genes that steer the development towards male heterosexuality. In these cases, the effect of the alleles would be increased sensitivity, empathy, tender-mindedness, and kindness (Miller, 2000). Heterosexual individuals (particularly males, in this case) with these traits would make for better caregivers and fathers, and subsequently a more attractive prospective mate (Miller, 2000), thereby resulting in an increase in their fitness. Males who inherit none of these alleles are “hypermasculine” and lack sensitivity and other traits that make them better partners which decreases fitness.
Miller (2000) hypothesizes the occurrence of a similar effect in genes that produce homosexuality in females. However, rather than empathy and kindness, it is believed that this would result in increased willingness to engage in non-committal sexual relations; thereby contributing to a mating advantage (Zietsch et al., 2008). It is in this way that Miller (2000) hypothesizes the alleles for homosexuality have been maintained: through a type of heterozygote advantage.
Zietsch et al. (2008) tested Miller’s theory regarding pleiotropic genes affecting homosexuality and the resulting heterozygote advantage. They conducted a twin study, whereupon they measured and compared sexual orientation, sexual identity, and the number of opposite-sex partners. Sexual identity measured the degree of participants’ masculine versus feminine self-concept using a numerical system; a higher total score reflected a more sex-atypical gender identity (Zietsch et al., 2008). The results in this study showed increased sex-atypical gender identity is more likely characteristic of nonheterosexual males and females; however, in heterosexual individuals that display this trait, there is a significant increase in the number of sexual partners. These findings are consistent with Miller’s hypothesis concerning genes predisposing to homosexuality resulting in an increased mating advantage in their heterosexual counterparts. Lastly, heterosexuals with a nonheterosexual twin reported a higher number of opposite-sex partners compared to heterosexual twin pairs; however, this result was only significant in females (Zietsch et al., 2008). This is also consistent with Miller’s hypothesis wherein genes predisposing to nonheterosexuality in females result in an increased willingness to engage in noncommittal sexual relations. This would result in an increase in the number of sexual partners, thereby increasing the mating advantage observed in heterosexual females with this trait.
Female Sexuality and Evolution
In a female twin study conducted by Burri et al. (2015), monozygotic and dizygotic twins were asked to complete a survey regarding sexual behaviour and sexual orientation, as well as a follow-up survey assessing CGN (childhood gender nonconformity) – a measure for sex typicality. Zygosity of the twins was established using standardized questions about physical similarity and was confirmed by multiplex DNA genotyping in cases of uncertainty. In the first survey, a Kinsey-like scale was used to score sexual attraction, ranging from 1 (only with males, never with females) to 5 (only with females, never males) with the option of “no sexual attraction” for asexual participants. As a measure of fitness, lifetime number of sexual partners was employed by the researchers. The results of the experiment showed low CGN (“masculine”) scores were associated with greater nonheterosexual attractions (score of 2-5 on the scale) and more sexual partners (Burri et al., 2015). Additionally, heterosexual women that scored “masculine” CGN were also associated with more sexual partners (Burri et al., 2015). Overall, the researchers concluded that CGN was associated with female homosexuality, more masculine heterosexual women had greater numbers of lifetime sexual partners, and that these relationships are influenced by common genetic factors via a single shared phenotype with a heritability of 40% (Burri et al., 2015). These results are consistent with the theory that the genetic variation underlying this fitness-reducing trait—female homosexuality—might be maintained through its reproductive benefits (higher number of lifetime partners) in heterosexual individuals. These results thereby corroborate the previously-proposed Miller (2000) hypothesis.
References
Ahmed, S. F., Morrison, S., & Hughes, I. A. (2004). Intersex and gender assignment; the third way? Archives of Disease in Childhood, 89, 847–850.
Avery, A., Chase, J., Johansson, L., Litvak, S., Montero, D., & Wydra, M. (2007). America's Changing Attitudes toward Homosexuality, Civil Unions, and Same-Gender Marriage: 1977-2004. Social Work, 52(1), 71-79. http://dx.doi.org/10.1093/sw/52.1.71
Bailey, J., Dunne, M., & Martin, N. (2000). Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. Journal Of Personality And Social Psychology, 78(3), 524-536. http://dx.doi.org/10.1037//0022-3514.78.3.524
Bailey, J. M., Willerman, L., & Parks, C. (1991). A test of the maternal stress theory of human male homosexuality. Archives of Sexual Behavior, 20(3), 277-293. Retrieved from https://www.researchgate.net/profile/J_Bailey2/publication/288653604_A_test_of_the_maternal_stress_theory_of_human_male_homosexuality/links/5694286f08ae425c68963693.pdf
Blanchard, R. (2001). Fraternal birth order and the maternal immune hypothesis of male homosexuality. Hormones and Behavior, 40, 105-114. https://doi.org/10.1006/hbeh.2001.1681
Bobrow, D., & Bailey, J. (2001). Is male homosexuality maintained via kin selection?. Evolution And Human Behavior, 22(5), 361-368. http://dx.doi.org/10.1016/s1090-5138(01)00074-5
Burri, A., Spector, T., & Rahman, Q. (2015). Common Genetic Factors among Sexual Orientation, Gender Nonconformity, and Number of Sex Partners in Female Twins: Implications for the Evolution of Homosexuality. The Journal of Sexual Medicine, 12(4), 1004–1011.
Byne, W., Mattice, L.A., Lasco, M.S., Kemether, E.K., Edgar, M.A., Morgello, S., Buchsbaum, M.S.,… Liesl, B. (2001). The Interstitial Nuclei of the Human Anterior Hypothalamus: An Investigation of Variation with Sex, Sexual Orientation, and HIV Status. Hormones and Behavior, 40, 86-92. doi:10.1006/hbeh.2001.1680
Camperio-Ciani, A., Corna, F., & Capiluppi, C. (2004). Evidence for maternally inherited factors favouring male homosexuality and promoting female fecundity. Proceedings of the Royal Society of London B: Biological Sciences, 271(1554), 2217-2221.
Diamond, M., & Sigmundson, K. (1997). Sex reassignment at birth. Long-Term review and clinical implications. Archives of Pediatrics & Adolescent Medicine, 151, 298–304.
Gates, G. (2017). In U.S., More Adults Identifying as LGBT. Gallup.com. Retrieved 13 November 2017, from http://news.gallup.com/poll/201731/lgbt-identification-rises.aspx
Hughes, I. A., Houk, C., Ahmed, S. F., et al. (2006). Consensus statement on management of intersex disorders. Archives of Disease in Childhood, 91, 554–563.
Kunzig, R. (2008, May/June). Finding the switch: homosexuality may persist because the associated genes convey surprising advantages on homosexuals' family members. Psychology Today Magazine. Retrieved from https://www.psychologytoday.com/us/articles/ 200805/finding-the-switch
Miller, E. M. (2000). Homosexuality, birth order, and evolution: Toward an equilibrium reproductive economics of homosexuality. Archives of sexual behavior, 29(1), 1-34.
Money, J., & Erhardt, A. A. (1972). Man and woman, boy and girl: The differentiation and dimorphism of gender identity from conception to maturity. Baltimore, MD: Johns Hopkins University Press.
Pearson Education. (n.d.). What is Human Sexuality? Retrieved from https://www.pearsonhighered.com/assets/samplechapter/0/2/0/5/0205909469.pdf
Rahman, Q., & Hull, M. (2005). An Empirical Test of the Kin Selection Hypothesis for Male Homosexuality. Archives Of Sexual Behavior, 34(4), 461-467. http://dx.doi.org/10.1007/s10508-005-4345-6
Sanders, A. R., Martin, E. R., Beecham, G. W., Guo, S., Dawood, K., Reiger, G., . . . Bailey, J. M. (2015). Genome-wide scan demonstrates significant linkage for male sexual orientation. Psychological Medicine, 45, 1379-1388. https://doi.org/10.1017/S0033291714002451
Savic, I., Garcia-Falgueras, A., & Swaab, D. (2010). Sexual differentiation of the human brain in relation to gender identity and sexual orientation. Sex Differences In The Human Brain, Their Underpinnings And Implications, 41-62. http://dx.doi.org/10.1016/b978-0-444-53630-3.00004-x
Schober, J. M., & Pfaff, D. (2007). The neurophysiology of sexual arousal. Best Practice & Research. Clinical Endocri nology & Metabolism, 21, 445–461.
SexInfo Online. (2017, March 14). Overview of Sexual Orientations | SexInfo Online. Retrieved from http://www.soc.ucsb.edu/sexinfo/article/overview-sexual-orientations
Suh, M. J. (2015, April 18). The truth about the “gay gene.” Innovation: Princeton Journal of Science and Technology. Retrieved from http://princetoninnovation.org/magazine/2015/04/18/truth-gay-gene/
Swaab, D. F. (2003). The human hypothalamus. Basic and clinical aspects. Part I: Nuclei of the hypothalamus. In M. J. Aminoff, F. Boller, & D. F. Swaab (Eds.), Handbook of clinical neurol ogy (pp. 127–140). Amsterdam, The Netherlands: Elsevier.
Swaab, D. F. (2004). The human hypothalamus. Basic and clinical aspects. Part II: Neuropathology of the hypothalamus and adjacent brain structures. In M. J. Aminoff, F. Boller, D. F. Swaab (Eds.), Handbook of clinical neurology (pp. 193–231). Amsterdam, The Netherlands: Elsevier.
World Health Organization. (2018). Defining sexual health. Retrieved from http://www.who.int/reproductivehealth/topics/sexual_health/sh_definitions/en/
Vasey, P., & VanderLaan, D. (2010). Monetary exchanges with nieces and nephews: a comparison of Samoan men, women, and fa'afafine. Evolution And Human Behavior, 31(5), 373-380. http://dx.doi.org/10.1016/j.evolhumbehav.2010.04.001
Zietsch, B. P., Morley, K. I., Shekar, S. N., Verweij, K. J. H., Keller, M. C., Macgregor, S., … Martin, N. G. (2008). Genetic factors predisposing to homosexuality may increase mating success in heterosexuals. Evolution and Human Behavior, 29(6), 424–433. https://doi.org/10.1016/j.evolhumbehav.2008.07.002
Zufall, F. (2005). Connexins and olfactory synchronicity: Toward the olfactory code. Neuron, 46, 693–694.