Table of Contents
Addison's Disease
Presentation 2: Addison's Disease Powerpoint File
Introduction
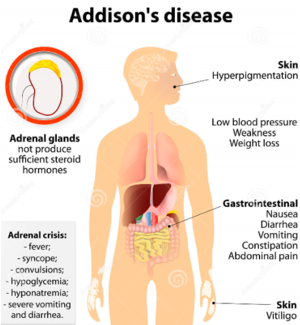
<style justify>Addison’s disease is an endocrine disorder that occurs when the adrenal glands do not produce enough of the hormones cortisol and in some cases aldosterone, due to the destruction or dysfunction of the adrenal cortex (National Institute of Diabetes and Digestive and Kidney Diseases, 2014). This disease may also be known as hypoadrenalism, hypocortisomalism or chronic or severe adrenal insufficiency. The adrenal glands are responsible for endocrine secretions in the body. In those with Addison’s disease, the adrenal glands are destroyed, and hence, nonfunctional (Neto & Carvalho, 2014). Hence, they are unable to produce the steroid hormones. This disease can be classified as primary or secondary, of which secondary adrenal insufficiency is more common (Neto & Carvalho, 2014). Addison's disease is often due to autoimmune causes is the developed world and tuberculosis in the developing part of the world (Adam & Dixon, 2008).
This condition is now highly treatable, if the proper treatment and management steps are taken. An adrenal crisis, also known as an Addisonian crisis, is a medical emergency that occurs when the body has insufficient levels of the hormone cortisol. This is usually triggered by high levels of stress, and can result in death if not treated (National Institute of Diabetes and Digestive and Kidney Diseases, 2014). </style>
Figure 1: Overview of Addison's Disease
Image from: http://www.organsofthebody.com
<br>
Epidemiology
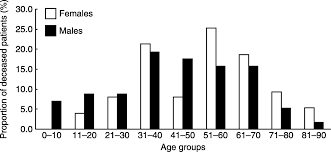
Figure 2: Age at diagnosis for deceased Addison patients.
Image from: http://www.eje-online.org/content/160/2/233/F2.expansion.html </style>
<style justify>The frequency rate of Addison's disease is approximately one in 100,000 (MedicineNet, 2007). In translating this to a population of millions, the prevalence is roughly 40-60 cases per 1 million (Odeke & Nagelberg, 2001). This disease typically presents in adults between 30 and 50 years of age (Volpe, 1990). It is also more common in females and children than it is in men. These findings are displayed in Figure 2. Furthermore, research has shown no predispositions based on ethnicity (Odeke & Nagelberg, 2001).
To find out if the incidence of Addison’s disease was changing around the world, researchers carried out an epidemiological study. In their results, they yielded a prevalence of 140 per million, whereas estimated prevalence in the past had varied from 29 to 117 million (Lovas & Husebye, 2002). These results supported the hypothesis of an increasing incidence of autoimmune adrenal insufficiency. Additionally, a higher prevalence of Addison’s disease was found in Norway than had been previously reported anywhere in the world (Lovas & Husebye, 2002).
</style>
<br>
Signs and Symptoms
Table 1: Michels & Michels, 2014
</style>
<style justify>The symptoms of Addison’s disease begin gradually. As the disease progresses and more of the adrenal glandular tissue is destroyed, symptoms tend to worsen from mild to severe. This can take up to a few years, and because these symptoms are generalized and similar to many other health conditions, Addison’s disease may not even be diagnosed until the patient suffers from an Addisonian crisis (Sarkar et al., 2012).
Early symptoms of Addison’s include worsening fatigue, generalized lethargy and muscle weakness, loss of appetite, leading to weight loss, hypotension, and low mood and irritability. Oftentimes, patients also present with hyponatremia and hyperkalemia (Sarkar et al., 2012). This is classified as pathological when serum sodium concentrations drop below 135 mmol/L (Simon, 2016) and serum potassium concentrations are higher than 5.5 mEq/L (Lederer, 2016). Although uncommon, hypoglycaemia can occur, which leads to common symptoms experienced with low blood sugar levels, like dizziness and, in extreme cases, loss of consciousness (Sarkar et al., 2012). The patient also complains of gastrointestinal upset, nausea, vomiting, diarrhea, dehydration, and a peculiar craving for salt. Dehydration and salt cravings are linked to the drop in aldosterone levels (Sarkar et al., 2012). Table _ gives a brief overview of the major symptoms experienced by patients, along with the prevalence of each one.
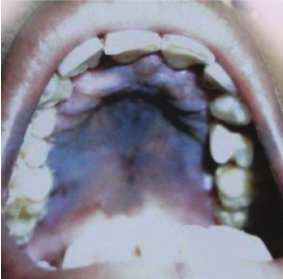
As the disease progresses, early symptoms worsen, along with the appearance of new symptoms. The most characteristic late symptom of Addison’s is generalized hyperpigmentation of the skin and mucosal surfaces (Sarkar et al., 2012). These include any sun-exposed skin and over pressure points, like elbows and knees. Observable hyperpigmentation of mucosal surfaces presents as intraoral and buccal pigmentation, as shown in Figure 3 (Sarkar et al., 2012). These are due to the abnormal interaction of ACTH with the melanocortin-1 receptor on keratinocytes. In autoimmune Addison’s, hyperpigmentation is seen in association with vitiligo due to the destruction of melanocytes (Sarkar et al., 2012). Because these symptoms are slow to progress and very generalized, an event of illness or accident can spontaneously worsen the condition and lead to an Addisonian crisis. The crisis is characterized by sudden, penetrating pains in the lower back, abdomen, or legs, combined with severe vomiting and diarrhea (Sarkar et al., 2012). This leads to severe dehydration, drastic drop in blood pressure, and loss of consciousness. Other associated symptoms include pale, cold, and clammy skin, sweating, and rapid, shallow breathing (Sarkar et al., 2012). It is important that a patient suffering from an Addisonian crisis seeks emergency medical assistance. If left untreated, it can lead to shock, seizures, coma, or even death (Sarkar et al., 2012).
Figure 3: Sarkar et al., 2012
</style>
<br>
Diagnosis
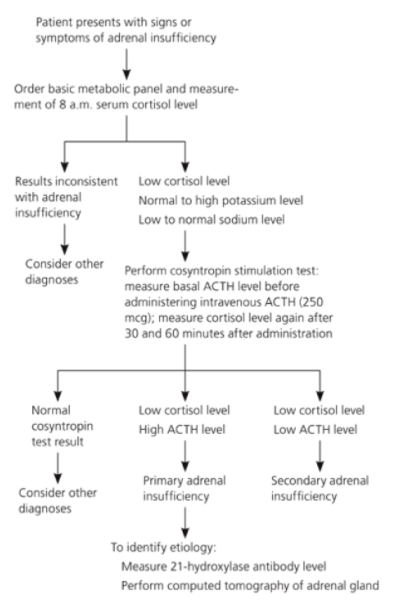
Figure 4: Michels & Michels, 2014
</style>
<style justify>Due to the subtlety and non-specificity of Addison’s disease, early diagnosis can be difficult. Presentation of the symptoms relates to the degree of cortisol, mineralocorticoid, and adrenal androgen deficiency (higher degree of deficiency equates to more severe symptoms) (Michels & Michels, 2014). There are three main types of tests that are used in combination to diagnose Addison’s:
1. Metabolic Tests
A metabolic panel is a complete blood analysis that serves as a broad-spectrum medical screening tool. This type of test provides a rough evaluation of kidney and liver function, and the balance of serum electrolytes and fluid, using a blood sample drawn from the patient in question (2005). Along with this, the physician also requests serum cortisol levels to be tested early in the morning (8 a.m.). Low cortisol levels are the most evident clinical feature of Addison’s (Michels & Michels, 2014). If the results demonstrate low serum cortisol concentrations (<83 nmol/L), hyponatremia (<135 mmol/L) (Simon, 2016), and hyperkalemia (>5.5 mEq/L) (Lederer, 2016), the physician then requests a cosyntropic stimulation test (Michels & Michels, 2014). This type of test measures cortisol response to ACTH levels. An abnormal rise in ACTH levels (>11 pmol/L) is indicative of underlying cortisol deficiency (Michels & Michels, 2014). Therefore, this type of test is the first-line test for confirming diagnosis of Addison’s disease. Initially, plasma levels of cortisol, ACTH, aldosterone, and renin should be measured, followed by administration of 250 mcg of synthetic ACTH (Michels & Michels, 2014). After 30-60 minutes of intravenous ACTH administration, serum cortisol levels are measured again (Michels & Michels, 2014). Less than 497-552 nmol/L peak cortisol levels in the blood is diagnostic for adrenal insufficiency (Michels & Michels, 2014). A summary of the metabolic panel is shown in Figure 4.
2. Immunologic Tests
The next test that supplements the cosyntropin stimulation test is an immunologic assay that measures the levels of 21-hydroxylase antibody in the blood (Michels & Michels, 2014). The enzyme 21-hydroxylase is critical from the synthesis of cortisol in the adrenal cortex. High levels of 21-hydroxylase antibodies indicate autoimmune reactivity, which is characteristic of Addison’s disease (Michels & Michels, 2014).
3. Imaging
Although radiographic imaging is helpful in diagnosing Addison’s, it is important to make the biochemical diagnosis prior to this. Computed tomography (CT) scans can demonstrate small adrenal glands in patients with autoimmune adrenal destruction (Michels & Michels, 2014). When Addison’s is comorbid with other conditions, like tuberculosis, the CT scan may show calcification of the adrenal glands. If Addison’s disease is the result of tumors, malignancies, or metastases, the CT scan shows masses or hemorrhaging of the adrenal glands (Michels & Michels, 2014). These tests supplement other metabolic tests. It is critical to note that a CT scan alone is not sufficient to diagnose Addison’s disease.
</style>
<br>
Adrenal Glands & Hormone Synthesis
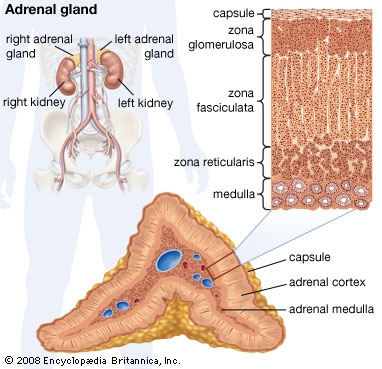
<style justify> The adrenal glands produce a variety of these hormones, that balance and regulate various functions in the body. Located above the kidneys, each gland has an outer (adrenal) cortex which produces steroid hormones and an inner medulla (Fujieda & Tajima, 2005). The adrenal cortex is divided into three zones including: zona glomerulosa, the zona fasciculata and zona reticularis. The adrenal cortex produces three main types of steroid hormones; mineralocorticoids, glucocorticoids and androgens (Fujieda & Tajima, 2005).
Figure 5: The three zones of the adrenal cortex.
Image from https://www.britannica.com/science/adrenal-gland.
1. Mineralocorticoids like aldosterone are produced in the zona glomerulosa and help in the regulation of blood pressure and electrolyte balance. Low aldosterone levels leads to a imbalanced regulation of salt and water levels by the kidneys causing blood pressure to drop (Fujieda & Tajima, 2005).
2. Glucocorticoids like cortisol and corticosterone, are synthesized in zone fasciculata and their functions include the regulation of metabolism and immune system suppression (Fujieda & Tajima, 2005). Cortisol is a steroid hormone secreted to help the body respond to stress. It also helps regulate the body’s use of protein, carbohydrates and fats and maintains blood pressure and controls inflammation. Cortisol is essential to health and the amount produced by the adrenal gland’s is precisely balanced. Like several other hormones, cortisol is regulated by the HPA axis (de Silva & Wijesiriwardene, 2009).
3. Androgens: the innermost layer of the cortex, the zona reticularis, produces androgens that are converted to fully functional sex hormones (de Silva & Wijesiriwardene, 2009).
The biosynthesis of these hormones depends on the available plasma cholesterol. Most of the cholesterol comes from low density lipoproteins (LDL). The uptake of LDL-cholesterol by adrenal cells is promoted by ACTH. Cholesterol is converted to the steroid molecule, pregnenolone through a series of biochemical reactions. Pregnenolone is then converted to mineralocorticoids, glucocorticoids, and sex steroids by a complex of biochemical events activated by enzymatic actions (Miller & Bose, 2011).
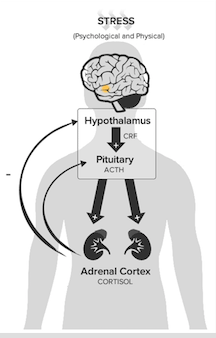
Hypothalamic–Pituitary–Adrenal axis (HPA axis) The HPA Axis is the body’s central stress response system and involves the interactions of 3 endocrine glands: the hypothalamus, the pituitary gland and the adrenal glands. CRH (corticotropin-releasing hormone) is a peptipe hormone released from the hypothalamus which stimulates ACTH (adrenocorticotropic hormone) from the anterior pituitary gland, which then stimulates the production and release of CORT (cortisol) from the adrenal cortex. Systemic levels of cortisol act in a negative feedback system throughout the HPA (hypothalamic–pituitary–adrenal) axis, wherein increased levels of cortisol signal the hypothalamus to stop secretion of CRH, and the anterior pituitary to stop secretion of ACTH. (Alshuler, 2016).
Figure 6: The hypothalamus, pituitary gland & adrenal glands make up the HPA axis.
Image from http://www.integrativepro.com/Resources/Integrative-Blog/2016/The-HPA-Axis. </style>
<br>
Pathophysiology
<style justify>
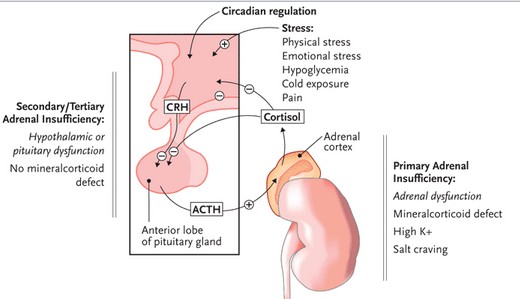
Addison’s disease can manifest in one of two ways, either as primary adrenal insufficiency, or secondary adrenal insufficiency.
Figure 7: Comparing Primary Adrenal Insufficiency to Secondary/Tertiary Adrenal Insufficiency.
Image from: http://www.prn.org/index.php/complications/article/hiv_osteoporosis_adrenal_insufficiency_85.
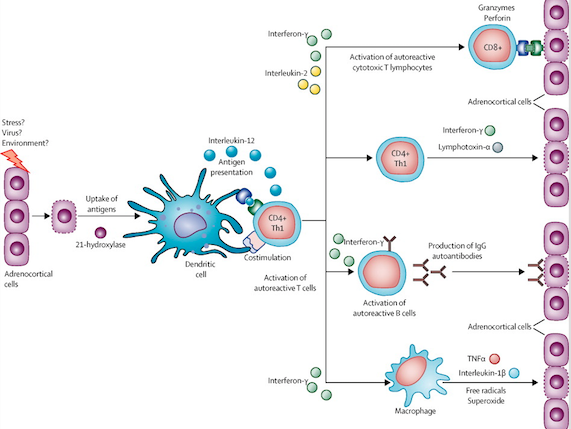
Primary adrenal insufficiency is characterized by the destruction of adrenal glands through the production of antibodies that destroy the adrenal cortex cells. The cause of autoimmune Addison disease is complex and clearly understood. Changes in multiple genes are associated with the development of this condition (Charmandari, Nicolaides & Chrousos, 2014). The most commonly associated gene changes are found in the human leukocyte antigen (HLA) complex. The HLA complex helps the immune system distinguish the body's own proteins from proteins made by foreign invaders. HLA proteins are expressed on the surface of antigen-presenting cells and display peptides (both self and non-self) to T cells for pathogenic surveillance by the immune system. Autoimmune Addison disease is most commonly attributed to a variant of the HLA-DRB1 gene called HLA-DRB1*04:04 located on chromosome 6 position 21.32 (“HLA DRB 1 gene”, 2014) An immune response is triggered by a normal adrenal gland protein called 21-hydroxylase which plays a key role in producing cortisol (Charmandari, Nicolaides & Chrousos, 2014). Circulating antibodies against 21-hydroxylase can be detected in around 85% of patients (Mitchell & Pearce, 2012). Steroid 21-hydroxylase autoantibodies have an IgG isotype and target the carboxy terminal of this enzyme thus damaging the adrenal glands by preventing cortisol production Mitchell & Pearce, 2012). A shortage of cortisol leads to appearance of AD symptoms. As shown in figure, the molecular immunopathogenesis of primary adrenal insufficiency initially involves sporadic adrenocortical cell apoptosis or necrosis which leads to activation of dendritic cells through presentation of peptides derived from 21-hydroxylase and activation of dendritic cells. Dendritic cells transport and present adrenocortical antigens to CD4-positive T-helper-1 (Th1) cells which also lead to auto-reactive B cells producing anti-21-hydroxylase antibodies. The figure shows several ways in which progressive destruction of the adrenal cortex is mediated including activation of autoreactive cytotoxic T lymphocytes , activation of autoreactive B cells, and or macrophages that target the destruction of adrenocortical cells (Charmandari, Nicolaides & Chrousos, 2014).
Figure 8: Molecular Immunopathogenesis of Primary Adrenal Insufficiency.
Image from: (Charmandari, Nicolaides & Chrousos, 2014)
Secondary/tertiary adrenal insufficiency results from hypothalamic or pituitary dysfunction which leads to decreased stimulation of the adrenal cortex (Pavlaki & Magiakou, 2011). This is typically caused by a lack of ACTH attributed to pituitary tumors, head injuries affecting the hypothalamus or even sudden withdrawal of steroid therapy. When steroids are taken to treat other medical conditions acting in the same way cortisol does , the brain stops sending the ACTH signal to the adrenals to make the body’s own cortisol. Once steroids are stopped, it can take a long time for the brain to resume sending this signal, which causes adrenal insufficiency (Pavlaki & Magiakou, 2011).
</style>
<br>
Etiology
<style justify> A number of genetic and environmental factors can lead to the development of Addison's disease. To help understand this condition better, the various etiologies of Addison’s disease can be grouped into three categories:
1. Adrenal Dysgenesis involves the inadequate development of the adrenal glands. This can be attributed to a number of reasons such as the deletion of DAX-1 during the development of adrenal glands (Mitchell & Pearce, 2012).
2.Impaired Steroidogenesis involves normally developed glands that are biochemically unable to produce cortisol. This include defects in cholesterol biosynthesis typically seen in Smith-Lemli-Opitz syndrome or and mutations in genes encoding steroidogenic enzymes as seen in congenital adrenal hyperplasia (Mitchell & Pearce, 2012).
3. Adrenal Destruction involves pathological conditions that lead to glandular damage. The most common cause of AD is autoimmune adrenal destruction (Mitchell & Pearce, 2012). Long lasting infections, such as tuberculosis (attributed as the second leading cause of AD), HIV and some fungal infections can also have of target effects and impact the normal functioning of the adrenal glands(Brooke & Monson, 2009). Metastatic malignancy of the lungs, breasts and kidneys that spread to adrenal glands can also cause insufficiencies seen with Addison's (Brooke & Monson, 2009). </style>
<br>
Treatment
Addison’s disease is incurable, however there are chronic hormone replacement therapies that work to replenish the body of its missing corticosteroids; cortisol and aldosterone.
Figure 9: Oral tablets which replenish bodily hormones.
Image from: http://www.mcguffmedical.com, http://www.drsfostersmith.com </style>
Oral Medications
<style justify> Oral tablets can be taken to compensate for the lack of cortisol and aldosterone in the body. Cortisol can be replenished with hydrocortisone, cortisone acetate, or prednisone tablets and is usually accompanied by fludrocortisone tablets to replenish aldosterone (Mayo Clinic, 2015).
Hydrocortisone and cortisone acetate are typically taken two to three times daily, in which the first dose would be taken in the morning and the last dose to be taken a few hours before bedtime (Husebye et al., 2013). Hydrocortisone tablets come in 2.5, 10 and 20 mg, whereas, cortisone acetate comes in 5 and 25 mg tablets (Husebye et al., 2013). Prednisone is suggested for those patients who experience energy fluctuations throughout the day, and the recommended dose is between 1-5 mg, once or twice daily (Husebye et al., 2013). Of these three drug therapies, studies indicate that one is no more effective than another (Husebye et al., 2013).
An over-replacement of cortisol with the use of these medications may lead to side effects including weight gain, peripheral edema and/or insomnia (Husebye et al., 2013). Consumption times and prescribed doses may have to be adjusted depending on the individual’s lifestyle, energy levels and sleeping habits (Husebye et al., 2013). In particular, if an individual works night shift their morning dose of medication should be taken before the commencement of their shift. If the patient is on other medications or has certain foods in their diet, the daily dosage for hydrocortisone or cortisone acetate may need adjusting (Husebye et al., 2013). For example, a greater dose may be needed if the individual is taking anti-epileptic or antifungal drugs, and a lesser dose may be needed if consuming grapefruit juice or licorice (Husebye et al., 2013).
Fludrocortisone should be taken in order to replenish aldosterone, which is essential for the distribution and absorption of sodium and maintenance of blood pressure in the body (Husebye et al., 2013). A daily morning dose of a 0.05 or 0.1 mg tablet is recommended, but may be modified depending on the fluid and electrolyte balance or age of the individual (Husebye et al., 2013). Some forms of drugs, such as diuretics, should be avoided when taking fludrocortisone, since both are associated with blood pressure and electrolyte adjustment (Husebye et al., 2013). </style>
Injection Alternatives
<style justify> Since some individuals experience nausea leading to vomiting as a symptom, intravenous or intramuscular cortisol injections can replace the oral tablets (Mayo Clinic, 2015).
It is strongly recommended that individuals with the disease carry a cortisol injection kit with them in case of Addisonian/Adrenal Crisis (Canadian Addison Society, 2017). In the appropriate hospital setting, receiving an intravenous dose of hydrocortisone and dextrose sugar in saline solution would be an ideal treatment for the adrenal crisis (Mayo Clinic, 2015). Otherwise, the individual or family member can follow the appropriate instructions to inject the hydrocortisone intramuscularly into a large muscle, typically the thigh (quadricep muscle) (Canadian Addison Society, 2017).
Figure 10: Injection kit should be carried on the person in case of Addisonian crisis.
Image from: http://www.addisons.org
Figure 11: Intramuscular injection into a large muscle is usually recommended; typically the thigh.
Image from: www.addisonsociety.ca
Considerations
Along with cortisol and aldosterone, individuals can also be deficient in androgens (Michels & Michels, 2014). This is more of a concern for women considering most of their androgen production is from the adrenals, as opposed to males who can be provided with androgen from the testes (Michels & Michels, 2014). Women can have the androgens replenished in their body by taking dehydroepiandrosterone (DHEA) in tablet form once daily (Michels & Michels, 2014).
If a patient with Addison’s disease has to partake in a medical procedure, such as surgery, it is essential for the patient to increase the amount of cortisol and aldosterone medication they are taking, in order for the body to cope with the increased stress (Husebye et al., 2013). Physicians will recommend increases in hydrocortisone doses preoperatively and postoperatively and the regimen with vary depending on the severity of the medical procedure (Husebye et al., 2013). For example, an individual receiving a minor surgery or major dental surgery will be recommended to take a 100 mg hydrocortisone intramuscular injection prior to going under anaesthesia, and also double their oral dose for 24 hours after the procedure (Husebye et al., 2013).
An increase in hydrocortisone, salt and fluid is also necessary if an individual with Addison’s disease wants to partake in vigorous exercise (Husebye et al., 2013). Since the individual will be sweating, the fluids and electrolytes will need to be replenished to maintain homeostasis of the body (Husebye et al., 2013).
Continuous Subcutaneous Hydrocortisone Injection versus Conventional Oral Hydrocortisone Replacement
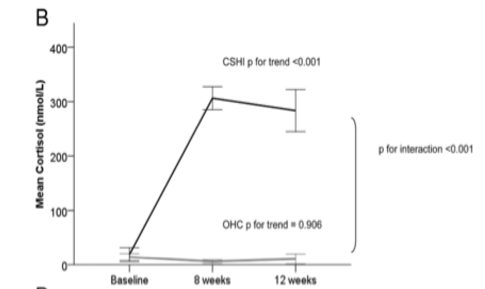
Oral tablets, intravenous injections and intramuscular injections are current hormone replacement therapies for Addison’s Disease, however, there have been many clinical trials considering continuous subcutaneous hydrocortisone infusions (CSHI) as a better treatment option. A 2014 study investigated the efficacy of CSHI versus conventional oral hydrocortisone (OHC) replacement and the subcutaneous infusions showed improvements in normalization of ACTH and cortisol levels and improvements in quality of life (Oksnes et al., 2014). As shown in figure 12, the infusions got the morning blood cortisol levels back to normal ranges as opposed to the oral tablets (Oksnes et al., 2014). Cortisol levels should be high in the morning and the infusions reestablished this to the normal range (Oksnes et al., 2014). </style>
Figure 12: Morning cortisol levels in the CSHI condition are up to normal circadian levels; OHC condition levels are still low.
Image from: (Oksnes et al., 2014)
<br>
Prognosis
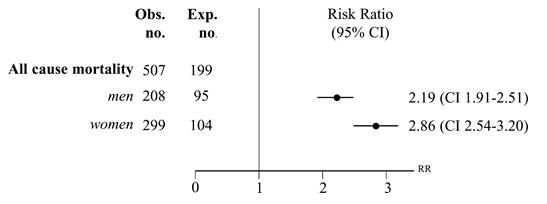
Figure 13: All-cause mortality in Addison's patients, between 1987-2001, in Sweden.
Image from: Bergthorsdottir et al., 2006 </style>
<style justify> The average Addison’s patient can anticipate a fairly good outcome, if the disease is treated adequately. A relatively normal quality of life can be expected for these patients (NHS Choices, 2017).
However, a recent, population-based study of mortality in Addison’s patients conducted by Bergthorsdottir et al. revealed that the risk ratio for death was more than 2-fold higher in patients with Addison’s disease. In this study, 1675 patients who were diagnosed with primary adrenal insufficiency, were followed-up for an average of 6.5 years. 507 patients died during the study period. The expected number of mortalities was 199 (Bergthorsdottir et al., 2006).
This increased rate of mortality in Addison’s patients was attributed to cardiovascular, malignant, and infectious diseases, or improper management or treatment of the disease (Bergthorsdottir et al., 2006). For example, excess glucocorticoid exposure from medication may induce hypertension or diabetes mellitus, which are strong independent risk factors for cardiovascular disease (Bergthorsdottir et al., 2006). This excess mortality may be prevented by adequately educating patients and health care workers.
Furthermore, patients tend to be at greatest risk of mortality when also afflicted with diabetes mellitus. In an observational cohort study by Chantzichristos et al., individuals afflicted with either type 1 or 2 diabetes and Addison’s were compared against a group of diabetic patients without the latter (Chantzichristos et al., 2016).
In this study, it was determined that patients afflicted with both diabetes mellitus (both type 1 or 2) and Addison’s Disease have an almost 4-fold increased risk of death compared to matched controls, despite similar glycemic control at baseline (Chantzichristos et al., 2016). This additional risk of mortality illuminates that a complex clinical picture of patients with diabetes and Addison’s exists, and improvement in management is required. </style>
<br>
Management
Figure 14: Medical bracelets help convey information.
Image from: http://www.myidentitydoctor.com </style>
<style justify> Along with taking prescribed daily medications, management of Addison’s disease involves a sufficient amount of self-maintenance. Taking charge of your healthcare is most important in managing Addison’s, and this involves appointments with an endocrinologist every 6-12 months. During these meetings, physicians may adjust medication and review progress of the patient (NHS Choices, 2017). Falling behind on taking medication or managing the disease with care can result in adrenal crisis.
Important daily management rituals of Addison’s patients include four main steps:
1. Managing Medication
- collecting repeat prescriptions
- keeping spare medication on their person, or packing extra - especially if they are traveling
- remembering to take medication daily (NHS Choices, 2017)
- falling behind on taking medication or not managing the disease with care can result in Addisonian crisis
2. Injectable forms of medication
- patients may benefit from a needle, syringe or injectable form of corticosteroids in case of an emergency or an adrenal crisis — It is important to request this from the physician (Mayo Clinic, 2017)
3. Doctor-Patient relationship
- an ongoing line of communication with the doctor about medication dosage and timing (Mayo Clinic, 2017)
- scheduling regular appointments with the endocrinologist every 6-12 months, as well as the GP, to adjust medication and review progress
4. Providing information to others
- keeping friends, family, and colleagues aware of the condition, in order to offer assistance as necessary (NHS Choices, 2017)
In addition to keeping others aware of the patient's condition, Addison’s patients typically maintain an information card or medical bracelet, equipped with all necessary information about their condition for emergency personnel (Michels and Michels, 2014). In this way, even if they are incapacitated, the medical personnel will know what care they require (Mayo Clinic, 2017).
Being pro-active about Addison’s disease makes living with it a simpler, better-managed experience. Taking initiative to monitor your own care and knowledge is crucial to the management and care of Addison’s disease. </style>
<br>
References
Adam, A., & Dixon, A. (2008). Preface. Grainger & Allison's Diagnostic Radiology, Xv. doi:10.1016/b978-0-443-10163-2.50002-6
Addison’s Disease. (2015). Mayo Clinic. Retrieved February 28, 2017 from, http://www.mayoclinic.org/diseases-conditions/addisons-disease/diagnosis-treatment/treatment/txc-20156064
“Addison Disease”. MedicineNet. Archived from the original on 24 June 2007. Retrieved 2007-07-25.
“Addison's disease - Treatment“. NHS Choices. Retrieved 2017-02-25.
Adrenal Insufficiency & Addison's Disease | NIDDK. (n.d.). Retrieved March 01, 2017, from https://www.niddk.nih.gov/health-information/endocrine-diseases/adrenal-insufficiency-addisons-disease#prepare
Alschuler, L. (2016). The HPA Axis. Retrieved from http://www.integrativepro.com/Resources/Integrative-Blog/2016/The-HPA-Axis
Bergthorsdottir, R., Leonsson-Zachrisson, M., Odén, A., & Johannsson, G. (2006). Premature mortality in patients with Addison’s disease: a population-based study. The Journal of Clinical Endocrinology & Metabolism, 91(12), 4849-4853.
Brooke, A. M., & Monson, J. P. (2009). Addison's disease. Medicine, 37(8), 416-419.
Chantzichristos, D., Persson, A., Eliasson, B., Miftaraj, M., Franzén, S., Bergthorsdottir, R., … & Johannsson, G. (2016). Patients with Diabetes Mellitus Diagnosed with Addison´ s Disease Have a Markedly Increased Additional Risk of Death. In Cushing Syndrome and Primary Adrenal Disorders (pp. OR25-4). Endocrine Society.
Charmandari, E., Nicolaides, N. C., & Chrousos, G. P. (2014). Adrenal insufficiency. The Lancet, 383(9935), 2152-2167.
de Silva, D., & Wijesiriwardene, B. (2009). The adrenal glands and their functions. Ceylon Medical Journal, 52(3). Chicago
Fujieda, K., & Tajima, T. (2005). Molecular basis of adrenal insufficiency. Pediatric research, 57, 62R-69R.
HLA-DRB1 gene. (2014). Genetics Home Reference. Retrieved 2 March 2017, from https://ghr.nlm.nih.gov/gene/HLA-DRB1
Husebye, E. S., Allolio, B., Arlt, W., Badenhoop, K., Bensing, S., Betterle, C., … Pearce, S. H. (2013). Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. The Journal of Internal Medicine, 275 (2): 104-115.
Injection Kit. (2017). Canadian Addison Society. Retrieved February 28, 2017 from http://www.addisonsociety.ca/injection-kit.html
Lederer, E. (2016). Hyperkalemia. Medscape.
Lovas, K., & Husebye, E. S. (2002). High prevalence and increasing incidence of Addison's disease in western Norway. Clinical Endocrinology,56(6), 787-791. doi:10.1046/j.1365-2265.2002.t01-1-01552.x
Michels, A., & Michels, N. (2014). Addison disease: early detection and treatment principles. Indian Journal of Clinical Practice, 25(6).
Miller, W. L., & Bose, H. S. (2011). Early steps in steroidogenesis: intracellular cholesterol trafficking thematic review series: genetics of human lipid diseases. Journal of lipid research, 52(12), 2111-2135.
Mitchell, A. L., & Pearce, S. H. (2012). Autoimmune Addison disease: pathophysiology and genetic complexity. Nature reviews Endocrinology, 8(5), 306-316.
N. A. Comprehensive Metabolic Panel. (2005). Lab Tests Online.
Neto, R. A., & Carvalho, J. F. (2014). Diagnosis and classification of Addison's disease (autoimmune adrenalitis). Autoimmunity Reviews, 13(4-5), 408-411. doi:10.1016/j.autrev.2014.01.025
Odeke, S., & Nagelberg, S. (2001). Addison disease. eMedicine Journal, 2, 5.
Oksnes, M., Björnsdottir S., Isaksson, M., Methlie, P., Carlsen, S., Nilsen, R. M., … Løvås, K. (2014). Continuous Subcutaneous Hydrocortisone Infusion versus Oral Hydrocortisone Replacement for Treatment of Addison's Disease: A Randomized Clinical Trial. The Journal of Clinical Endocrinology & Metabolism, 99 (5): 1665-1674.
“Overview: Addison’s disease”. Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/addisons-disease/manage/ptc-20156068.
Pavlaki, A. N., & Magiakou, M. A. (2011). Glucocorticoid Therapy and Adrenal Suppression.
Sarkar, S. B., Sarkar, S., Ghosh, S., & Bandyopadhyay, S. (2012). Addison’s disease. Contemporary Clinical Dentistry, 3(4), 484–486. http://doi.org/10.4103/0976-237X.107450
Simon, E. (2016). Hyponatremia. Medscape.
Ten, S., New, M., & Maclaren, N. (2001). Addison’s disease 2001. The Journal of Clinical Endocrinology & Metabolism, 86(7), 2909-2922.
Volpé, R. (1990). Autoimmune diseases of the endocrine system. Boca Raton: CRC Press.
<br>