This is an old revision of the document!
Table of Contents
Amyotrophic lateral sclerosis (Lou Gehrig's Disease)
<style justify> Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that results in degeneration and death of motor neurons leading to muscle wasting and an inability to control voluntary movement (Leigh & Wijesekera, 2009). The ‘living wires’ which connect your brain to your muscles degenerate, leading to a loss of mobility, loss of speech and eventually impact the ability to breathe. A proposed explanation for the progression of this disease focuses on the effects of the hyperactivation of motor neurons, by the amino acid glutamate, on neuronal pathways. Treatment options include drug therapy, which interferes with the action of glutamate, and the potential to use stem cell therapy to replace malfunctioning astrocytes.
</style>
History
<style justify> Migraines are one of the oldest documented illness being described as early as 1200 BCE by the Egyptians. Arataeus of Cappadocia is however the one credited with the discovery of migraines after having extensively described unilateral headaches with aura. Migraines have plagued humans throughout our history with little progress made in the area of treatment and cures. Several treatments have been tried with no avail, including hot irons, bloodletting, inserting garlic into incisions made in the temple and applying opium and vinegar solutions directly to the skull. Some have even gone as far as to perform surgeries such as lobotomies on migraine sufferers. Trepanation involves making a hole in the skull to let out evil spirits that presumably were causing the migraine. It is possibly the oldest surgery in history and may date as far back as 6500 BCE. The first known use of medication that has shown some success is use of ergotamine in the 1930s. Ergotamine constricts blood vessels in the brain and has been shown to provide pain relief in some individuals. Many drugs have been researched and experimented with since with little success. The first and currently only class of drugs developed and used solely for the treatment of migraines are Triptans, which were developed in the early 1990s (American Migraine Foundation, 2016).
</style>
Epidemiology
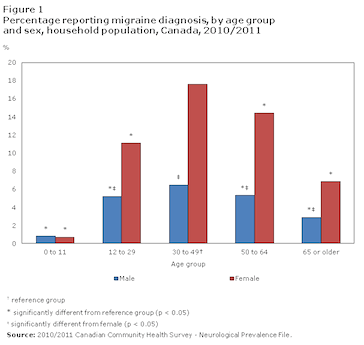
<style justify> Migraines are more common in males before puberty and in females after puberty with 19% of adult women and 11% of adult males being affected (Vos, 2010). Migraines commonly begin between the ages of 15 and 24 and have an increased incidence in adults aged 35 to 45 (Bartleson, 2010). The incidence of migraines are slightly lower in Asian and African countries, but this may be partially due to lack of diagnostic resources in these areas (Wang, 2003). In the United States, 18% of men and 43% of women will experience migraines at some point in their lives with about 6% of men and 18% of women experiencing migraines in a given year. Europeans have a lifetime risk of migraine between 12 and 28%. Chronic migraines occur in about 1.4 to 2.2% of the population. Worldwide, migraines affect about 15% of the population (Vos, 2010).
</style>
Figure 1: Prevalence of migraines in Canada by age group and sex
Phases and Symptoms
<style justify>
Initial Phase: Symptoms of the initial phase include pain, feeling weak, fatigue, muscle twitching, and muscle atrophy. These symptoms lead to one tripping, dropping things, having slurred speech, having trouble swallowing or breathing, muscle cramping, and weight loss. 75% of people experience the initial symptoms one of their limbs first, which is known as “limb-onset”. 25% of the people experience trouble with speech, swallowing, and breathing first, which is known as “bulbar-onset”. (Mioshi, E. et al., 2014)
Progression: As the disease progresses, patients lose their ability to use their limbs, and therefore become unable to walk. Patients also lose their ability to speak or swallow food. Patients who experience the “limb-onset” have their symptoms progress from one limb to the other then to the rest of the body regions. Patients who experience the “bulbar-onset” have their symptoms progress to their arms then to their legs. (Hillel, A. D. et al., 1989)
Late Stages: Almost all patients with ALS end up with a respiratory failure that leads to their death within two to five years after diagnosis. However, there are some patients, around 4%, that lived with ALS for ten years or longer. (Turner, M. R. et al., 2003)
ALS does not affect the five senses of sight, hearing, taste, smell and touch, nor does it normally affect the eye muscles, heart, bladder, bowel, or sexual muscles.
</style>
Pathophysiology
<style float-centre>
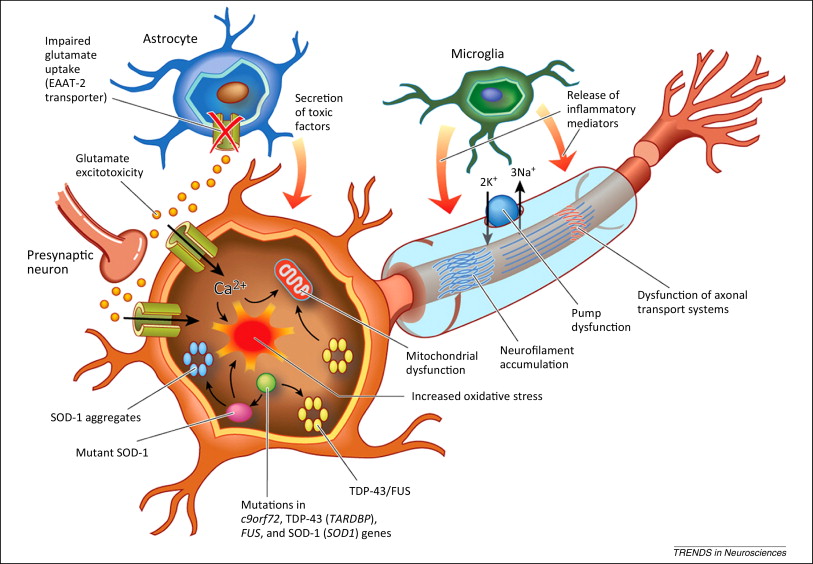
Figure 3: The Pathophysiology of ALS
</style>
<style justify>
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that primarily involves the motor neuron system. It is characterized by the loss of motor neurons in both the brain and spinal cord (Majoor-krakauer et. al, 2011) There is no single cause of ALS, as the disease is currently characterized by its major symptom: the death of motor neurons. For this reason, it is classified as a Complex Genetic Disorder, with no single genetic or environmental factor causing onset, and a combination of factors uniquely contributing to each case (Gordon, 2011). Due to the variety of underlying causes and mechanisms that result in ALS, there are two accepted “forms” of the disease that result in similar pathophysiology. In approximately 10% of cases, ALS is a mostly genetic disease and is inherited, this is known as Familial ALS, or FALS. The other 90% is thought to be caused by a unknown mix of environmental factors as well as genetic predisposition, and is known as sporadic ALS or SALS (Kato, 2008). Both forms are poorly understood, and the major differentiator between the two is observable family history. </style>
Genetic abnormality
<style justify>
Although a consistent genetic marker has yet to be observed in FALS patients, approximately 20% of families with a history of ALS have a mutation on chromosome 21 for a gene known as SOD1, thought to code for the enzyme superoxide dismutase (Saccon et. al, 2013). The mutation on SOD1 is thought to be autosomal dominant, meaning it only has to inherited from one parent to trigger expression in the phenotype. Due, to this mutation is present in only 20% of FALS cases, it currently has no diagnostic use.
</style>
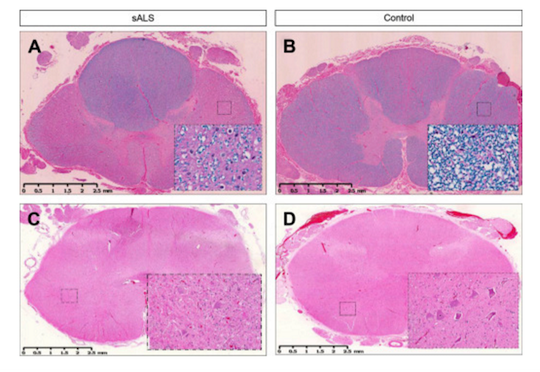
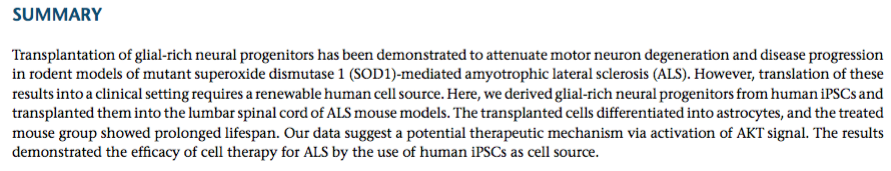
Figure 4: Lateral sclerosis is shown in the thoracic spinal cord in SALS (A) and compared with control (B). Inserts show loss of myelin in the white matter tracts under higher power (20X). Loss of motor neurons is shown in the lumbar spinal cord in SALS (C) and compared with control (D).
Bunina Bodies
<style justify>
One of the hallmarks of ALS diagnosis is the presence of Bunina Bodies, or round to oval eosinophilic intracellular inclusions in the motor neurons of the spinal cord and brain stem of both patients with sporadic ALS (SALS) and patients with familial ALS (FALS). Although Bunina bodies are present in 85-90% of ALS cases (Saberi et. al, 2015), their relationship to the underlying cause of ALS is unknown, as they test negative for a variety of biomarkers associated with neuro-degeneration. Interestingly, the only cases where Bunina bodies are absent are FALS cases that include the SOD1 mutation (Kato, 2008). Bunina bodies are shown below (denoted by black arrows) (Saberi et. al, 2015).
</style>
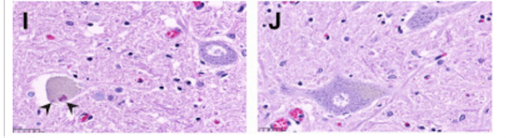
Figure 5: Black arrows indicate the presence of bunina bodies
Proteinopathy- TDP43
<style justify> A third observation of ALS proposes that the disease is a due to the presence of prions or misfolded proteins, as seen in many other neurological diseases, has been proposed. Large protein inclusions were commonly seen in the neurons of ALS patients, and in 2006, TDP-43 was identified as the major component of these inclusions. TDP-43 is a heterogeneous nuclear ribonucleoprotein and has many different cellular functions, including the stability, transport, and modification of mRNA. Mutations in the gene for TDP-43, TARDBP, have been implicated in facilitating the neurotoxic conditions of ALS, particularly in the glycine rich area of the gene (Guo et. al, 2011). This has led to the inference that variation in the mRNA metabolism is an area for further research. Similarly, to Bunina bodies, this pathology has been almost exclusively seen in ALS patients without the SOD1 mutation, suggesting that ALS can have multiple mechanisms that result in the same symptoms. </style>
Proposed Mechanism: <style justify> In healthy individuals, glutamate is released from the presynaptic neuron and activates receptors on the postsynaptic membrane (Foran & Trotti, 2009). This causes ion-gated calcium and sodium channels to open up and initiate an action potential. Glutamate transporters, located in adjacent astroglia cells, remove excess glutamate left in the synapse in order to prevent any further activation (Foran & Trotti, 2009). In patients with ALS, there is overactivation of these glutamate receptors, which can be caused by excessive glutamate release or a malfunctioning of the reuptake system on the presynaptic membrane (Figure 3) (Foran & Trotti, 2009). This increased activation results in higher intracellular calcium concentrations that exceed the buffering capacity of the mitochondria and endoplasmic reticulum and ultimately lead to neuronal excitotoxicity and neuron death (Figure 3) (Foran & Trotti, 2009). This abnormal increase in glutamate concentration can be linked to the presence of mutations in associated molecular factors.
Astrocytes, which absorb and recycle neurotransmitters, containing a superoxide dismutase 1 mutation (SOD1) are thought to be implicated in this hyperexcitability phenotype observed in individuals with ALS. To demonstrate the involvement of the SOD1 mutation, researchers cultured induced pluripotent stem cell-derived motor neurons with the SOD1 mutation and compared the spontaneous firing of these neurons to controls (Wainger et.al, 2014). They were able to show that SOD1 mutant neurons fired significantly more than neurons lacking this mutation supporting the idea that astrocytes are not executing their normal function (Wainger et al, 2014). When researchers corrected the mutant SOD1 gene to a wild-type, this overactivation was no longer observed (Wainger et al, 2014). Overall, the toxicity effects of this mutation are seen primarily in familial cases of ALS although astrocytes in general, play a role in both familial and sporadic forms (Wainger et al, 2014).
</style>
Treatment
<style justify> To date, there is no actual cure for individuals diagnosed with ALS. Much of the treatment available is targeted around managing the symptoms and attempting to slow down the progression of the disease. There has only been one FDA approved drug therapy available with many clinical trials taking place to test more options. Overall, management of patients with ALS requires a multidisciplinary approach, with the expertise of many different health care professionals to provide the best care and least amount of suffering for these individuals. </style>
Pharmacologic treatment: Riluzole
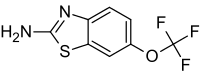
Figure 6: Chemical structure of the drug Riluzole
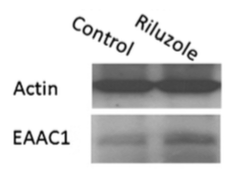
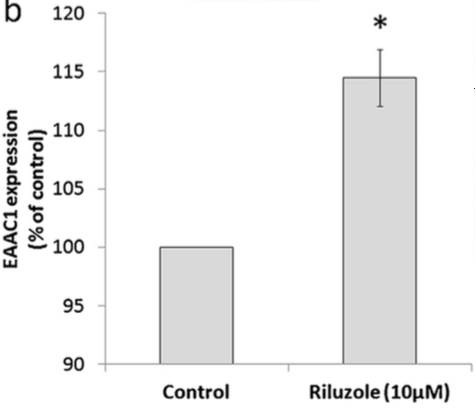
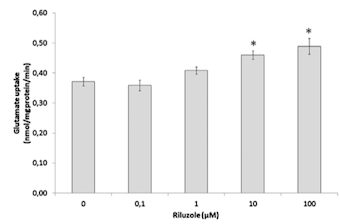
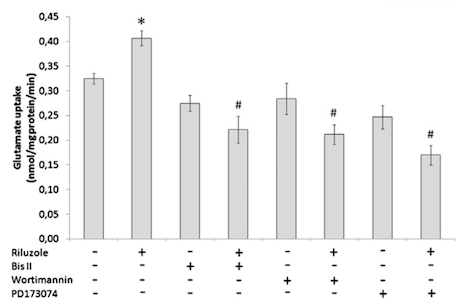
</style> <style justify> The only FDA approved treatment available to date is the drug Riluzole. It belongs to the class of drugs known as antiglutamates and whose chemical formula is C8H5F3N2OS (Figure 6). It functions to prevent the overactivation of neurons by regulating neurotransmitter activity and inhibiting neuronal firing (Dall’Igna et al, 2013). Dall’Igna et. al (2013) studied the influence of riluzole on glutamate uptake at doses that did not induce damage on the studied cells within a 1 h time frame (0.1–100 M). One hour pre-incubation of riluzole significantly enhanced glutamate uptake in C6 astroglial cell cultures (Figure 8a). In further studies, the researchers chose the lowest effective dose of riluzole to alter glutamate uptake (10 M). Because EAAC1 is the main glutamate transporter in C6 astroglial cells, they analyzed the effect of riluzole on EAAC1 expression (Figure 7). Treatment with 10 M riluzole for 1 h induced an 18% increase in the expression of EAAC1 transporter as detected by Western blotting, indicating a possible mechanism for the increase in glutamate uptake (Figure 7). The increase in glutamate uptake was demonstrated to be dependent on PKC, PI3K and ERK intracellular pathways; riluzole had the opposite effect on glutamate uptake when it was added simultaneously with inhibitors of these enzymes (Figure 8b). Co-incubation of riluzole with the inhibitors of PKC (bisindolylmaleimide II), PI3K (wortmannin) or FGFR (PD173074) not only blocked the stimulatory effect of riluzole on glutamate uptake, but the riluzole plus inhibitor combination also decreased glutamate uptake levels to below the basal controls (Figure 8b). Such effects occurred in doses of bisindolylmaleimide II, wortmannin and PD173074 that alone had no significant effect on basal glutamate uptake (Dall'Igna, 2013). </style>
Figure 7: EAAC1 expression, measured by Western blotting with specific antibody, in C6 astroglial cells in the presence or absence of riluzole. Cells were pretreated with riluzole for 1 h in DMEM without serum.
Figure 8: (a) Glutamate uptake in C6 astroglial cells in the presence or absence of riluzole. (b)Glutamate uptake in C6 astroglial cells in the presence or absence of riluzole and/or of the inhibitor of protein kinase C (PKC) bisindolylmaleimide II (Bis II), the inhibitor of phosphatidylinositol 3-kinase (PI3K) wortimannin or the inhibitor of extracellular-signal regulated kinase (ERK) PD173074.
Management strategies
Managing ALS is a continually changing challenge. Although ALS is a degenerative disease, the rate at which neurons and muscles degenerate is unpredictable and varies greatly from one individual to another. In some cases the disease seems to have reached a plateau, while in others it reaches a standstill for varying lengths of time. Also, ALS can progress steadily at a rapid or slow rate. Whatever the rate of muscle degeneration, individuals should remain as active as possible, without causing fatigue in affected muscles. Many health care professionals are involved including: the family doctor, neurologist, palliative care doctor, nurse clinicians, occupational therapist, physiotherapist, psychiatrist, dietician, respiratory therapist, speech-language pathologist and social worker (ALS Society of Canada, 2012). This speaks to the diverse nature of the symptoms and the multitude and magnitude of expertise required.
Management options focus on aiding the individual with:
- changes in mobility
- swallowing problems and maintaining good nutrition (i.e. tube feeding)
- changes in speech and maintaining communication
- changes in breathing and maintaining lung function
Future directives: stem cell therapy
Another potential, but preclinical therapeutic option is the use of human induced pluripotent stem cells (hIPSCs) that can differentiate to become healthy astrocytes (Kondo et al, 2014). Researchers transplanted hIPSCs into the spine of mSOD1 mice subjects after disease onset and observed differentiation of these cells into astrocytes and an overall prolonged lifespan (Kondo et al, 2014). The ability to generate functional astrocytes, to compensate for mutated ones, can be advantageous in rescuing motor neurons as it will allow for glutamate to be properly removed from the synapse, which will prevent hyperexcitability of the postsynaptic membrane.
Conclusion
Overall, ALS is a rapidly, progressing disease that greatly impacts the everyday functioning of the individual who has it. glutamate excitotoxicity in ALS patients can be linked to mutant astrocytes that malfunction and this can be the target of potential therapies. Drug therapy has shown promising temporary effects and although more research needs to be done, there is the potential to use innovative stem cell therapeutics to introduce healthy astrocytes into neuronal regions for longer-term effects.