This is an old revision of the document!
Table of Contents
Epilepsy: Childhood Absence Epilepsy
Presentation 1: Epilepsy: Childhood Absence Epilepsy Powerpoint File
Introduction
Figure 1: Epileptic seizures involve disruption in brain cell activity.
Image from: http://www.webmd.com/epilepsy/types-of-seizures-their-symptoms </style>
<style justify> Epilepsy is the fourth most common neurological disorder in people of all ages, and affects the central nervous system. This disorder is chronic, and entails recurrent, unprovoked seizures lacking visible cause (Mayo Clinic, 2013). These seizures are the most characteristic symptom of this disorder, and are due mainly to the disruption of nerve cell activity, ranging from mild to severe (Mayo Clinic, 2013). Epilepsy may also constitute a range of other health problems, and can affect the safety, social relationships, and treatment of afflicted individuals (Epilepsy Foundation, 2014). Diagnoses of epilepsy are based on medical and family history, descriptions of episodic seizures, as well as various diagnostic tests, such as electorencephalograms (EEGs). Epileptic seizures are readily controllable with medications, or various other treatment options (Epilepsy Ontario, 2016).
Childhood Absence Epilepsy (CAE), or petit mal epilepsy, typically occurs in otherwise normal, school-aged children, with peak onset occurring at 6-7 years old. It is observed more frequently in young girls. This disorder is characterized by frequent, daily absence seizures (Loiseau et al., 2002). Absences differ from regular epileptic seizures in that they are non-convulsive and typically accompanied by a brief loss of consciousness. Absence seizures can occur in epileptic children up to 200 times a day (Crunelli and Leresche, 2002). They are unassociated with visual and sensory stimuli, and are generally deemed as unprovoked. CAE typically tends to recede in adolescence for most affected individuals (Crunelli and Leresche, 2002). </style>
<br>
Epidemiology
Approximately 1 in 100 Canadians currently live with epilepsy (Epilepsy Ontario, 2016). The yearly incidence of epilepsy is deemed to be approximately 50 cases per 100 000 individuals in developed and industrialized countries. In areas of socioeconomic deprivation, these risks tend to be higher. Generally, in these regions, the yearly incidence tends to hover around 150 cases per 100 000 individuals (Sander, 2003). Resource-poor countries are believed to demonstrate higher incidences of the disorder due, in part, to the lack of medical facilities and improper diagnoses. However, despite these factors, it is widely believed that rates in developing countries are genuinely higher (Sander, 2003).
Globally, 50 million people are currently afflicted by epilepsy. Active epilepsy — constituting recurrent seizures and use of medication — is estimated at 4-10 individuals per 1000 in higher income countries, while the rate proves higher in low income areas, at about 7-14 per 1000 (World Health Organization, 2012). Endemic conditions such as malaria, injury, and medical infrastructure are believed to contribute to these rates of disease (World Health Organization, 2012).
New diagnoses of epilepsy are approximated at 2.4 million annually (World Health Organization, 2012).
In the case of Childhood Absence Epilepsy, the annual incidence has been reported to fall within the range of 2-8% per 100,000 children under the age of 16 years old. Among children that are already afflicted with epilepsy, rates can go up 10% (Crunelli and Leresche, 2002). Furthermore, girls are deemed to be at twice the risk of boys in developing Childhood Absence Epilepsy, however, equal incidences have thus far been reported (Crunelli and Leresche, 2002).
<br>
Signs and Symptoms
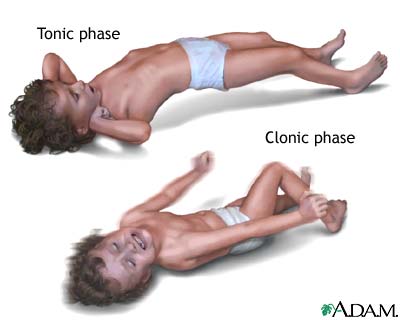
Figure 2: A depiction of tonic and clonic seizure types in children.
Image from: https://therefusers.com/cdc-says-seizures-can-occur-after-vaccination/ </style>
The symptoms of epilepsy are a result of experiencing seizures, which is the defining feature of this neurological disorder. A seizure occurs when there is an excessive synchrony of activity between neurons within the brain (McCormick & Contreras, 2001).
Seizures can be classified broadly as either partial or generalized (McCormick & Contreras, 2001). Partial seizures occur when the onset of abnormal activity is in one cortical region of the brain; typically within one cerebral hemisphere (McCormick & Contreras, 2001). Generalized seizures occur when the onset of abnormal activity is in both cerebral hemispheres (McCormick & Contreras, 2001). There are different subcategories of partial and generalized seizures and each with their own set of symptoms.
Partial seizures can either be simple or complex in which the later involves a loss in consciousness or cognitive abilities and the former does not (McCormick & Contreras, 2001). Simple partial seizures may also involve an “aura” which is a sensory experience prior to the onset of the seizure (McCandless, 2011). These sensory experiences could involve: perceived buzzing or whistling noises, a bad taste in the mouth, visions of flashing lights, hallucinations, sweating, or nausea, etc., depending on the brain region involved (McCandless, 2011). Clonic movements (constant contraction and relaxation of muscles) of the neck, face or whole extremities may also appear as a symptom in simple partial seizures (McCandless, 2011). Complex partial seizures may involve the experience of an aura prior to the onset of the seizure, motionless staring involving a loss of consciousness or repetitive movements of the extremities (McCandless, 2011).
Generalized seizures are further divided into 6 subcategories each with differing symptomatology. They are: Clonic seizures, tonic seizures, absence seizures, atonic seizures, myoclonic seizures and tonic-clonic seizures (Mayo Clinic, 2017).
Clonic seizures: involve repetitive jerking movements usually of the face, neck and extremities associated with alternating muscle contraction and relaxation.
Tonic seizures: involves the stiffening of the muscles usually in the back, legs or arms, lasting for less than 20 seconds.
Absence seizures: involve a brief loss of consciousness leading to motionless staring. Simple absence seizures last for less than 10 seconds while complex absence seizures last up to 20 seconds and are accompanied by unintentional bodily movements.
Atonic seizures: involve a loss of muscle control for less than 15 seconds, which could lead to the individual collapsing to the ground.
Myoclonic seizures: involves very brief twitching or jerking of the neck, shoulders, arms or legs. It will seem as though the individual is being electrically shocked. These seizures will last no longer than a couple seconds.
Tonic-clonic seizures: this type of generalized seizures has two phases associated with it. First is the tonic phase in which the individual’s muscles will tighten and then they will lose consciousness. Second is the clonic phase where the individual’s muscles will contract and relax in a rhythmic fashion. These seizures typically last for 1 to 3 minutes, however if prolonged to over 5 minutes this becomes a medical emergency. (Mayo Clinic, 2017; Devinsky & Sirvin, 2013)
Childhood Absence Epilepsy Symptoms:
The symptoms reported for childhood absence epilepsy in particular are a result of the absence seizures experienced. The seizures present as staring spells lasting approximately 10 to 20 seconds as a result of a brief lack of consciousness (Holmes and Fisher, 2013). The child will not know that they have experienced the seizure and will continue with the activity they were previously involved in or they may experience some brief confusion (Donner, 2010). These seizures could occur up to one hundred times daily and are typically spurred on by exercise (Holmes and Fisher, 2013). A child may also experience automatisms, which are actions performed by the body unintentionally (Donner, 2010). These actions may include, tongue movement, chewing, swallowing, raising eyelids and fiddling with hands (Donner, 2010).
<br>
Diagnosis
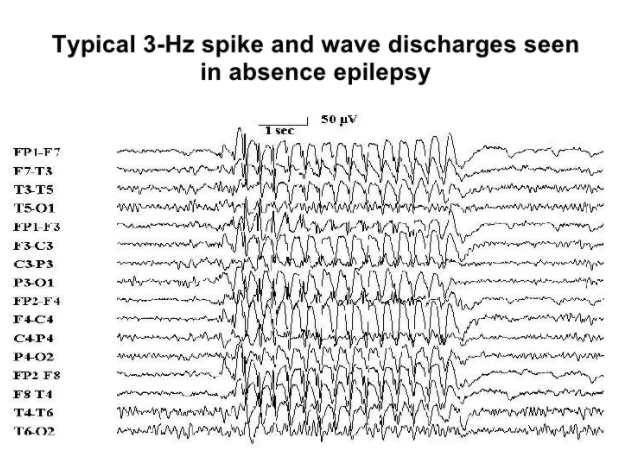
Figure 3: Typical EEG spikes in CAE.
Image from: http://www.slideshare.net/Kouya71/epilepsy-4502939 </style>
Doctors will take a number of steps to come to the proper diagnosis of epilepsy. Diagnostic procedures include:
Electroencephalogram (EEG): the EEG is the most common tool used in the diagnosis of epilepsy. The detection of an interictal spike by the EEG will assist in the confirmation of epilepsy as a diagnosis (Mayo Clinic, 2017). This interictal spike is caused by abnormal hypersynchrony among neurons in the brain (Pillai & Sperling, 2006). The EEG is also useful in determining whether the seizure is of partial or generalized origin (Pillai & Sperling, 2006).
Computerized Tomography (CT): the cross-sectional images of the brain taken by the CT scan can be used to investigate if there is any anatomical damage or abnormalities within the brain leading to the onset of the seizures (Mayo Clinic, 2017). Physicians will be able to detect if there are any tumors or cysts within the brain that may be associated with the seizures (Mayo Clinic, 2017).
Magnetic Resonance Imaging (MRI): MRI can provide more detailed images of the brain tissue with the use of strong magnets and radio waves (Mayo Clinic, 2017). Physicians can potentially detect lesions and abnormalities within the brain leading to the onset of the seizures (Mayo Clinic, 2017).
Positron Emission Tomography (PET): measures metabolic activity in the body with the use of a radioactive tracer that is injected into a vein (Mayo Clinic, 2017). PET can be used to detect the activity levels of brain regions that may be abnormal and leading to the seizures (Mayo Clinic, 2017).
Functional Magnetic Resonance Imaging (fMRI): technique that follows the path of oxygenated blood in the body to indicate where increased levels of activity reside (Mayo Clinic, 2017). Research is underway to combine fMRI with EEG in the diagnosis of epilepsy to get a more precise understanding of where abnormal activity is occurring in the brain during a seizure (Gotman, 2008).
History check: A physician will ask questions about the symptoms that have been occurring to raise the health concern. The physician will also ask about family history to identify whether there are any genetic underpinnings associated with the onset of the patient’s seizures (Mayo Clinic, 2017).
Examinations (Neurological & Neuropsychological): The physician can test motor, cognitive, memory, and speech functions to narrow down the type of seizures the patient may be experiencing (Mayo Clinic, 2017).
Laboratory tests: Physicians may request a blood sample from patients to look into whether they may have a genetic condition, toxin, or infection leading to the onset of the seizures (Mayo Clinic, 2017). The blood test can also help to determine if the seizures are merely a byproduct of another disease, such as diabetes.
Childhood Absence Epilepsy Diagnosis:
To diagnose childhood absence epilepsy, physicians will start off with asking the caregiver about the history of the symptoms being presented and any other associated health problems (Holmes & Fisher, 2013). They will then proceed in a physical examination to see if there is any bodily damage that could be leading to the seizures (Holmes & Fisher, 2013). An electroencephalogram (EEG) on the child’s brain activity is essential in detecting the presence of seizures. The child will be diagnosed with childhood absence epilepsy if there is generalized neuronal activity at 3 Hz spike and wave discharges as indicated by the EEG (Holmes & Fisher, 2013). This 3 Hz spike is caused by a depolarization via excessive excitatory neuronal activity among pyramidal cells in cortical structures (McCormick & Contreras, 2001). In order to study the child’s seizure episodes with the EEG or to merely observe their symptoms, the child will be asked to intentionally hyperventilate (Holmes & Fisher, 2013). Hyperventilation has been shown to induce an absence seizure in most children diagnosed with CAE (Holmes & Fisher, 2013). CT and MRI scans appear to be normal in these patients, indicating that there doesn’t appear to be anatomical damage or abnormalities in the brains of these children (Holmes & Fisher, 2013).
<br>
The Action Potential
A quick review of the normal transmission of the action potential aids in understanding the pathophysiology behind ictogenesis, or the production of a seizure. The resting potential in a neuron that is not firing is -70 millivolts (mV). A higher concentration of sodium (Na+) is found outside the cell, and a higher concentration of potassium (K+) is found inside the cell (Stafstrom, 1998).
Once stimulated, the action potential is an “all-or-none” event. Depolarization occurs as there is an influx of Na+ ions through voltage-gated ion channels (Stafstrom, 1998). The membrane potential at the end of the depolarization stage is +30 mV, at which point K+ ions exit the cell. After repolarization, the membrane reaches a stage of hyperpolarization (Stafstrom, 1998). This stage is dependent of intracellular calcium (Ca2+) levels and is mediated by the action of the Ca2+-dependent K+ channels. These channels regulate the refractory period so that the cell cannot generate another action potential. After the production of one action potential is complete, the cell then enters the refractory period, which restores the normal balance of intracellular and extracellular ions (Stafstrom, 1998).
<br>
Synaptic Transmission
As action potentials arrive at the end of the axon, the influx of Ca2+ prompts vesicles to release one of two neurotransmitters: glutamate or gamma-amino butyric acid, or GABA. In an inhibitory post-synaptic potential (IPSP), GABA is released into the synapse (Stafstrom, 1998). GABA receptors activate chloride channels. Influx of Cl- ions increases the negative charge of the neuron which results in hyperpolarization, thus inhibiting the passage of the action potential. In an excitatory post-synaptic potential (EPSP), glutamate is released into the synapse (Stafstrom, 1998). Glutamate binds to one of its many receptors on the post-synaptic terminal, which activates another ion channel. Depending on the type of receptor activated, Na+, Mg2+, or Ca2+ may enter the cell and initiate a depolarization event (Stafstrom, 1998).
<br>
Etiology
Etiology underlying epilepsy is categorized into three main types: idiopathic, remote symptomatic, and cryptogenic (Berg et al., 1999).
Idiopathic epilepsy contributes to 40% of the diagnoses (Engelborghs et al., 2000). Patients show no neurological abnormalities but have a strong genetic predisposition for the disorder (Berg et al., 1999). The most common examples include benign rolandic epilepsy, juvenile myoclonic epilepsy, and childhood absence epilepsy. Underlying genetic bases for idiopathic epilepsy are not very well understood because of the polygenic nature of its inheritance (Engelborghs et al., 2000). Although there is very little literature on humans with absence epilepsy, studies conducted on rat models have demonstrated an autosomal dominant mode of inheritance of one main gene, the WAG/Rij strain, along with significant interactions of a few other genes (Renier & Coenen, 2000). Various replications of this study have been conducted and literature reviews claim that these studies are validated with similar patterns of inheritance seen in patients with absence epilepsy (Coenen & Van Luijtelaar, 2003). Underlying changes in the biochemical microenvironment can also play a role in the etiology of epilepsy. These include increase in glutamate levels, decrease in GABA, and in some cases, blockage of the Na+-K+ pump (Engelborghs et al., 2000). Genetic alterations in T-type calcium channels have been associated with most generalized epilepsy syndromes, including childhood absence epilepsy (Stafstrom & Rho, 2016).
Remote symptomatic epilepsy is less common and has no known genetic bases. This type of epilepsy is characterized by the presence of a neurological abnormality, a history of brain injury, or comorbidities with other disorders. Patients are diagnosed with symptomatic epilepsy if they have had more than one sporadic, unprovoked seizure (Berg et al., 1999). Symptomatic mechanisms may be caused by a process known as “kindling” (Engelborghs et al., 2000). Kindling refers to the process of permanently decreasing the threshold potential for normal neuronal transmission. This causes the membrane to depolarize at a lower potential charge and may lead to structural and functional changes in glutamatergic synapses (Engelborghs et al., 2000).
Finally, some types of epilepsy have no known causes or underlying mechanisms and these are known as cryptogenic (Berg et al., 1999).
<br>
Pathophysiology
The pathophysiology of epilepsy is characterized by two distinct but related hallmarks: hyperexcitability and hypersynchrony. Hyperexcitability is when a neuron abnormally responds to incoming excitatory stimuli and fires multiple discharges at once instead of just one (Stafstrom, 1998). Hypersynchrony is when a large number of neighboring neurons discharge into one neuron simultaneously. Epilepsy is the result of a combination of both these characteristics (Stafstrom, 1998).
The main distinguishing factor of a seizure from a normal depolarization event can be seen in an EEG. Normally, excitation and inhibition are balanced and when neurons are not needed, they are silent (Stafstrom, 1998). Normal brain activity is low-voltage and desynchronized. If neurons start firing discharges at an abnormal rate, this classifies as a paroxysmal depolarization shift (PDS) and can be seen in an EEG as a “spike” of electrical activity. This is known as the interictal state (Stafstrom, 1998). In the ictal state, there is a flood of repeated EEG spikes which may continue for several second and can last up to a few minutes (Stafstrom, 1998).
<br>
Ictogenesis
Abnormal excitation of neurons may be attributed to a combination of factors. In the axonal membrane, there may be an abnormally large amount of K+ ions outside the cell, which switches around the normal concentration gradient (Stafstrom, 1998). After depolarization, there will be no efflux of K+ and Na+ would remain within the cell. The cell membrane will then remain in a state of depolarization, during which an abnormal number of action potentials will discharge within one large depolarization event (Stafstrom, 1998). This shift in depolarization is what characterizes the PDS, depicted in the EEG as the interictal spike. In some cases, the PDS is followed by a state of “post-PDS hyperpolarization” during which the cell may temporarily hyperpolarize (Stafstrom, 1998). However, if the PDS progresses, it leads to the barrage of synchronized neuronal firing characteristic of the ictal state (Stafstrom, 1998).
Another factor that characterizes a seizure is when the action potential reaches the end of the axon. Due to the presence of genetically altered T-type calcium, a large number of Ca2+ enters the cell, which induces the release of a large amount of neurotransmitters into the synapse. Epileptic neurons also tend to have chronically elevated Ca2+ levels inside the cell to begin with (Stafstrom & Rho, 2016). This, in combination with the excess of glutamate and low GABA levels, leads to overstimulation and depolarization of a multitude of surrounding neurons (Engelborghs et al., 2000). All components of normal neurotransmission are intricately linked together in a delicate balance of electric potential within the brain. A disruption in any one of these checkpoints can have a devastating domino effect which may lead to the production of an epileptic seizure.
In childhood absence epilepsy, these events take place in thalamocortical circuitry. Specific pathogenesis of an absence seizure results from the effects of a few abnormalities listed previously (Stafstrom & Rho, 2016). Namely, the T-type Ca2+ channels are altered, so that they are activated by smaller membrane depolarizations. Changes in other subtypes of channels that play a role in normal transmission of potentials in the thalamus are also seen in this type of epilepsy. Other synaptic influences include antagonists of GABA and agonists of glutamate (Stafstrom & Rho, 2016).
<br>
Susceptibility of the Immature Brain
Seizure incidence is highest in the early years of life and in some cases, especially childhood absence epilepsy, the disorder seems to disappear right after puberty. This is a result of multiple physiological factors that contribute to increased susceptibility (Stafstrom & Rho, 2016).
- Ion channels that mediate depolarization events usually develop earlier than those that are responsible for repolarization. In conjuncture with this, excitatory neurotransmitters are produced earlier in development than inhibitory neurotransmitters (Stafstrom & Rho, 2016).
- Early in development, GABA actually induces an excitatory effect rather than an inhibitory effect (Stafstrom & Rho, 2016).
- There are more synapses found in the immature brain than the mature one. This increases the amount of fast-acting electrical signals to be transmitted that may facilitate the production of seizures (Stafstrom & Rho, 2016).
These are just some examples that may increase a child’s susceptibility to developing epilepsy. Each of these factors alters the brain’s delicate balance of excitation and inhibition, in the favor of excitation (Stafstrom & Rho, 2016).
<br>
Prognosis and Prevention
Overall, the remission rate for CAE is 80% by early puberty, although these rates vary widely. Approximately, 11-18% of children who have CAE develop tonic-clonic seizures, which begin at puberty. If the child has tonic-clonic seizures as well as absence seizures, these are less likely to go away. However, they are usually easy to control. Early treatment to the anti-epileptic drugs may contribute to the permanent disappearance of the seizures. Drugs may be discontinued if a child has been seizure free for two-three years, but early discontinuation may trigger seizures.
A study conducted by Wirrell et al found that, in a study size of 81 children, forty-seven (65%) were in remission at the time of follow-up, which was 20.4 years on average. 17% of this population were taking AEDs but continued to have seizures, while 13% were taking AEDs and 15% had progressed to juvenile myoclonic epilepsy (JME). This ecidence suggests that when AEDs are taken, chances of remission into adulthood are high. Of 81 children with CAE, 72 (89%) were contacted for follow-up. Mean age at seizure onset was 5.7 years (range, 1 to 14 years) and at follow-up was 20.4 years (range, 12 to 31 years). Forty-seven (65%) were in remission. Twelve others (17%) were not taking AEDs but continued to have seizures. Thirteen (18%) were taking AEDs; five were seizure-free over the last year (in four of these a trial without AEDs had previously failed). Fifteen percent of the total cohort had progressed to juvenile myoclonic epilepsy (JME). Multiple clinical and EEG factors were examined as predictors of outcome. Factors predicting no remission (p < 0.05) included cognitive difficulties at diagnosis, absence status prior to or during AED treatment, development of generalized tonic clonic or myoclonic seizures after onset of AEDs, abnormal background on initial EEG, and family history of generalized seizures in first-degree relatives.
Furthermore, in a retrospective analysis of a cohort of 163 patients, 64 of which had CAE, were followed for a duration of 25.8 years. It was found that 58% of patients with CAE were in remission, and had been seizure free for a period of at least two years.
<br>
Treatment
Antiepileptic Drug (AED) monotherapy is the mainstay treatment measure for patients diagnosed with recurrent, unprovoked seizures or epilepsy. With the various types of seizures and epilepsy syndromes, a broad-spectrum of AEDs are available that are selective in targeting certain types of seizures/epilepsy syndromes then others (Ha & Bellanger, 2013). While AEDs do not cure epilepsy, instead are taken to prevent the onset of a seizure by altering and reducing the excessive electrical activity (or excitability) of the neurons that normally cause a seizure (Goldenberg, 2010). Different AEDs work in different ways and have different effects on the brain. AEDs are taken prophylactically either once or twice a day (Ha & Bellanger, 2013).
Childhood Absence Epilepsy and AEDs:
The three medications of choice commonly used as initial monotherapy to treat childhood absence epilepsy are Ethosuximide (Zarontin), Valporic acid (Epilim), and Lamotrigine (Lamictal).
Ethosuximide selectively blocks a type of calcium channel called the T-type calcium currents in the thalamic neurons, which inhibits the thalamocortical circuits responsible for generating the EEG spike-wave complex underlying and directly reducing absence seizures (Peterson & Albertson, 1998). Ethosuximide is given orally to patients as an initial dose of 250 mg capsule per day for 3-6 year olds and two 500mg capsules per day for children above 6 years of age (Goldenberg, 2010). Common side effects include anorexia and drowsiness and in extreme and less common cases liver toxicity and drug rash (Goldenberg, 2010).
Valproic acid decreases the breakdown of GABA, resulting in an increased inhibitory effect. Valproic acid is available in liquid or capsule form with a starting dose of 15 mg/kg/day or maintenance dose of 60mg/kg/day. Common side effects include abdominal discomfort, nausea, and weight gain (Goldenberg, 2010).
Lamotrigine works by blocking the sodium channels and is available in chewable and pill form. It is usually started at 0.5mg/kg/day and can be increased to a maximum of 5 to 10 mg/kg/d (Goldenberg, 2010).
The most efficacious and tolerable treatment has not been defined with very little guidelines for physicians to utilize when prescribing one AED over the other as each of these medications have different side effects and drug interaction profiles (Buchhalter, 2011).
However, as of a double blind, randomized control trial conducted in 2013, Ethosuximide is now the recommended first-line therapy in children with childhood absence epilepsy (Glauser et al., 2013). The study looked at the efficacy, tolerability and neuropsychological effects of ethosuximide, valproic acid, and lamotrigine in children diagnosed with CAE. 435 children were eligible and randomly assigned to one of three groups; drug doses were titrated for clinical response (until the child was free of seizures). The primary outcome was freedom from treatment failure (a defined criterion) after 16 weeks of therapy; the secondary outcome was attentional dysfunction.
The researchers found that Ethosuximide and Valproic acid are more effective than Lamotrigine in the treatment of childhood absence epilepsy. Ethosuximide was also associated with fewer adverse attentional effects (in 33%) where as attentional dysfunction was more common with valproic acid (in 49% children). This study address the ambiguity clinicians experienced when treating patients and is the first of its kind providing an evidence-based standpoint to initiate Ethosuximide as a first line drug choice. Valproic acid and lamotrigine are options when seizures are refractory to Ethosuximide (Glauser et al., 2013).
For patients who refuse pharmacological treatment, a Ketogenic diet, special high fat, low-carbohydrate diet, is available as an alternative regimen to control and manage seizures. The ketogenic diet may prove to be beneficial in comparison to the antiepileptic drugs. Studies find that approximately 50% reduction in the frequency of seizures. In a recent study of 317 Chinese children, 35.0%, 26.2%, and 18.6% children showed >50% seizure reduction at three, six, and 12 months, respectively. Furthermore, in a systematic review conducted by Keene et al, with a total collective population of 972, an average of 15.6% of the patients had become seizure-free at the 6-month mark, and 33.0% had more than 50% reduction in seizure frequency after incorporating the ketogenic diet.
<br>
References
Berg, A. T., Shinnar, S., Levy, S. R., & Testa, F. M. (1999). Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia, 40(4), 445-452.
Buchhalter, J. (2011). Treatment of Childhood Absence Epilepsy—An Evidence-Based Answer at Last!. Epilepsy Currents, 11(1), 12-15.
Coenen, A. M. L., & Van Luijtelaar, E. L. J. M. (2003). Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behavior genetics, 33(6), 635-655.
Crunelli, V., & Leresche, N. (2002). Childhood absence epilepsy: genes, channels, neurons andnetworks. Nature Reviews Neuroscience, 3(5), 371-382.
Devinsky, O., & Sirven, J. I. (2013). Myoclonic Seizures and Tonic-Clonic Seizures. Epilepsy Foundation. Retrieved January 24, 2017, from http://www.epilepsy.com/learn/types-seizures/myoclonic-seizures
Donner, E. J. (2010). Absence Seizures. about kid’s health. Retrieved January 24, 2017 from, http://www.aboutkidshealth.ca/En/ResourceCentres/Epilepsy/UnderstandingEpilepsyDia gnosis/TypesofSeizures/Pages/Absence-Seizures.aspx
Engelborghs, S., D’hooge, R., & De Deyn, P. P. (2000). Pathophysiology of epilepsy. Acta neurologica belgica, 100(4), 201-213.
Epilepsy. (2013, May 31). Mayo Clinic. Retrieved January 21, 2017, from http://www.mayoclinic.org/diseases-conditions/epilepsy/basics/definition/CON-20033721?p=1
“Epilepsy”. Fact Sheets. World Health Organization. October 2012. Retrieved January 21, 2017.
Glauser, T. A., Cnaan, A., Shinnar, S., Hirtz, D. G., Dlugos, D., Masur, D., … & Adamson, P. C. (2013). Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia, 54(1), 141-155.
Goldenberg, M. M. (2010). Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. Pharmacy and Therapeutics, 35(7), 392.
Gotman, J. (2008). Epileptic Networks studied with EEG-fMRI. Epilepsia. 49 (s3), 42-51.
Ha, H., & Bellanger, R. (2013). Epilepsy: treatment and management. US Pharm, 38(1), 35-39.
Holmes, G. L. & Fisher, R. S. (2013). Childhood Absence Epilepsy. Epilepsy Foundation. Retrieved January 24, 2017, from http://www.epilepsy.com/learn/types-epilepsy-syndromes/childhood-absence-epilepsy
Loiseau, P., Panayiotopoulos, C. P., & Hirsch, E. (2002). Childhood absence epilepsy and related syndromes. Epileptic syndromes in infancy, childhood and adolescence, 3, 285-304.
McCandless, D. W. (2011). Epilepsy. Simple Partial Seizures (143-152) Chicago. IL: Springer Science+Business Media.
McCormick, D. A., & Contreras D. (2001). On the Cellular and Network Bases of Epileptic Seizures. Annul. Rev. Physiol, 63: 815-846.
Peterson, S. L., & Albertson, T. E. (Eds.). (1998). Neuropharmacology methods in epilepsy research. CRC Press.
Pillai, J. & Sperling, M. R. (2006). Interictal EEG and the Diagnosis of Epilepsy. Epilepsia. 47 (s1), 14-22.
Renier, W. O., & Coenen, A. M. L. (2000). Human absence epilepsy: the WAG/Rij rat as a model. Neuroscience Research Communications, 26(3), 181-191.
Sander, J. W. (2003). The epidemiology of epilepsy revisited. Current opinion in neurology, 16(2), 165-170.
Stafstrom, C. E. (1998). Back to Basics: The Pathophysiology of Epileptic Seizures: A Primer For Pediatricians. Pediatrics in Review, 19 (10).
Stafstrom, C. E., Rho, J. M. (2016). Pathophysiology of seizures and epilepsy. URL: https://www.uptodate.com/contents/pathophysiology-of-seizures-and-epilepsy
What Is Epilepsy? (2014, January). Epilepsy Foundation. Retrieved January 21, 2017, http://www.epilepsy.com/learn/epilepsy-101/what-epilepsy
What is Epilepsy? (2016). Epilepsy Ontario. Retrieved January 21, 2017, from http://epilepsyontario.org/about-epilepsy/what-is-epilepsy/
<br>