Table of Contents
Malaria
Malaria and malaria-causing parasites have been known to mankind for thousands of years. Around 7,000-12,000 years ago the increasing temperatures in Africa caused a rise in humidity, thus creating many new water bodies and allowing for agricultural development in the Middle East and North East Africa to occur (Srinivas, 2016). This led to the formulation of a favourable climate and area for breeding and transmission of malaria parasites and its carrier, the mosquito.
PowerPoint Presentation: malaria_-_final_.pdf
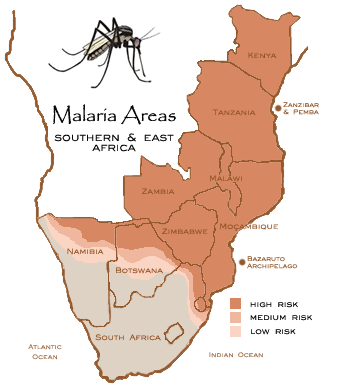
Figure 1: Malaria Areas. Retrieved from: http://www.thesafaricompany.co.za/images/Africa%20Malaria.gif
History
Discovery of the Malaria Parasite (1880)
Charles Louis Alphonse Laveran, a French army surgeon stationed in Constantine, Algeria, was the first to notice parasites in the blood of a patient suffering from malaria. This occurred on the 6th of November 1880. For his discovery, Laveran was awarded the Nobel Prize in 1907 (Lambert, 2016).
Differentiation of Species of Malaria (1886)
<style justify>
Camillo Golgi, an Italian neurophysiologist, established that there were at least two forms of the disease, one with tertian periodicity (fever every other day) and one with quartan periodicity (fever every third day). He also observed that the forms produced differing numbers of merozoites (new parasites) upon maturity and that fever coincided with the rupture and release of merozoites into the blood stream. He was awarded a Nobel Prize in Medicine for his discoveries in neurophysiology in 1906 (Fagan, 2016).
</style>
Naming of Human Malaria Parasites (1890, 1897)
<style justify>
The Italian investigators Giovanni Batista Grassi and Raimondo Filetti first introduced the names Plasmodium vivax and P. malariae for two of the malaria parasites that affect humans in 1890 (Mandal, 2016). Laveran had believed that there was only one species, Oscillaria malariae. An American, William H. Welch, reviewed the subject and, in 1897, he named the malignant tertian malaria parasite P. falciparum.
</style>
Discovery That Mosquitoes Transmit Malaria Parasites (1897-1898)
<style justify>
On August 20th, 1897, Ronald Ross, a British officer in the Indian Medical Service, was the first to demonstrate that malaria parasites could be transmitted from infected patients to mosquitoes (Lambert, 2016). For his discovery, Ross was awarded the Nobel Prize in 1902.
</style>
Transmission and Mechanism of Action
Life Cycle
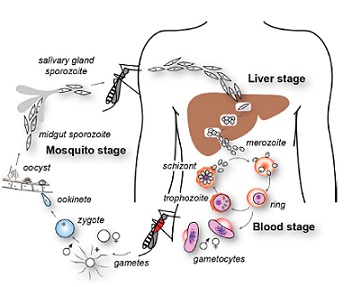
The malarial life cycle begins when the P. falcifarum sporozoites enter the bloodstream of the human host from the Anopheles mosquito (Haldar, Murphy, Milner, Taylor, 2007) . The sporozoites are the stage in parasite development that is infectious to humans (“Anopheles Mosquitoes”, 2015), and they briefly circulate in the body upon entering the hepatocytes. Within the hepatocytes, the sporozoites undergo asexual reproduction for 10 to 12 days, after which the liver cells rupture and release merozoites (their daughter cells) into the bloodstream (Haldar et al., 2007). These merozoites are non-motile and therefore likely interact with the erythrocytes by the occurrence of a random collision (Wiser M.F., 2016). Although the exact mechanism of the entry into the erythrocyte is still not fully understood, it has been found that the merozoite surface proteins (MSPs), specifically MSP-1, interact with the glycoprotein band 3 on the red blood cells (Haldar et al., 2007). A tight junction forms between the apical end of the merozoite and the erythrocyte, as the apical organelles as the apical membrane antigen-1 (AMA-1) interacts with the rhoptry neck proteins that are inserted into the host cells (Cowman, Berry, Baum 2012) Once the merozoites have invaded the surrounding erythrocytes, they undergo asexual reproduction for the next two days. These merozoites then develop into ring stage trophozoites, the feeding or growing stage of the parasite development, as they feed off the host resources within this time (Haldar et al., 2007). The trophozoites then mature into schizonts, which develop into 16 to 32 daughter merozoites. These merozoites undergo another round of asexual reproduction, with some differentiating into female and male gametes. The gametes then can be ingested by the female mosquito, where they fuse and reproduces into infective sporozoites via sexual reproduction (Haldar et al., 2007). These sporozoites are then passed on to the next person at the female mosquitos’ next blood meal, and the cycle repeats.
Figure 2: Life cycle of P.falciparum parasite. Retrieved from “The cellular and molecular basis for malaria parasite invasion of the human red blood cell”, Cowman A.F, Berry D, Baum J, 2012, The Journal of Cell Biology, 198, p. 962. www.jcb.org/cgi/doi/10.1083/jcb.201206112
Transmission
Malaria parasites are transmitted to humans via female Anopheles mosquitoes. Anopheles mosquitoes can be identified through specific characteristics such as spotted wings, the absence of a buzzing sound, and when at rest their body is inclined at 45 degrees to the surface. Only Anopheles mosquitoes can transmit malaria, and they must have been infected through a previous blood meal taken from an infected person. Since the malaria parasite is found in the red blood cells, malaria can also be transmitted through blood transfusions, organ transplants, or the use of shared syringes/needles that are contaminated with blood. It can also be transmitted from a mother to her unborn baby in utero or during delivery. Malaria is not contagious, meaning that it cannot spread from casual contact from person to person and cannot be sexually transmitted. (Euroclinix, 2016)
Symptomatology of Malarial Infection
Clinical Symptoms
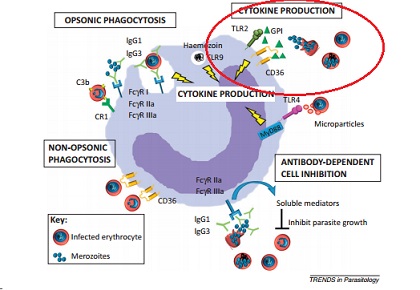
The clinical symptoms of malaria arise during the blood cell infection stage. Parasite-associated products such as glycophosphatidylinositol (GPI), hemozoin, and other substances accumulate in the infected erythrocyte and when the cell ruptures these toxins are released along with the merozoites (Chua, Brown, Hamilton, Rogerson, Boeuf, 2013). The presence of these toxins induce the macrophages to release inflammatory cytokines (Chua et al., 2013), which in turn produce a fever, chills, and weakness. (“Malaria”, 2015) These first symptoms of fever, chills, aches, and vomiting appear roughly 7 days after the parasite first enters the body. (“Malaria, 2016)
Figure 3: Mechanisms involved in malarial prevention by leukocytes. Circled area indicates the section of interest, where glycophosphatidylinositol (GPI) and hemozoin activate macrophages to produce cytokines, which induce an inflammatory response. Adapted from “Monocytes and macrophages in malaria: Protection or pathology?”Chua, C. L. et al., 2013, Trends in Parasitology, 29, p. 27. doi:10.1016/j.pt.2012.10.002
Stages
There are three stages of a malarial infection: the initial cold stage, where the person feels cold and shivers; the following hot stage where the person develops a fever and may experience vomiting; and the final sweating stage, where the body temperature returns to normal and the individual experiences extreme tiredness. This entire experience lasts anywhere from 6 to 10 hours (“Malaria, 2015), which makes it very difficult to isolate the symptoms as a result of a malarial infection. If the infection is not treated within 24 hours, the person is at risk of their infection progressing to a severe stage, which could result in death (“Malaria”, 2016).
Severe Malaria
Severe malaria infections are defined when the patient experiences serious organ failure or blood abnormalities in. Symptomatology of severe malaria include cerebral malaria, where the patient experiences abnormal behaviour, seizures, and even falls into a coma. An aggressive infection can cause hemolysis, which will lead to severe anemia. Additionally, when more than 5% of the erythrocytes are infected with the malaria parasite, the person is deduced to have hyperparasitemia. Severe malaria can also lead to acute respiratory distress syndrome (ARDS), an inflammatory reaction in the lungs that prevents effective oxygen exchange between the lung tissue and blood (“Malaria”, 2015). A buildup of fluid in the alveoli inhibits oxygen moving into the bloodstream, and this fluid buildup also stiffens and hardens the lungs, which prevents their ability to properly expand. (Hadjiliadis, 2015)
Epidemiology
<style justify>
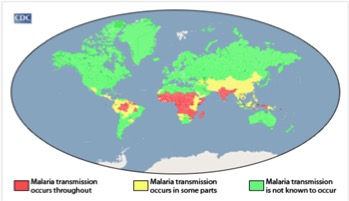
Malaria is most prevalent in tropical areas of the world, with a particular increase seen in impoverished areas. Mosquitos thrive in warm and humid climates and therefore malaria is increasingly seen tropical and subtropical areas of the world. Africa has a disproportionally high prevalence of malaria compared to the rest of the world. Its warm climate allows for substantial mosquito breeding which partially explain why this area has the highest incidence of malaria. The warm weather allows malaria-infected mosquitoes to thrive all year long. In addition, Africa has a lack of resources and finances to combat the issue. The continent is home to the Anopheles gambiae mosquito, which is known to be very efficient and confer high transmission rates. The factors described make certain areas more susceptible to malaria occurrence, Africa has a combination of all these factors which therefore explains why it has the highest prevalence (CDC, 2016). </style>
<style justify> Rates of malaria infection have significantly decreased since the year 2015 due to better protective practices and preventative measures such as greater access to protective bed nets. Globally, there has been a 37% decrease in the number of individuals affected by malaria since the year 2000. In addition there has been a 60% decrease in the number of malaria deaths since 2000, meaning treatment has become more advanced and accessible throughout the world. 3.2 billion people worldwide are at risk for being infected with malaria. The number of people affected by malaria worldwide in the year 2015, was 214 million individuals with 438,000 of those cases resulting in death. The highest prevalence of malaria was seen in Africa and South-East Asia, however Africa was still significantly higher than any other country. In 2015, malaria was the third leading cause of death in Africa. However, before 2004, malaria was the leading cause of death in Africa therefore its incidence has been on the decline. North and South America combined had only 660 cases of malaria in 2015, most of which were reported in South America, an area with a more tropical climate and therefore higher susceptibility to malaria (WHO, 2016). </style>
<style justify> Individuals who have the highest risk of contracting malaria are children, pregnant women and travelers. Children and pregnant women have decreased immune systems and therefore are less able to fight off the disease. Men and women have the same probability of contracting malaria; this excludes women who are pregnant in which case the incidence is higher. In addition, travelers from different countries where malaria is not prevalent have not developed sufficient immune responses towards the parasitic disease and therefore are at higher risk for disease contraction. Individuals with HIV/AIDS (which is extremely prevalent in Africa) also have increased susceptibility to malaria due to their weakened immune systems. The overlap between HIV/AIDS and malaria prevalence within Africa is a factor that leads to the increased mortality of malaria seen in this continent. (CDC, 2016). </style>
Figure 4:Malaria Prevalence Worldwide. Retrieved from: https://www.cdc.gov/malaria/malaria_worldwide/impact.html
Treatments
Antimalarials can be prescribed as a form of pre-exposure prophylaxis, or upon infection, depending on the geographical location that the individual is in. People living in areas of little to no malarial transmission, such as countries in North America, are prescribed oral medications only if they are travelling to areas with high malarial transmission, such as South Asian and African countries. This prevents future malarial infection, which is important because people living in these Western countries do not receive partial immunity to malaria, as they are not exposed to malaria. People living in these areas of high malarial transmission will only be prescribed antimalarial medication if they are infected with the parasite (World Health Organiation, 2006). Due to the highly pathogenic nature of the protozoan parasite, both pre- and post-exposure antimalarial medications are administered as a form of combination therapy. This includes a fast-acting anti-malarial, and a slow-acting antimalarial. Fast acting antimalarials reduce the main parasitic load in the blood, to prevent progression of the disease and provide symptomatic relief. Slow-acting antimalarials target the residual parasitic load with the goal of completely eliminating the parasite (World Health Organization, 2006; Tripathi, 2013).
Pre-exposure Prophylaxis:
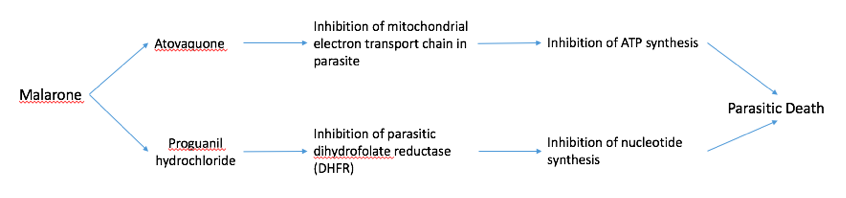
Malarone Malarone is a common drug given to people who are travelling to areas with high malarial transmission, such as India, Sub-Sahel Africa, and other African countries. Malarone is a form of combination therapy which consists of a fast- and slow-acting antimalarial (Figure 5). The fast-acting drug, atovaquone acts by selectively inhibiting the electron transport chain in the parasitic mitochondria. This inhibits ATP synthesis which leads to death of the parasite. Proguanil hydrochloride is a slow-acting drug that inhibits the parasitic dihydrofolate reductase (DHFR) enzyme. DHFR is essential to converting folic acid into purine and pyrimidines for DNA synthesis. Inhibition of this enzyme prevents parasitic replication and growth, also contributing to parasitic death. Both of these drugs are included in a pill which is to be taken every day that the traveler is in the foreign country (“Malarone”,2016).
Figure 5: A schematic diagram showing the mechanisms of the fast-acting drug, atovaquone, and the slow-acting drug, proguanil hydrochloride, in malarone.
Fast-Acting Antimalarials
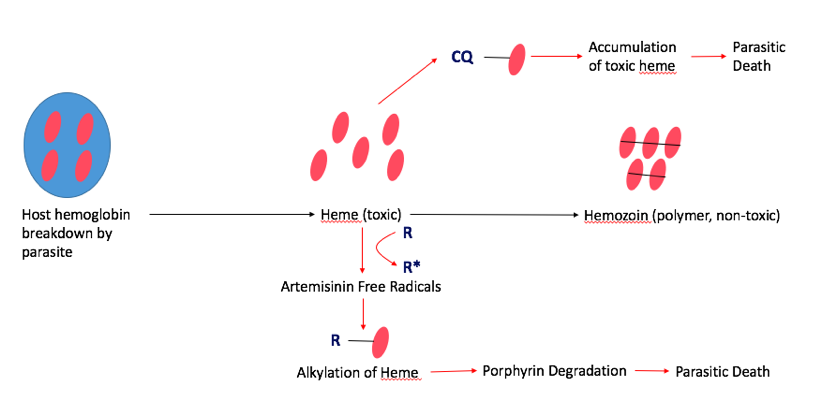
Fast-acting antimalarials act by disrupting the heme levels in the parasite. Malaria depends heavily on host heme groups for its survival, which makes this pathway an appropriate target for treatments. In the normal pathway, the parasite first breaks down host hemoglobin via proteolysis releasing several heme groups inside the parasite. However, individual heme groups are toxic to malaria. In order to combat this, malaria developed a mechanism to sequester and polymerize the individual heme groups to create hemozoin, which is non-toxic to the parasite. Common fast-acting antimalarials, such as Artemisinin and Chloroquine interfere with hemozoin formation, which is fatal to the parasite (Figure 6) (Tripathi, 2013).
Artemisinin: Artemisinin is the fast-acting antimalarial components of the most widespread form of treatment, called artemisinin-based combination therapy (ACT). This treats the most common form of malaria, known as uncomplicated P. Falciparum Malaria. Artemisinin was actually used in traditional Chinese medicine, and famous scientist, Tu Youyou, discovered its use as an antimalarial in the 1980s, which awarded her the Nobel Prize in Medicine in 2015 (World Health Organization, 2006). Artemisinin disrupts the hemozoin pathway by binding to the parasitic heme-iron group. This creates a toxic level of free-radicals within the parasite, which damages several intracellular proteins via alkylation, including heme itself. Ultimately, this results in the degradation of the porphyrin ring in the heme group, which is fatal to the parasite (Meshnick, 2002).
Chloroquine Chloroquine is another fast-acting drug that is used for treatment against P.vivax, the second most common form of malaria. Chloroquine also targets the parasitic hemoglobin pathway. It acts similarly to artemisinin in that it binds to the toxic heme groups, preventing heme polymerization into hemozoin within the parasite. This leads to accumulation of the toxic heme groups leading to parasite and cell lysis (Tripathi, 2013).
Figure 6: The mechanisms of two fast-acting antimalarials, artemisinin (R) and chloroquine (CQ) via the parasitic hemoglobin pathway.
Slow-Acting Antimalarials:
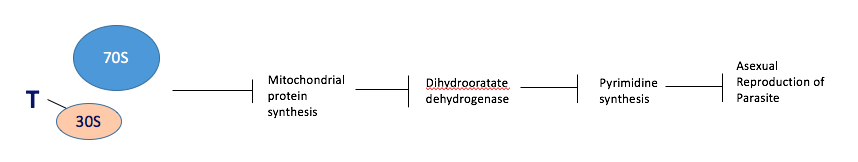
Tetracycline Tetracycline is a slow-acting drug that is used in combination with fast-acting drugs. It acts by binding to the 30S ribosomal subunit inside the parasite’s mitochondria, preventing translation of mRNA transcripts to polypeptides. This prevents the synthesis of key enzymes, especially those involved in pyrimidine synthesis, such as dihydrooratate dehydrogenase. Ultimately this prevents the asexual reproduction of the parasite (Figure 7) (Gaillard, 2015).
Figure 7: The mechanism of a slow-acting antimalarial, tetracycline, via inhibition of parasitic asexual reproduction.
References
- “Anopheles Mosquitoes”. (2015). Retrieved November 22, 2016, from https://www.cdc.gov/malaria/about/biology/mosquitoes/index.html
- CDC (2016). Impact of Malaria. Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/malaria/malaria_worldwide/impact.html
- CDC (2015). Malaria: Disease. Centers for Disease Control and Prevention. Retrieved from http://www.cdc.gov/Malaria/about/disease.html
- Chua, C. L., Brown, G., Hamilton, J. A., Rogerson, S., & Boeuf, P. (2013). Monocytes and macrophages in malaria: Protection or pathology? Trends in Parasitology, 29(1), 26-34. doi:10.1016/j.pt.2012.10.002
- Cowman, A. F., Berry, D., & Baum, J. (2012). The cellular and molecular basis for malaria parasite invasion of the human red blood cell. The Journal of Cell Biology, 198(6), 961-971. doi:10.1083/jcb.201206112
- Euroclinix (2016). Malaria Transmission - Malaria Life Cycle. Retrieved 23 November 2016, from https://www.euroclinix.net/en/travel-health/malaria/transmission
- Fagan, T. (2016). When was malaria first discovered and by whom? How is the disease transmitted? What are its effects?. Scientific American. Retrieved 28 November 2016, from https://www.scientificamerican.com/article/when-was-malaria-first-di/
- Gaillard, T., Madamet, M., & Pradines, B. (2015). Tetracyclines in malaria. Malaria journal, 14(1), 1.
- Hadjiliadis, D. (2015). Acute respiratory distress syndrome: MedlinePlus Medical Encyclopedia. Retrieved November 22, 2016, from https://medlineplus.gov/ency/article/000103.htm
- Haldar, K., Murphy, S. C., Milner, D. A., & Taylor, T. E. (2007). Malaria: Mechanisms of Erythrocytic Infection and Pathological Correlates of Severe Disease. Annual Review of Pathology: Mechanisms of Disease, 2(1), 217-249. doi:10.1146/annurev.pathol.2.010506.091913
- Lambert, P. (2016). Malaria - History of Malaria. Nobelprize.org. Retrieved 28 November 2016, from https://www.nobelprize.org/educational/medicine/malaria/readmore/history.html
- Malarone (Atovaquone and Proguanil Hcl) Drug Information: Clinical Pharmacology - Prescribing Information at RxList. (2016, October 26). Retrieved November 23, 2016, from http://www.rxlist.com/malarone-drug/clinical-pharmacology.htm
- Mandal, A. (2016). Malaria History. News-Medical.net. Retrieved 28 November 2016, from http://www.news-medical.net/health/Malaria-History.aspx
- Meshnick, S. R. (2002). Artemisinin: mechanisms of action, resistance and toxicity. International journal for parasitology, 32(13), 1655-1660.
- Srinivas,. (2016). Evolution of Malaria Parasites – Malaria Site. Malariasite.com. Retrieved 28 November 2016, from http://www.malariasite.com/history-parasites/References
- Tripathi, K. D. (2013). Essentials of medical pharmacology. JP Medical Ltd.
- Hemingway, J. & Bates, I. (2003). Malaria: past problems and future prospects. EMBO Reports, 4(Supp1), S29-S31. http://dx.doi.org/10.1038/sj.embor.embor841
- Wiser, M. F. (2016). Cell Biology of Plasmodium. Retrieved November 21, 2016, from http://www.tulane.edu/~wiser/malaria/cmb.html
- Wiser, M. F. (2016). Plasmodium Life Cycle. Retrieved November 20, 2016, from http://www.tulane.edu/~wiser/protozoology/notes/mal_lc.html
- World Health Organization. (2006). Guidelines for the treatment of malaria. World Health Organization. Fagan, T. (2016). When was malaria first discovered and by whom? How is the disease transmitted? What are its effects?. Scientific American. Retrieved 28 November 2016, from https://www.scientificamerican.com/article/when-was-malaria-first-di/
- World Health Organization. 2016. Malaria. [Fact sheet]. Retrieved from http://www.who.int/mediacentre/factsheets/fs094/en/
- WHO. (2016). World Malaria Report. World Health Organization. Retrieved from: http://apps.who.int/iris/bitstream/10665/205224/1/WHO_HTM_GMP_2016.2_eng.pdf?ua=