This is an old revision of the document!
Table of Contents
Primary Systemic Amyloid Light-Chain (AL) Amyloidosis
PDF Presentation: pdf_-_final_version_ls_4m03_al_amyloidosis.pdf
PowerPoint Presentation: final_version_ls_4m03_al_amyloidosis.pptx
Introduction
What Is AL Amyloidosis?final_version_ls_4m03_al_amyloidosis.pptx:
History
To understand the history of AL Amyloidosis, it is necessary to investigate the history of the term, ‘amyloid’. This term was first used in 1854 by Rudolph Virchow to define extracellular accumulation in the liver (Nyirady, 2016). Thus, this was the first instance of this term being used in the same context as it is now presently. However, before Virchow coined this term, in 1838 a German botanist by the name of Mathias Schleiden used the term to refer to the, “normal amylaceous constituent of plants” (Nyirady, 2016). Interestingly, if one were to look further back into history, it becomes apparent that amyloids and the disease amyloidosis were known by several different names by doctors conducting post-mortem autopsies (Sipe, 2008). In these autopsies, abnormal deposits of amyloids in the liver or spleen were identified (Sipe, 2008). For example, even as far back as Nicolaus Fontanus in 1639, identified during an autopsy that a patient’s spleen had abnormal white stones (Kyle, 2001). Thomas Bartholin, Antoine Portal, and Jeremiah Wainewright are all other examples of doctors during different periods in history that all identified similar symptoms in patients or noticed similar characteristics when conducting autopsies (Kyle, 2001). Eventually, it was in 1856 when a doctor reported symptoms of something typical of primary amyloidosis (Kyle, 2001). This doctor was Samuel Wilks and he remarked on the autopsy of a 52-year-old man who died with, “lardaceous viscera” that the man’s kidneys were white (Kyle, 2001). Up to this point, there was no test to positively identify tissues that display amyloids (Beckerman, 2015). This changed in 1927 when Paul Divry and associates discovered that Congo red dye could be used to stain tissue samples suspected of containing amyloids (Beckerman, 2015). These tissue samples would glow green when viewed under polarized light (Beckerman, 2015). Finally, in 1959 Cohen and Calkins discovered that amyloidosis demonstrated a fibril structure using an electron microscope (Nyirady, 2016).
Classification
Systemic amyloidosis can be classified into 3 categories: primary systemic amyloidosis (PSA), amyloidosis associated with multiple myeloma and secondary systemic amyloidosis (Nyirady, 2016). Primary systemic amyloidosis is also known as AL Amyloidosis.
Epidemiology
AL Amyloidosis is the most common type of Systemic Amyloidosis with an estimated incidence of 9 cases per-million inhabitants each year (Real de Asúa et al., 2014). In the USA there are approximately 1275-3200 new cases annually (Real de Asúa et al., 2014). These values however are not considered precise due to often un-diagnosis and misdiagnosis. A reported 56% of all systemic cases are classified as AL Amyloidosis (Nienhuis, Bijzet & Hazenberg, 2016). In the UK 65% of the Amyloidosis diagnosed patients have AL Amyloidosis while in China 93% are Amyloidosis(Desport et al., 2012).
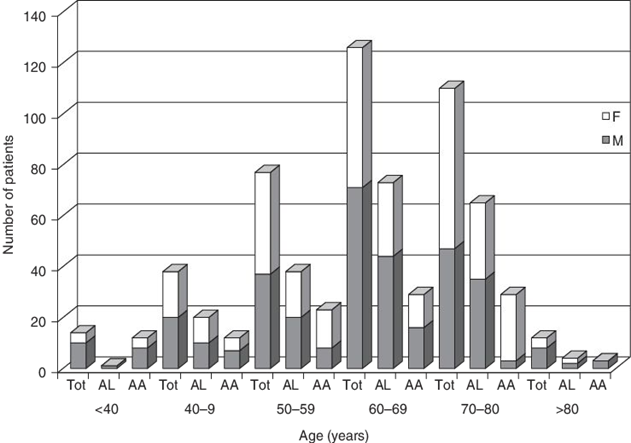
Figure 1: Patients age at time of diagnosis for AL/AA Amyloidosis along with patients sex.
AL Amyloidosis is a disease of adulthood with an incidence rate highest amongst adults aged 60 – 80 years old with the average age of diagnoses being 65 years (Real de Asúa et al., 2014). Only 10% of all patients are under the age of 50 and an even lower 5% of total patients are under the age of 40 (Real de Asúa et al., 2014). There have ever only been a handful of cases where the patient diagnosed with AL Amyloidosis was in their late 20’s. Although the reasoning to it is still unknown two-thirds of all patients are male. There has been no racial predilection reported in developing AL Amyloidosis (Nienhuis, Bijzet & Hazenberg, 2016).
AL Amyloidosis is not considered hereditary; it is not secondary to other diseases and cannot be passed on to others through aerosols/touch/sex. There are however a few families that have increases incidence to blood diseases which would include myeloma and AL Amyloidosis amongst others. This is not considered a norm (Desport et al., 2012).
Signs and Symptoms
According to the Cedar-Sinai Cancer Institute (“Amyloidosis”, 2017) the signs and symptoms of a patient potentially afflicted with Amyloidosis are hard to identify. This is because the disease is so rare and that many of the symptoms overlap with the characteristics displayed by patients with other diseases. Similarly, because Amyloidosis can affect different organs of the body, this too affects what symptoms a patient may display. Further, it is possible that a patient might not display any symptoms whatsoever although they may have the disease. Thus, all these factors contribute to the complexity of diagnosing this disease solely through signs and symptoms (“Amyloidosis”, 2017).
Nevertheless, there are some key symptoms, which are generally associated with the disease (“Amyloidosis”, 2017). These are:
“
- An enlarged liver
- An enlarged tongue
- An irregular heartbeat
- Diarrhea alternating with constipation
- Difficulty swallowing
- Dizziness or feeling faint
- Loss of weight
- Numbness or tingling in the hands or feet
- Severe fatigue
- Shortness of breath
- Skin changes
- Swelling of the ankles and legs
- Weakness…
- A burning sensation
- Bowel obstruction
- Carpal tunnel syndrome
- Nervous system disrupted” (“Amyloidosis”, 2017).
It is also important to note that if the disease is affecting the heart or kidney, the symptoms displayed may be more severe and will pertain to the respective organ, which is being affected (“Amyloidosis”, 2017). Usually in these cases the disease is life threatening (“Amyloidosis”, 2017).
Prognosis
http://circ.ahajournals.org/content/133/3/245
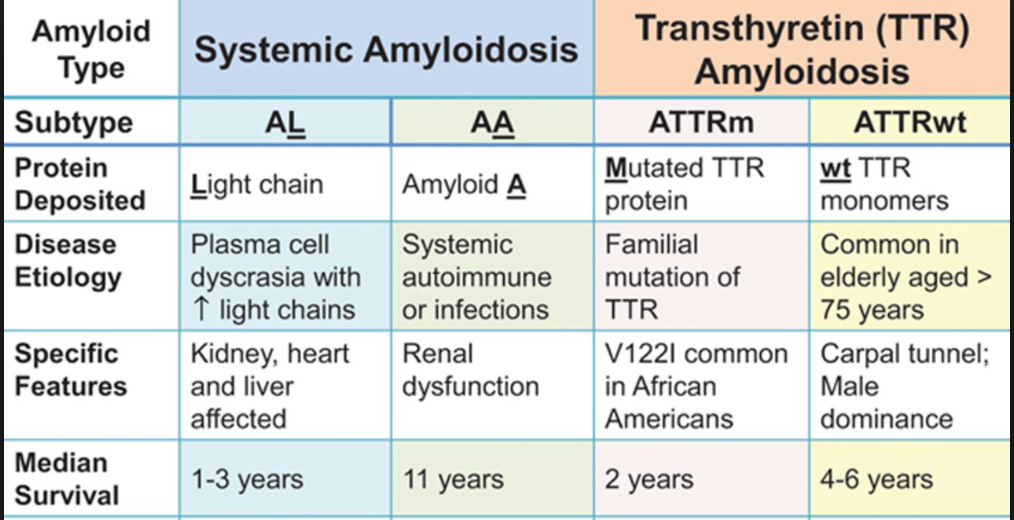
Table 1: The prognosis of different amyloidosis. (Liu, P.P., & Smyth, D., 2015).
If we would like know the prognosis of Amyloidosis, it is basically depends on the forms of amyloidosis and its response to the treatment. Also, vital organ involvement will have adversely effected on the life expectancy (Liu, P.P., & Smyth, D., 2015; Shiel, C.W., & Balentine, R. J., 2017; Papadakis, M.A., & McPhee, S. H., 2017).Median survival for AL (primary) amyloidosis is approximately 1-3 years. If patients do not treat the disease, it will slowly progress and become fatal. (Ma, G., & Ra, K., 1994; Tidy, C., 2014; Liu, P.P., & Smyth, D., 2015; Shiel, C.W., & Balentine, R. J., 2017). AL Amyloidosis is fatal in 80% of cases (Tidy, C., 2014).Sometimes, AL Amyloidosis will affect different parts of our body. If it affects our cardiac or autonomic nerve, people generally have survivals of 3 to 9 months; similarly, for Carpal tunnel syndrome or nephrosis, they have survivals of 1.5-3 years. In addition, if people have peripheral neuropathy, they may have survivals of 5 years by using multiple treatment (Papadakis, M.A., & McPhee, S. H., 2017). These data demonstrated the survival rates based on the patients who received the treatment.
Nowadays, for the patients who have AL Amyloidosis, if they can get treat by autologous hematopoietic stem cell transplantation properly, they have the median survival approaches 5 years now. Moreover. If patients get a complete hematologic remission, they approaches 10 years (Papadakis, M.A., & McPhee, S. H., 2017). Moreover, if patients have amyloidosis is related to the kidney, some researches illustrated that patients have dialysis and kidney transplantation; they will have further improved the prognosis (Tidy, C., 2014).
Targeted Organs:
http://www.bloodjournal.org/content/119/19/4343?sso-checked=true
Figure 2: Percentages referring to mortality rate (death within 3 years) upon development of AL Amyloidosis.
AL Amyloidosis can target a variety of organs. Mortality rates vary depending on the organ that the amyloid fibrils form within. The heart is the most sensitive to fibril formation and such mortality is very high if amyloid fibrils form. Figure 00 outlines some of the main organs that AL Amyloidosis target and the respected mortality rate that comes along with it. Every organ has a respectful threshold to the amount of amyloid it can withstand before losing functionality and failing. The heart and the kidney's have the lowest such threshold (Gertz et al., 2016).
Diagnosis
Patients who have unexplained proteinuria (presence of excess proteins in the urine), cardiomyopathy (diseases of the heart muscle), neuropathy, or hepatomegaly and in patients with multiple myeloma that has atypical manifestations all should be considered for having AL Amyloidosis (Sanchorawala, 2006). Diagnosis requires two criteria, the first being a demonstration of amyloid in the tissue and the second being a demonstration of a plasma cell dyscrasia (Sanchorawala, 2006). In about 15-20% of patients the most common skin signs include petechieae, purpura and ecchymoses (Nyirady, 2016). Petechieae are considered to be small purpura where purpura are areas of discolouration on the skin or mucous membranes that comes about because of hemorrhages from small blood vessels (Ngan, 2005). Ecchymoses also known as bruises are larger extravasations of blood (Ngan, 2005). These skin signs all occur spontaneously or after minor trauma (Nyirady, 2016).
Pathophysiology / Biochemical Mechanisms
Pathophysiology
Amyloid Light Chains
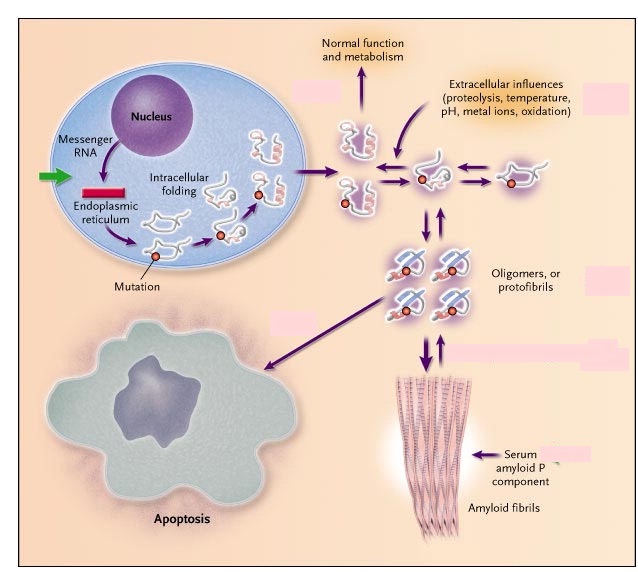
Amyloid Light-chain (AL) amyloidosis is a bone marrow disorder, specifically a plasma cell dyscrasia, which forms misfolded immunoglobulin light chains that accumulate in large quantities and deposit in the extracellular matrix of many organs, rendering them dysfunctional (Zhang, Huang & Li, 2017).
Amyloidogenic Immunoglobulin light chains are produced by abnormally, over-proliferative monoclonal populations of plasma cells that mass-produce antibodies like immunoglobulin(Ig)(Ramirez-Alvarado, 2012, Zhang, Huang & Li, 2017). Once in blood circulation, these Ig light chains unfold, bind to other light chains and extracellular matrix (ECM) components and deposit as insoluble amyloid fibrils in tissues and organs (Ramirez-Alvarado, 2012).
http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html
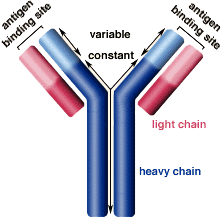
Figure 3: Structure of the Immunoglobulin antibody
The Ig antibodies produced by plasma cells are composed of two light chains, classified as kappa and lambda, and two heavy chains linked via sulfide bonds (Ramirez-Alvarado, 2012). The tendency of Ig light chains to develop fibrillogenesis depends on the primary structure of the light chains (Zhang, Huang & Li, 2017). The ratio of the two light chain classes, kappa and lambda, in normal adults is approximately 2:1 however, in AL patients, the lambda light chains are seen four times as much compared to kappa (Zhang, Huang & Li, 2017, Weiss et al., 2016). In the Immunoglobulin light chains, the variable regions are highly mutated relative to the closest germline genes (Perfetti et al., 1998).
http://http://www.nejm.org/doi/full/10.1056/NEJMra023144
Figure 4: ECM components and Ig light chains combine to form a β-super-secondary structure (Merlini & Belloti, 2003). These β- sheet polypeptides form a protofilament which then wraps around other protofilaments to shape the amyloid fibrils (Merlini & Belloti, 2003).
The amyloid precursors form and are secreted as native proteins, however, they escape the intracellular quality control system where the mutant light chains are not recognized (Merlini & Bellotti, 2003). Once in the ECM, these light chains unfold and reach an equilibrium between fully folded and partially folded state (Merlini & Bellotti, 2003). Local factors such as low pH, oxidative stress, high temperature and limited proteolysis shift this equilibrium towards the partially folded state, forming the protofibrils (Merlini & Bellotti, 2003). The protofibrils then go onto forming amyloid fibrils and they also cause cellular toxicity by inducing apoptosis in target tissue cells (Merlini & Bellotti, 2003).
Factors Influencing Fibrillogenesis
Plasma Cells
- Malignant plasma cells can be identified by low levels of glycoproteins CD19, CD27, CD38, CD45 and high levels of CD28, CD 33, CD56 compared to normal plasma cells (Zhang, Huang & Li, 2017, Paiva et al., 2010).
- In a study conducted on 17 AL amyloidosis patients, 81% had cytogenetic abnormalities such as translocation of chromosome 14q32, monosomy 13, trisomies 9, 15, 11 and 3 (Zhang, Huang & Li, 2017 Warsame et al., 2015).
Post Translational Modifications
- Glycosylation may promote amyloid fibrillogenesis by altering protein solvation energy, increasing binding strength at certain sites and protecting some regions from degradation by proteases (Zhang, Huang & Li, 2017, Petukhov, Cregut, Soares, & Serrano, 1999; Schachter, 1984; Wallick, Kabat, & Morrison, 1988).
Extracellular Matrix Components
- Serum Amyloid P protects the amyloid fibrils from proteolytic degradation (Zhang, Huang & Li, 2017, Tennent, Lovat & Pepys, 1995)
- Heparan Sulfate proteoglycans(HSPG) interact with ligands such as growth factors, cytokines, enzymes, etc (Zhang, Huang & Li, 2017). They induce precursors to take-up amyloidogenic conformations and also promote protofilament assembly into mature fibrils through stabilization (Zhang, Huang & Li, 2017, McLaurin, Franklin, Zhang, Deng, & Fraser, 1999; Ren et al., 2010)
Extracellular Chaperones (ECs)
- ECs have a controversial role in AL amyloidosis (Zhang, Huang & Li, 2017). Some studies show that alpha2-macroglobulins ( a type of EC) facilitate degradation of beta-amyloid by receptor-mediated endocytosis and degradation by lysosome with the help of clusterin (EC) (Zhang, Huang & Li, 2017, Narita, Holtzman, Schwartz, & Bu, 1997). However, other studies have shown that ECs bind to amyloid fibrils and protect them from degradation by proteases (Zhang, Huang & Li, 2017).
Prevention
In the case of Alzheimers, Transthyretin prevents amyloid formation (Schwarzman et al., 1994). Alzheimers is part of the same family as AL amyloidosis, with the family being systemic amyloidosis. Colchicine prevents amyloidosis of familial mediterranean fever (Zemer et al., 1986). This fever is also within the family of systemic amyloidosis like AL amyloidosis. Colchicine is a drug that is administered to the individual.
Treatment / Management
Recently, limited treatment is available that specifically targets the amyloid and most of them are supportive measure (Ma, G., & Ra, K., 1994; Tidy, C., 2014) In order to treat this disease, the aim is to suppress the development of the amyloid-forming protein and manage the symptoms. (Tidy, C., 2014)Therefore, people may use different treatments depends on what type of amyloidosis and the stabilization of organs (Papadakis, M.A., & McPhee, S. H., 2017).
http://www.amyloidosis.org.uk/al-amyloidosis/treatment/
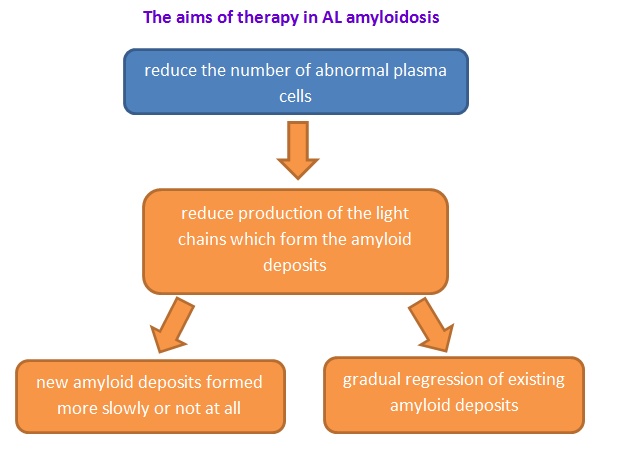
Figure 6: The aims of therapy in AL amyloidosis.(Wechalekar, A., 2017).
Chemotherapy and Autologous Stem-Cell Transplantation:
High-dose chemotherapy medicines (melphalan) with autologous stem cell transplantation is the current treatment for the patients who have AL Amyloidosis.(Derrer, T.D., 2015; NHS choices, 2017; Shiel, C.W., & Balentine, R. J., 2017) The reason is that AL Amyloidosis is a protein abnormally deposits in some tissues of our human body; therefore, it causes some organ dysfunction (i.e. heart, liver, kidney etc.) (Papadakis, M.A., & McPhee, S. H., 2017). By using this treatment, the aim is to reduce the light chain protein production (the substance that can form amyloid) and deposition which can cause AL Amyloidosis. On the other hand, this treatment can arrest progressive of the organ dysfunction (Papadakis, M.A., & McPhee, S. H., 2017). Using high doses of chemotherapy medicines (melphalan), we observed a high rate of remission (Child et al., 2003).Since this treatment comes with autologous stem cell transplantation, but not all the patients can accept it. For this point of view, people should consider the toxicity of autologous stem cell transplantation such as febrile neutropenia, mucositis, delirium and atrial dysrhythmias (Bernard et al., 2015). As a result, patients may have dialysis-dependent renal failure (Bernard et al., 2015). If people cannot use the treatment properly, they may use oral melphalan and dexamethasone (Derrer, T.D., 2015; NHS choices, 2017; Shiel, C.W., & Balentine, R. J., 2017). These two chemotherapy medicine can give good hematological and organ response(Mahmood et al, 2014). As to chemotherapy, physician usually use melphalan, cyclophosphamide, dexamethasone, lanalidomide and bortezomib (Papadakis, M.A., & McPhee, S. H., 2017). However, this treatment has the related mortality about 8% in patients with AL Amyloidosis, because they do not have the organ improvement after therapy. Moreover, some patients are being treated very well. The reason is that the treatment can facilitate amyloid dissolution and correct the amyloid protein folding. Therefore, the protein will fold in normal form (Papadakis, M.A., & McPhee, S. H., 2017).
Anti-Inflammatory Medicines:
As to the Secondary (AA) amyloidosis, it usually comes with some inflammation. Therefore, physicians normally treat it with chemotherapy and the anti-inflammatory medicines (Papadakis, M.A., & McPhee, S. H., 2017). For this kind of medicine, they are basically used steroids (Derrer, T.D., 2015; NHS choices, 2017) . Using steroids sometimes may have some bad reaction which is caused by the effect of the chemotherapy (NHS choices, 2017), so people should use it properly and monitor the reaction. On the other hand, secondary amyloidosis may occur with another illness including chronic infections (i.e. Tuberculosis and osteomyelitis) and chronic inflammatory diseases (i.e. Rheumatoid arthritis). For Rheumatoid arthritis, physicians usually treat it by using Chlorambucil (Derrer, T.D., 2015).
Organ Transplant:
http://www.mayoclinic.org/tests-procedures/liver-transplant/details/what-you-can-expect/rec-20211848
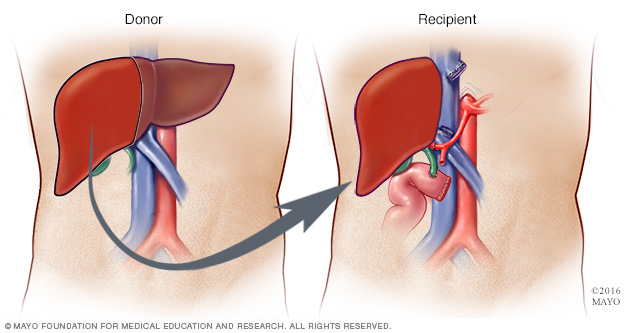
Figure 7: The Liver Transplant (Mayo, 2017).
Liver transplant is also one of the treatment for AL Amyloidosis. Depends on the condition of this disease, some patients may have liver failure (Derrer, T.D., 2015). Therefore, liver transplant may become one of the best treatment. Also, some patients may have hereditary amyloidosis or ATTR (Derrer, T.D., 2015). For this disease, liver will produce mutated transthyretin which will form amyloids (Papadakis, M.A., & McPhee, S. H., 2017). In order to arrest this process, physicians will strongly suggest the patients to do liver transplant ( NHS choices, 2017). However, we need to consider the rejection of our human body.
Like the information provided above, depends on the condition of amyloidosis, the amyloid protein may affect some vital organs such as kidney and heart. If the vital organs get infection, organ transplant may become a reasonable treatment (i.e. kidney or heart transplant) (Derrer, T.D., 2015; NHS choices, 2017).
Colchicine:
For Familial Mediterranean fever, means 2nd amyloidosis in inflammatory bowel disease, physicians will suggest to use Colchicine (Shiel, C.W., and Balentine, R. J., 2017).
Other Treatments:
Amyloidosis can happen in different conditions. It may affect different vital organs. If the disease affects the kidney and cause kidney problem, people may use diuretic medicines in order to remove excess water from the body (Tidy, C., 2014; Derrer, T.D., 2015). Secondly, people may have swelling of the tongue, therefore, they can add some thickeners to the fluids in order to prevent choking (Derrer, T.D., 2015). Thirdly, people may have swelling in their legs and feet, then they may use compression stockings. It can helop them to relieve swelling (Derrer, T.D., 2015). Last but not least, if people have gastrointestinal amyloidosis, they can modify their diet in order to prevent the disease progressive (Derrer, T.D., 2015).
Social Implications
AL Amyloidosis or Amyloidosis in general is a disease not known by the majority of society. Among those who do there are often a lot of misconceptions and wrongful information. A lot is being done by corporations/foundations in order to spread correct information about the disease to the general public in order to create awareness. The Amyloidosis Foundation is one such foundation looking to create awareness, collect donation for patients and invest in research that will go a long in helping those affected by the disease.
Further Research / Applications
There is still a lot of research that needs to be done on AL Amyloidosis in order successfully diagnose and treat everyone who happens to obtain the disease with a high rate of success. The research being conducted spans a variety of platforms such as alternate treatments, enhancements to current treatments, earlier diagnosis methods and prevention methods. Of all such platforms the focus is on discovering tools in which to diagnose patients earlier even before the appearance of symptoms in order to apply treatment earlier which helps increase success and survivor-ship.
Epigallocatechin-3-Gallate (EGCG):
Epigallocatechin-3-Gallate (EGCG) is being looked at as a potential treatment for AL Amyloidosis. EGCC is the most abundant catechin in tea. EGCC is studied greatly today for its potential effects on human health and diseases. It is used in many dietary supplements. It is found in high concentrations in the dried leaves of green tea, white tea and black tea (Andrich et al., 2016). Trace amounts are found in foods such as apple skins, plums, onions and pecans (Andrich et al., 2016). EGCC is currently the subject of pre-clinical studies involving autoimmune diseases, inflammation and rheumatoid arthritis (Andrich et al., 2016). Recently, EGCC is being looked at as a secondary treatment for AL Amyloidosis. Research so far has suggested that EGCG specifically inhibits the second aggregation phase causing the formation of more stable non-amyloid light chain aggregates (Andrich et al., 2016). Scientists are most intrigued by the fact that EGCG intervention does not depend on the individual sequences of the light chains and thus can potentially be universally used as a treatment (Andrich et al., 2016). Further research is required in order to determine if EGCG would work at larger quantities and if it sustain inhibition for a prolonged period of time.
http://toptestosteronesupplements.com/epigallocatechin-gallate-egcg-ingredient-breakdown/
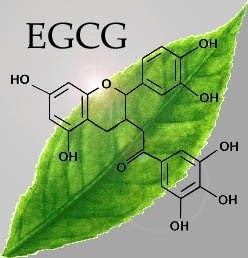
Figure 8: Molecular structure of EGCG. EGCG found commonly in a variety of dried tea leaves. Looked at as a potential treatment for AL Amyloidosis.
Monoclonal Antibodies - NEOD001:
The most cutting edge approach to AL Amyloidosis is the use of monoclonal antibodies which would clear the amyloid from the affected organs. Recent studies looking at NEOD001 have been very promising. NEOD001 is a monoclonal antibody that specifically targets the aggregated amyloid that accumulates in AL patients which would be eliminated (Gertz et al., 2016). In one study 69 patients were administrated NEOD001 post chemotherapy (Gertz et al., 2016). Response rate was 53% in cardiac patients and 64% among renal patients with little to no adverse side-effects (Gertz et al., 2016). NEOD001 also demonstrated improved neuro-pathy (Gertz et al., 2016). There are high hopes for this treatment and is currently in phase 2 of pre-clinical trials with phases 3 study planned.
Genetically Engineered Cells:
Pre-Clinical testing is un-going on a potential treatment for AL Amyloidosis where a patient’s own immune system is genetically engineered to recognize and kill abnormal blood cells when reintroduced into the body (Rosenzweig, M., 2017). The goal of the research currently is to evaluate blood cells of patients with AL Amyloidosis and to find a specific protein that could be targeted (Rosenzweig, M., 2017). This protein must be unique to infected blood cells of AL Amyloidosis patients. Once this key step is completed in laboratory testing of genetically engineered cells be able to occur.
Study of Gene Expression Changes/Mutations:
To date, mutations/proteins involved in AL Amyloidosis are poorly understood and the lack of knowledge is holding back scientists from studying a broader range of treatments as well as being able to diagnose patients much earlier in life. A large portion of scientific research on AL Amyloidosis is spent on understanding and locating varies proteins, mutations and gene expressions that play a role in the diseases rise (Zhou et al., 2012). Identifying and understanding such aspects not only helps with further treatment development but also helps diagnosis patients early in the disease stage or ideally before symptoms even take place. A large-scale characterization of the mutations and gene expression changes that occur when developing AL Amyloidosis is currently occurring (Zhou et al., 2012).
Clear Amyloid Protein Deposits (Immunotherapy / Physical):
Clearing Amyloid protein deposits directly was seen as impossible but recent research has now proves this misconception incorrect. It has been discovered that some immune cells known as Scavenger Cells have the capabilities of engulfing and breaking apart Amyloid deposits (Rosenzweig et al., 2017). However, the production and effectiveness of such cells are not at the levels required to clear large deposits of amyloids (Rosenzweig et al., 2017). New immunotherapy treatments are trying to accelerate the productiona nd functionality of such immune cells in order to be able to clear larger deposits as well as clear them quicker (Rosenzweig et al., 2017). 3 of such immuno-therapies are currently in stage 3 of clinical trails. There is a lot of hope in this form of treatment due to it being both efficient and safe (Rosenzweig et al., 2017).
Basic research has also begun on constructing machinery that can physically be inserted into the body, target amyloid deposits, break the amyloid deposits into smaller piece and finally remove all traces of the amyloid deposits (Rosenzweig et al., 2017). Constructing such machinery while ensuring the patients safety is not an easy task. Research into such machinery has only recently begun and is a ways away from being a realistic treatment method for AL Amyloidosis.
Proteasome Inhibitors:
There are currently 2 drugs in clinical trails that specifically target proteasomes required in the formation of the amyloid aggregates (Sanchorawala et al., 2017). Inhibition of such prevents such proteasomes from doing their role of degrading specific proteins (Sanchorawala et al., 2017). The proteins they normally degrade are proteins that stop the aggregation and formation of amyloid fibrils. Thus the 2 drugs would keep the preventive proteins in tact and amyloid fibrils would be prevented from every forming (Sanchorawala et al., 2017). The two drugs carfilzomib and ixazomib are very close to market release.
Other:
There are several other viable treatments being researched today in hopes of finding better treatments for AL Amyloidosis. A few others include:
1. Antibiotics: New antibiotics that interfere with Amyloid deposit formation. Does not stop poor light-chain formation but prevents the formation of dangerous and large Amyloid deposits (Gertz et al., 2016).
2. Targeting of SAP Protein: Potential new drug treatments are targeting the SAP protein which is a very specific protein structure found exclusively on Amyloid deposits and no other human cells/structures. Targeting such a protein will prevent any harm being done on normal healthy cells (Wechaleka et al., 2016).
3. Vaccines: The formation of a new vaccine is being studies that when administrated would protect vital organs such as the heart from Amyloid Formation. The basic idea is that such antibodies would coat and organs and prevent amyloid fibrils from attaching and growing (Sanchorawala et al., 2017).
Conclusion
Bringing it all together AL Amyloidosis is a dangerous bone marrow disorder that due to abnormal antibody light-chains forms lethal amyloids that disrupt the health of organs (Nienhuis, Bijzet & Hazenberg, 2016). AL Amyloidosis is still rather lethal even with newer treatments. It is important for older individuals to be aware, educated and take initiative. The Amyloidosis Foundation website is a great source for more information - provides background info, charities/fundraiser info, potential grants and future planned research. Researchers believe AL Amyloidosis will no longer be a threat in 10 years due to the current success rate of research and the release of more treatments.
References
- Amyloidosis Foundation. (n.d.). Retrieved from http://www.amyloidosis.org/
- Andrich, K., Hegenbart, U., Kimmich, C., Kedia, N., Bergen III, C.R., Schonland, S., Wanker, E.E., & Bieschke, J. (2016). Aggregation of full length immunoglobulin light chains from AL Amyloidosis patients is remodeled by epigallocatechin-3-gallate. Journal of Biological Chemistry, 292, 2328-2344. https://doi.org/10.1074/jbc.M116.750323
- Bernard, R., Chodirker, L., Masih-khan, E., Jiang, H., Franke, N, Kukreti, v., . . .Chen, C. (2015). Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone Marrow Transplantation, 50, 95-99. https://doi.org/10.1038/bmt.2014.226
- Biology.arizona.edu. (2017). Antibody Structure. [online] Available at: http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html [Accessed 6 Oct. 2017].
- Child, J.A., Morgan, J.G., Davies, E.F., Owen, R.G., Bell, S.E., Hawkins, K., Brown, J., Drayson, M.T., & Selby, P.J. (2003). High-Dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med, 348, 1875-1883. https://doi.org/10.1056/NEJMoa022340
- Derrer, T.D. (2015). Amyloidosis. WebMD. Retrieved from: http://www.webmd.com/cancer/lymphoma/amyloidosis-symptoms-causes-treatments#1
- Desport, E., Bridoux, F., Sirac,C., Delbes,S., Bender, S., Fernandez, B., … Jaccard, A. (2012). AL Amyloidosis. Orphanet Journal of Rare Diseases, 7, 54. https://doi.org/10.1186/1750-1172-7-54
- Ebert, E. C., & Nagar, M. (2008). Gastrointestinal manifestations of amyloidosis. The American Journal of Gastroenterology. 103(3), 776–787. https://doi.org/10.1111/j.1572-0241.2007.01669.x
- Gandelman, G. (2012). Cardiac Amyloidosis. The New York Times. Retrieved from: http://www.nytimes.com/health/guides/disease/cardiac-amyloidosis/overview.html?mcubz=0
- Gertz, M.A., Landau, H., Comenzo, R.L., Seldin, D., Weiss, B., & Zonder, J. (2016). First-in-Human Phase I/II Study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. Journal of Clinical Oncology, 34, 1097-1103. https://doi.org/10.1200/JCO.2015.63.6530
- Jantunen, E., Kuittinen, T., Penttilä, K., Lehtonen, P., Mahlamäki, E., & Nousiainen, T. (2006). High-dose melphalan (200 mg/m2) supported by autologous stem cell transplantation is safe and effective in elderly (greater than or equal to65 years) myeloma patients: comparison with younger patients treated on the same protocol. Bone Marrow Transplantation, 37, 917-922. https://doi.org/doi:10.1038/sj.bmt.1705360
- Liu, P.P., & Smyth, D. (2015). Wild-Type transthyretin amyloid cardiomyopathy a missed cause of heart failure with preserved ejection fraction with evolving treatment implications. Circulation,133,245-247. https://doi.org/10.1161/CIRCULATIONAHA.115.020351
- Ma, G., & Ra, K. (1994). Amyloidosis: prognosis and treatment. Semin Arthitis Rheum., 24(2), 124-138. https://doi.org/10.1016/S0049-0172(05)80006-X
- McLaurin, J., Franklin, T., Zhang, X., Deng, J., & Fraser, P. E. (1999). Interactions of Alzheimer amyloid-beta peptides with glycosaminoglycans effects on fibril nucleation and growth. European Journal of Biochemistry, 266(3), 1101–1110. https://doi.org/10.1046/j.1432-1327.1999.00957.x
- Merlini, G., & Bellotti, V. (2003). Molecular Mechanisms of Amyloidosis. New England Journal of Medicine, 349(6), 583–596. https://doi.org/10.1056/NEJMra023144
- Narita, M., Holtzman, D. M., Schwartz, A. L., & Bu, G. (1997). Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. Journal of Neurochemistry, 69(5), 1904–1911. https://doi.org/10.1046/j.1471-4159.1997.69051904.x
- Ngan, V. (2015). Purpura. DermNet New Zealand. Retrieved from https://www.dermnetnz.org/topics/purpura/
- NHS choices. (2017). Amyloidosis. NHS choices. Retrieved from: http://www.nhs.uk/Conditions/amyloidosis/Pages/Introduction.aspx#treatment
- Nienhuis H, L., A, Bijzet J., & Hazenberg B, P, C. (2016). The prevalence and management of systemic amyloidosis in western countries. Kidney Dis, 2, 10-19. https://doi.org/10.1159/000444206
- Nyirady, J. (2016). Primary systemic amyloidosis. MedScape. Retrieved from http://emedicine.medscape.com/article/1093258-overview
- Paiva, B., Almeida, J., Pérez-Andrés, M., Mateo, G., López, A., Rasillo, A., Orfao, A. (2010). Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry. Part B, Clinical Cytometry, 78(4), 239–252. https://doi.org/10.1002/cyto.b.20512
- Palladini, G., Perfetti, V., Obici, L., Caccialanza, R., Semino, A., Adami, F.,Cavallero, G., Rustichelli, R., Virga, G., & Merlini, G. (2004). Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood, 103, 2936-2938. https://doi.org/10.1182/blood-2003-08-2788
- Papadakis, M.A., & McPhee, S.J. (2017) Current: medical diagnosis & treatment. USA: McGraw-Hill Education
- Perfetti, V., Ubbiali, P., Vignarelli, M. C., Diegoli, M., Fasani, R., Stoppini, M.,Merlini, G. (1998). Evidence that amyloidogenic light chains undergo antigen-driven selection. Blood, 91(8), 2948–2954. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9531605
- Petukhov, M., Cregut, D., Soares, C. M., & Serrano, L. (1999). Local water bridges and protein conformational stability. Protein Science: A Publication of the Protein Society, 8(10), 1982–1989. https://doi.org/10.1110/ps.8.10.1982
- Ramirez-Alvarado, M. (2012). Amyloid formation in light chain amyloidosis. Current Topics in Medicinal Chemistry, 12(22), 2523–2533. https://doi.org/10.2174/1568026611212220007
- Real de Asúa, D., Costa, R., Galván, J. M., Filigheddu, M. T., Trujillo, D., & Cadiñanos, J. (2014). Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clinical Epidemiology, 6, 369–377. http://doi.org/10.2147/CLEP.S39981
- Ren, R., Hong, Z., Gong, H., Laporte, K., Skinner, M., Seldin, D. C., … Trinkaus-Randall, V. (2010). Role of glycosaminoglycan sulfation in the formation of immunoglobulin light chain amyloid oligomers and fibrils. The Journal of Biological Chemistry, 285(48), 37672–37682. https://doi.org/10.1074/jbc.M110.149575
- Rosenzweig, M., Urak R., Walter, M., Lim, L., Sanchez, J.F., Krishnan, A., Forman, S., & Wang, X. (2017). Preclinical data support leveraging CS1 chimeric antigen receptor T-cell therapy for systemic light chain amyloidosis. Cytotherapy, 19(7), 861-866. https://doi.org/10.1016/j.jcyt.2017.03.077
- Rosenzweig, M., Urak, R., Walter, M., Lim, L., Sanchez, J.F., Krishnan, A., Forman, S., & Wang, X. (2017). Preclinical data support leveraging CS1 chimeric antigen receptor T-cell therapy for systemic light chain amyloidosis. Cytotherapy, 19, 861-866. https://doi.org/10.1016/j.jcyt.2017.03.077
- Sanchorawala, V. (2006). Light-Chain (AL) Amyloidosis: diagnosis and treatment. Clinical Journal Of The American Society Of Nephrology, 1(6), 1331-1341. http://dx.doi.org/10.2215/cjn.02740806
- Sanchorawala, V., Palladini, G., Kukreti, V., Zonder, J.A., Cohen, A.D., Seldin, D.C., . . . Merlini, G. (2017). A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory light-chain (AL) amyloidosis. Blood, 130, 597-605. https://doi.org/10.1182/blood-2017-03-771220
- Schachter, H. (1984). Glycoproteins: their structure, biosynthesis and possible clinical implications. Clinical Biochemistry, 17(1), 3–14. https://doi.org/10.1016/S0009-9120(84)90360-6
- Schwarzman, A., Gregori, L., Vitek, M., Lyubski, S., Strittmatter, W., & Enghilde, J. et al. (1994). Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proceedings Of The National Academy Of Sciences, 91(18), 8368-8372. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC44607/
- Shiel, C.W., & Balentine, R. J. (2017). Amyloidosis. MedicineNet. Retrieved from: http://www.medicinenet.com/amyloidosis/article.htm#what_is_the_treatment_for_amyloidosis
- Tennent, G. A., Lovat, L. B., & Pepys, M. B. (1995). Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proceedings of the National Academy of Sciences of the United States of America, 92(10), 4299–4303. Received from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC41931/pdf/pnas01486-0235.pdf
- Tidy, C. (2014). Amyloidosis. Patient. Retrieved from: https://patient.info/doctor/amyloidosis-pro
- Wallick, S. C., Kabat, E. A., & Morrison, S. L. (1988). Glycosylation of a VH residue of a monoclonal antibody against alpha (1-6) dextran increases its affinity for antigen. The Journal of Experimental Medicine, 168(3), 1099–1109. . https://doi.org/10.1084/jem.168.3.1099
- Warsame, R., Kumar, S. K., Gertz, M. A., Lacy, M. Q., Buadi, F. K., Hayman, S. R., … Dispenzieri, A. (2015). Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer Journal, 5, 310. https://doi.org/10.1038/bcj.2015.34
- Wechalekar, A. (2017). Treatment of AL Amyloidosis. NAC. Retrieved from: http://www.amyloidosis.org.uk/al-amyloidosis/treatment/
- Wechalekar, A.D., Gilmore, J.D., & Hawkins P.N. (2016). Systemic amyloidosis. The Lancet, 387(38), 2641-2654. https://doi.org/10.1016/S0140-6736(15)01274-X
- Weiss, B. M., Wong, S. W., & Comenzo, R. L. (2016). Beyond the plasma cell: emerging therapies for immunoglobulin light chain amyloidosis. Blood, 127(19), 2275–2280. https://doi.org/10.1182/blood-2015-11-681650.
- Zemer, D., Pras, M., Sohar, E., Modan, M., Cabili, S., & Gafni, J. (1986). Colchicine in the prevention and treatment of the amyloidosis of familial mediterranean fever. New England Journal Of Medicine, 314(16), 1001-1005. http://dx.doi.org/10.1056/nejm198604173141601
- Zhang, C., Huang, X., & Li, J. (2017a). Light chain amyloidosis: Where are the light chains from and how they play their pathogenic role?. Blood Reviews, 31(4), 261–270. https://doi.org/10.1016/j.blre.2017.03.002
- Zhou, P., Hoffman, J., Landau, H., Hassoun, H., Lyer, L., & Comenzo, R.L. (2012). Clonal Plasma Cell Pathophysiology and clinical features of disease Are linked to clonal plasma cell Expression of cyclin D1 in systemic light-chain amyloidosis. Clinical Lymphoma Myeloma and Leukemia, 12, 49-58. https://doi.org/10.1016/j.clml.2011.09.217