This is an old revision of the document!
Table of Contents
Malaria
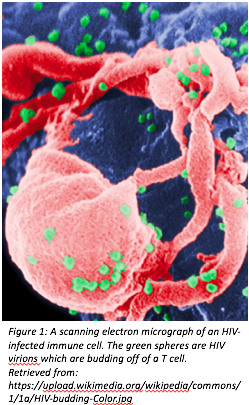
Human Immunodeficiency Virus, or HIV, is a retrovirus that causes AIDS, or acquired immune deficiency syndrome. It does this by infecting immune cells in the body, specifically CD4+ T cells, making the infected host vulnerable to infection. Without proper treatment of HIV, AIDS can take up to 8 years to develop, which can make diagnosis difficult (Sharp, 2011). An image of an infected CD4+ T cell can be seen in Figure 1 (right).
Introduction
Origins
HIV has two known strains, HIV-1 and HIV-2. The strain that is most commonly associated with AIDS pathogenicity is HIV-1. These strains originated from the Simmian Immunodeficiency Virus, or SIV, which initially infected several primates in Africa. Through cross-species transmission, several genetic modifications were made to SIV, allowing it to infect humans, leading to the classification of HIV (Sharp, 2011).
Once in the human population, the incredible pathogenicity of the virus allowed it to turn into a pandemic eventually killing millions of people around the world. It is thought that Hunters in West Africa were exposed to infected blood in primates, and initially contracted the virus. After this, through international travel and changes in patterns of sexual behaviour, the virus was able to transmit into several areas in the world (Sharp, 2011).
History of HIV/AIDS
<style justify>
In 1981, AIDS first broke out in the US amongst gay men in New York and San Francisco. They were initially classified as having a mysterious pneumonia-like disease that presented with a rare from of skin cancer, thrush, and other characteristic symptoms. Since this was highly prevalent in gay men, the name for this disease was called GRID, or Gay-related Immune Deficiency (Holland, 2013).
In a matter of 2 years, AIDS became a pandemic. This was largely due to the fact that research funding was compromised due to the prejudice surrounding gay men at the time. This halt in research gave the virus time to spread across the world, such as Africa, Europe, and Asia. More importantly, the disease was found to be prevalent in both sexes, which contradicted the initial theory that gay interactions lead to the disease, and so it was re-labelled to AIDS. Unfortunately, AIDS became the leading cause of death in people aged 25-44, however, this statistic accelerated research surrounding the disease (Holland, 2013)
Since finding a cure to AIDS was incredibly difficult, the goal was shifted to AIDS prevention. In 1984, the first method of prevention was established based on the finding that AIDS was primarily transmitted through sexual intercourse associated with mucosal membrane abrasions, and blood transfusions. Thus, prevention methods included the promotion of safe sex (especially through the use of condoms), testing blood in blood banks, and giving clean needles to drug users (“A Timeline of HIV/AIDS”, n.d).
By the late 1980s, HIV was officially labelled as a retrovirus that causes AIDS. As research surrounding the properties of retrovirus continued, the first anti-retroviral drugs were created and administered. One of these drugs was called zidovudine, or AZT. This prevented AIDS progression, rather than providing a cure (Holland, 2013).
However, despite advances in AIDS therapy, the majority of people suffering from AIDS did not have proper health care or access to treatment. The region with the highest prevalence of AIDS was in Sub-Saharan Africa. By 1999, around 33 million people, including women, children and men, were living with HIV. Since the first reported case of AIDS in the 1980s, around 14 million people had died (“Global HIV/AIDS Overview”, 2016).
Today, testing methods for AIDS are quick, and easily determined which allows for early diagnosis. These people must continue to take anti-retroviral therapy for the rest of their lives to prevent AIDS progression, but they can live healthy lives. However, there are still a large proportion of people, especially in Sub-Saharan Africa, who do not have access to either prevention methods (like safe sex), or anti-retroviral treatment (Holland, 2013).
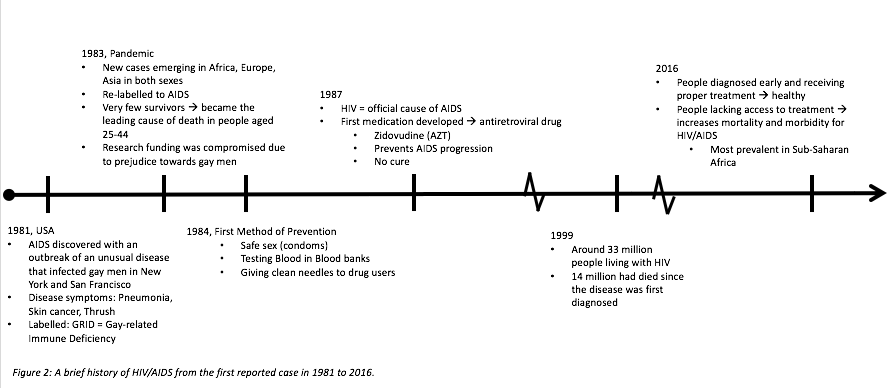
Currently, 36.7 million people are living with AIDS, 1.8 million of cases are in children. Since the start of the epidemic, 35 million people have died. However, the number of people receiving treatment drastically increases every year, with 17 million people receiving anti-retroviral treatment this year, which is 10 million higher than the statistic 2 years ago. Of the total pregnant women suffering from HIV, 77% of them are being treated, and there has a 50% decline in the rate of incidence in newborns, which again, is a huge advancement (“Global HIV/AIDS Overview”, 2016). A summarized version of the timeline can be seen in Figure 2 (below).
</style>
Epidemiology
<style justify>
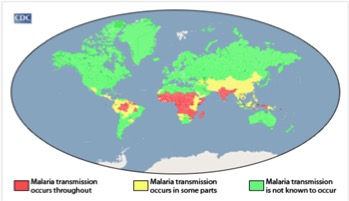
Malaria is most prevalent in tropical areas of the world, with a particular increase seen in impoverished areas. Mosquitos thrive in warm and humid climates and therefore malaria is increasingly seen tropical and subtropical areas of the world. Africa has a disproportionally high prevalence of malaria compared to the rest of the world. Its warm climate allows for substantial mosquito breeding which partially explain why this area has the highest incidence of malaria. The warm weather allows malaria-infected mosquitoes to thrive all year long. In addition, Africa has a lack of resources and finances to combat the issue. The continent is home to the Anopheles gambiae mosquito, which is known to be very efficient and confer high transmission rates. The factors described make certain areas more susceptible to malaria occurrence, Africa has a combination of all these factors which therefore explains why it has the highest prevalence (CDC, 2016). </style>
<style justify> Rates of malaria infection have significantly decreased since the year 2015 due to better protective practices and preventative measures such as greater access to protective bed nets. Globally, there has been a 37% decrease in the number of individuals affected by malaria since the year 2000. In addition there has been a 60% decrease in the number of malaria deaths since 2000, meaning treatment has become more advanced and accessible throughout the world. 3.2 billion people worldwide are at risk for being infected with malaria. The number of people affected by malaria worldwide in the year 2015, was 214 million individuals with 438,000 of those cases resulting in death. The highest prevalence of malaria was seen in Africa and South-East Asia, however Africa was still significantly higher than any other country. In 2015, malaria was the third leading cause of death in Africa. However, before 2004, malaria was the leading cause of death in Africa therefore its incidence has been on the decline. North and South America combined had only 660 cases of malaria in 2015, most of which were reported in South America, an area with a more tropical climate and therefore higher susceptibility to malaria (WHO, 2016). </style>
<style justify> Individuals who have the highest risk of contracting malaria are children, pregnant women and travelers. Children and pregnant women have decreased immune systems and therefore are less able to fight off the disease. Men and women have the same probability of contracting malaria; this excludes women who are pregnant in which case the incidence is higher. In addition, travelers from different countries where malaria is not prevalent have not developed sufficient immune responses towards the parasitic disease and therefore are at higher risk for disease contraction. Individuals with HIV/AIDS (which is extremely prevalent in Africa) also have increased susceptibility to malaria due to their weakened immune systems. The overlap between HIV/AIDS and malaria prevalence within Africa is a factor that leads to the increased mortality of malaria seen in this continent. (CDC, 2016). </style>
Figure:Malaria Prevalence Worldwide. Retrieved from: https://www.cdc.gov/malaria/malaria_worldwide/impact.html
Transmission and Mechanism of Action
Life Cycle
The malaria life cycle begins when the P. falcifarum sporozoites enter the bloodstream of the human host from the Anopheles mosquito (Haldar, Murphy, Milner, Taylor, 2007) . The sporozoites are the stage in parasite development that is infectious to humans (“Anopheles Mosquitoes”, 2015), and they briefly circulate in the body upon entering the hepatocytes. In the hepatocytes, the sporozoites undergo asexual reproduction for 10 to 12 days, after which the liver cells rupture and release merozoites, their daughter cells (STAN) into the bloodstream (Haldar et al., 2007). These merozoites are non-motile and therefore likely interact with the erythrocytes by random collision (Wiser M.F., 2016). Although the exact mechanism of the entry into the erythrocyte is still not fully understood, it has been found that the merozoite surface proteins (MSPs), specifically MSP-1, interact with the glycoprotein band 3 on the red blood cells (Haldar et al., 2007). A tight junction forms between the apical end of the merozoite and the erythrocyte, as the apical organelles as the apical membrane antigen-1 (AMA-1) interacts with the rhoptry neck proteins that are inserted into the host cells (Cowman, Berry, Baum 2012) Once the merozoites have invaded the surrounding erythrocytes, they undergo asexual reproduction for the next two days. These merozoites then develop into ring stage trophozoites, the feeding or growing stage of the parasite development, as they feed off the host resources within this time (Haldar et al., 2007). The trophozoites then mature into schizonts, which develop into 16 to 32 daughter merozoites. These merozoites undergo another round of asexual reproduction, with some differentiating into female and male gametes. The gametes then can be ingested by the female mosquito, where they fuse and reproduces into infective sporozoites via sexual reproduction (Haldar et al., 2007). These sporozoites are then passed on to the next person at the female mosquitos’ next blood meal, and the cycle repeats.
HIV Life Cycle
<style justify>
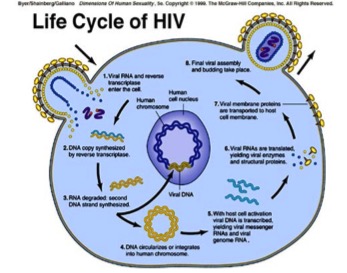
The HIV virus first gains entry into the host cell where it is able to stimulate certain signaling cascades that assist in viral replication. Specifically, HIV attacks CD4 immune cells, which are a type of white blood cell that protects the body from infection. HIV viral cells have two molecules attached to its’ surface, GP120 and GP41 cells. GP120 binds to CD4 receptor cells; this causes conformational changes and spore formation that allow the virus to gain entry into the CD4 immune cells. Once in the cell, the viral genome is released and it is reverse transcribed into DNA by the viral enzyme, reverse transcriptase. The viral genome is then inserted into the host chromosome via the viral enzyme integrase. The DNA is transcribed and translated into proteins and exported out of the host cell via the vesicular sorting pathway. These new HIV viral particles are immature and noninfectious; therefore it cannot infect another cell (Simon, Ho & Karim, 2006). However, once the noninfectious HIV has left the host CD4 cell, the HIV releases protease, which breaks up longer protein chains within the noninfectious virus, thus forming the mature and infectious form of HIV (AidsInfo, n.d.).
</style>
Figure 5: Life Cycle of the HIV virus. Retrieved from: http://nigeriahivinfo.com/wp-content/uploads/2015/10/hiv-virus-life-cycle.jpg
Symptomatology of Malarial Infection
Clinical Symptoms
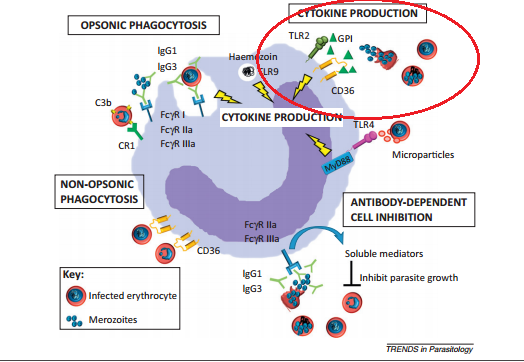
The clinical symptoms of malaria arise during the blood cell infection stage. Parasite-associated products such as glycophosphatidylinositol (GPI), hemozoin, and other substances accumulate in the infected erythrocyte and when the cell ruptures these toxins are released along with the merozoites (Chua, Brown, Hamilton, Rogerson, Boeuf, 2013). The presence of these toxins induce the macrophages to release inflammatory cytokines (Chua et al., 2013), which in turn produce a fever, chills, and weakness. (“Malaria”, 2015) These first symptoms of fever, chills, aches, and vomiting appear roughly 7 days after the parasite first enters the body. (“Malaria, 2016)
Stages
There are three stages of a malarial infection: the initial cold stage, where the person feels cold and shivers; the following hot stage where the person develops a fever and may experience vomiting; and the final sweating stage, where the body temperature returns to normal and the individual experiences extreme tiredness. This entire experience lasts anywhere from 6 to 10 hours (“Malaria, 2015), which makes it very difficult to isolate the symptoms as a result of a malarial infection. If the infection is not treated within 24 hours, the person is at risk of their infection progressing to a severe stage, which could resul</style>t in death (“Malaria”, 2016).
Severe Malaria
Severe malaria infections are defined when the patient experiences serious organ failure or blood abnormalities in. Symptomatology of severe malaria include cerebral malaria, where the patient experiences abnormal behaviour, seizures, and even falls into a coma. An aggressive infection can cause hemolysis, which will lead to severe anemia. Additionally, when more than 5% of the erythrocytes are infected with the malaria parasite, the person is deduced to have hyperparasitemia. Severe malaria can also lead to acute respiratory distress syndrome (ARDS), an inflammatory reaction in the lungs that prevents effective oxygen exchange between the lung tissue and blood (“Malaria”, 2015). A buildup of fluid in the alveoli inhibits oxygen moving into the bloodstream, and this fluid buildup also stiffens and hardens the lungs, which prevents their ability to properly expand. (Hadjiliadis, 2015)
Epidemiology
<style justify>
Malaria is most prevalent in tropical areas of the world, with a particular increase seen in impoverished areas. Mosquitos thrive in warm and humid climates and therefore malaria is increasingly seen tropical and subtropical areas of the world. Africa has a disproportionally high prevalence of malaria compared to the rest of the world. Its warm climate allows for substantial mosquito breeding which partially explain why this area has the highest incidence of malaria. The warm weather allows malaria-infected mosquitoes to thrive all year long. In addition, Africa has a lack of resources and finances to combat the issue. The continent is home to the Anopheles gambiae mosquito, which is known to be very efficient and confer high transmission rates. The factors described make certain areas more susceptible to malaria occurrence, Africa has a combination of all these factors which therefore explains why it has the highest prevalence (CDC, 2016). </style>
<style justify> Rates of malaria infection have significantly decreased since the year 2015 due to better protective practices and preventative measures such as greater access to protective bed nets. Globally, there has been a 37% decrease in the number of individuals affected by malaria since the year 2000. In addition there has been a 60% decrease in the number of malaria deaths since 2000, meaning treatment has become more advanced and accessible throughout the world. 3.2 billion people worldwide are at risk for being infected with malaria. The number of people affected by malaria worldwide in the year 2015, was 214 million individuals with 438,000 of those cases resulting in death. The highest prevalence of malaria was seen in Africa and South-East Asia, however Africa was still significantly higher than any other country. In 2015, malaria was the third leading cause of death in Africa. However, before 2004, malaria was the leading cause of death in Africa therefore its incidence has been on the decline. North and South America combined had only 660 cases of malaria in 2015, most of which were reported in South America, an area with a more tropical climate and therefore higher susceptibility to malaria (WHO, 2016). </style>
<style justify> Individuals who have the highest risk of contracting malaria are children, pregnant women and travelers. Children and pregnant women have decreased immune systems and therefore are less able to fight off the disease. Men and women have the same probability of contracting malaria; this excludes women who are pregnant in which case the incidence is higher. In addition, travelers from different countries where malaria is not prevalent have not developed sufficient immune responses towards the parasitic disease and therefore are at higher risk for disease contraction. Individuals with HIV/AIDS (which is extremely prevalent in Africa) also have increased susceptibility to malaria due to their weakened immune systems. The overlap between HIV/AIDS and malaria prevalence within Africa is a factor that leads to the increased mortality of malaria seen in this continent. (CDC, 2016). </style>
Figure:Malaria Prevalence Worldwide. Retrieved from: https://www.cdc.gov/malaria/malaria_worldwide/impact.html
Treatments
<style justify>
Antimalarials can be prescribed as a form of pre-exposure prophylaxis, or upon infection, depending on the geographical location that the individual is in. People living in areas of little to no malarial transmission, such as countries in North America, are prescribed oral medications only if they are travelling to areas with high malarial transmission, such as South Asian and African countries. This prevents future malarial infection, which is important because people living in these Western countries do not receive partial immunity to malaria, as they are not exposed to malaria. People living in these areas of high malarial transmission will only be prescribed antimalarial medication if they are infected with the parasite. Due to the highly pathogenic nature of the protozoan parasite, both pre- and post-exposure antimalarial medications are administered as a form of combination therapy. This includes a fast-acting anti-malarial, and a slow-acting antimalarial. Fast acting antimalarials reduce the main parasitic load in the blood, to prevent progression of the disease and provide symptomatic relief. Slow-acting antimalarials target the residual parasitic load with the goal of completely eliminating the parasite.
Pre-exposure Prophylaxis:
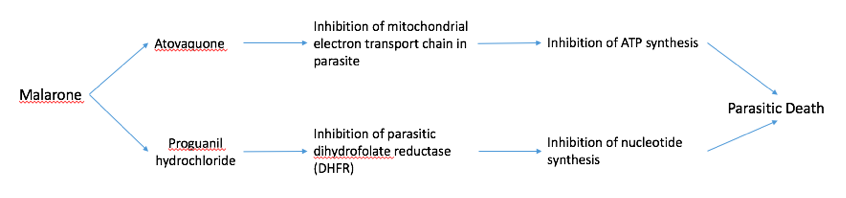
Malarone Malarone is a common drug given to people who are travelling to areas with high malarial transmission, such as India, Sub-Sahel Africa, and other African countries. Malarone is a form of combination therapy which consists of a fast- and slow-acting antimalarial (Figure #). The fast-acting drug, atovaquone acts by selectively inhibiting the electron transport chain in the parasitic mitochondria. This inhibits ATP synthesis which leads to death of the parasite. Proguanil hydrochloride is a slow-acting drug that inhibits the parasitic dihydrofolate reductase (DHFR) enzyme. DHFR is essential to converting folic acid into purine and pyrimidines for DNA synthesis. Inhibition of this enzyme prevents parasitic replication and growth, also contributing to parasitic death. Both of these drugs are included in a pill which is to be taken every day that the traveler is in the foreign country.
Figure #: A schematic diagram showing the mechanisms of the fast-acting drug, atovaquone, and the slow-acting drug, proguanil hydrochloride, in malarone.
Fast-Acting Antimalarials
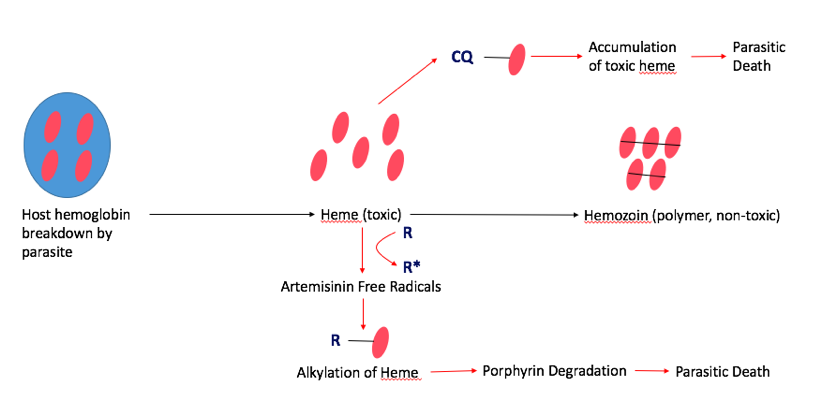
Fast-acting antimalarials act by disrupting the heme levels in the parasite. Malaria depends heavily on host heme groups for its survival, which makes this pathway an appropriate target for treatments. In the normal pathway, the parasite first breaks down host hemoglobin via proteolysis releasing several heme groups inside the parasite. However, individual heme groups are toxic to malaria. In order to combat this, malaria developed a mechanism to sequester and polymerize the individual heme groups to create hemozoin, which is non-toxic to the parasite. Common fast-acting antimalarials, such as Artemisinin and Chloroquine interfere with hemozoin formation, which is fatal to the parasite (Figure #).
Artemisinin: Artemisinin is the fast-acting antimalarial components of the most widespread form of treatment, called artemisinin-based combination therapy (ACT). This treats the most common form of malaria, known as uncomplicated P. Falciparum Malaria. Artemisinin was actually used in traditional Chinese medicine, and famous scientist, Tu Youyou, discovered its use as an antimalarial in the 1980s, which awarded her the Nobel Prize in Medicine in 2015. Artemisinin disrupts the hemozoin pathway by binding to the parasitic heme-iron group. This creates a toxic level of free-radicals within the parasite, which damages several intracellular proteins via alkylation, including heme itself. Ultimately, this results in the degradation of the porphyrin ring in the heme group, which is fatal to the parasite.
Chloroquine Chloroquine is another fast-acting drug that is used for treatment against P.vivax, the second most common form of malaria. Chloroquine also targets the parasitic hemoglobin pathway. It acts similarly to artemisinin in that it binds to the toxic heme groups, preventing heme polymerization into hemozoin within the parasite. This leads to accumulation of the toxic heme groups leading to parasite and cell lysis.
Figure #: The mechanisms of two fast-acting antimalarials, artemisinin (R) and chloroquine (CQ) via the parasitic hemoglobin pathway.
Slow-Acting Antimalarials:
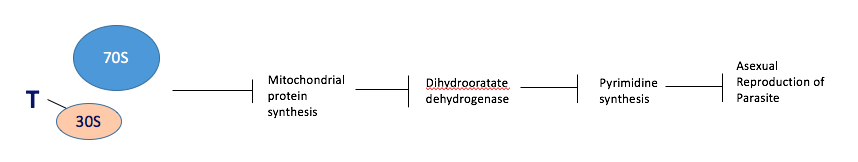
Tetracycline Tetracycline is a slow-acting drug that is used in combination with fast-acting drugs. It acts by binding to the 30S ribosomal subunit inside the parasite’s mitochondria, preventing translation of mRNA transcripts to polypeptides. This prevents the synthesis of key enzymes, especially those involved in pyrimidine synthesis, such as dihydrooratate dehydrogenase. Ultimately this prevents the asexual reproduction of the parasite (Figure #).
Figure #: The mechanism of a slow-acting antimalarial, tetracycline, via inhibition of parasitic asexual reproduction.
Current Research
<style justify>
As of November 2nd 2016, the first HIV Vaccine Trial has been initiated in South Africa, which has 7 million HIV-infected individuals, or one-fifth of the world HIV-positive population. Approximately 5400 men and women are enrolled in Phase III clinical trials of the HVTN (HIV Vaccine Trials Network) 702 vaccine (Surugue, 2016). The vaccine regimen actually consists of two different vaccines: the first is a canarypox-based vaccine, and the second is a bivalent GP120 subunit with an adjuvant to elicit an immune response. The canarypox-based vaccine is viral vaccine that elicits a cytotoxic T cell response towards HIV-infected cells. The bivalent GP120 vaccine with and adjuvant also helps boost the host's immune system by developing antibodies to recognize and remove viral particles if they enter the body. The type of GP120 subunit used is known to be associated with the strain of HIV that is the most prevalent in South Africa (“Large Scale HIV Vaccine Trial”, 2016). This provides the population with pre-exposure prophylaxis to prevent the the incidence and spread of HIV. The trial will last from November 2016 to December 2020 (Surugue, 2016).
</style>
References
<style justify>
- A Timeline of HIV/AIDS. (n.d.). Retrieved October 17, 2016, from https://www.aids.gov/hiv-aids-basics/hiv-aids-101/aids-timeline/index.html
- AIDS.gov. (2015). HIV Test Types. Retrieved from https://www.aids.gov/hiv-aids-basics/prevention/hiv-testing/hiv-test-types/AIDS.gov. (2015). HIV Test Types. Retrieved from https://www.aids.gov/hiv-aids-basics/prevention/hiv-testing/hiv-test-types/
- AIDS.gov. (2015). Stages of HIV Infection. Retrieved from https://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/hiv-in-your-body/stages-of-hiv/
- AIDSInfo. (2016). HIV Prevention. Retrieved from https://aidsinfo.nih.gov/education-materials/fact-sheets/20/48/the-basics-of-hiv-prevention
- AidsInfo. N.d. HIV Life Cycle. Retrieved from: https://aidsinfo.nih.gov/education-materials/fact-sheets/19/45/hiv-aids--the-basics
- Berger, E., Garrett, L., MacGregor, R. R., Vonmuller, E., Weiner, D. 2016. HIV and AIDS. Annenberg Learner. 91-106.
- Centers for Disease Control and Prevention. (2016). HIV/AIDS Testing. Retrieved from http://www.cdc.gov/hiv/basics/testing.html
- Global HIV/AIDS Overview. (2016, September 28). Retrieved October 20, 2016, from https://www.aids.gov/federal-resources/around-the-world/global-aids-overview/index.html
- Hall, H., Geduld, J., Boulos, D., Rhodes, P., An, Q., & Mastro, T. et al. (2009). Epidemiology of HIV in the United States and Canada: Current Status and Ongoing Challenges. JAIDS Journal Of Acquired Immune Deficiency Syndromes, 51(Supplement 1), S13-S20. http://dx.doi.org/10.1097/qai.0b013e3181a2639e
- Holland, K., & Krucik, G. (2013, July 12). The History of HIV. Retrieved October 17, 2016, from http://www.healthline.com/health/hiv-aids/history#EarliestCase1
- Large-Scale HIV Vaccine Trial to Launch in South Africa | NIH: National Institute of Allergy and Infectious Diseases. (2016, May 18). Retrieved November 04, 2016, from https://www.niaid.nih.gov/news-events/large-scale-hiv-vaccine-trial-launch-south-africa
- Nordqvist, C. (2016, May 11). “HIV/AIDS: Causes, Symptoms and Treatments.” Medical News Today. Retrieved 26 October 2016, from http://www.medicalnewstoday.com/articles/17131.php.
- Robinson, J. (2016). Understanding Treatment of AIDS/HIV. WebMD. Retrieved 26 October 2016, from http://www.webmd.com/hiv-aids/guide/understanding-aids-hiv-treatment?page=3
- Sharp, P. M., & Hahn, B. H. (2011). Origins of HIV and the AIDS pandemic. Cold Spring Harbor perspectives in medicine, 1(1), a006841.
- Simon, V., Ho, D. D., Karim, Q. A. 2006. HIV/AIDS Epidemiology, pathogenesis, prevention and treatment. The Lancet, 368: 489-504.
- Surugue, L. (2016, November 2). First large-scale HIV vaccine trial in seven years to start in South Africa. International Business Times. Retrieved November 2, 2016, from http://www.ibtimes.co.uk/first-large-scale-hiv-vaccine-clinical-trial-seven-years-start-south-africa-1589468
- University of California San Francisco. (2006). HIV Antibody Assays. Retrieved from http://hivinsite.ucsf.edu/InSite.jsp?page=kb-02-02-01
</style>