This is an old revision of the document!
Table of Contents
Juvenile Idiopathic Arthritis
Introduction
Juvenile idiopathic arthritis (JIA), also known as juvenile rheumatoid arthritis (JRA) is a chronic form of arthritis that can be seen in children between the ages of 1-16 (Shiel, n.a). This disease refers to a group of conditions that pertain to joint inflammation (Figure 1). According to The Genetics Home Reference, “It is classified as an autoimmune disorder which means that the immune system malfunctions and attacks the body’s organs and tissues, in this case joints.” Through much research, it has been determined that there are seven types of JIA which are classified in accordance to their signs and symptoms, number of affected joints, results from medical tests and familial history. All seven types of JIA are chronic, thus individuals must develop coping methods that are long lasting and effective. The prevalence of the seven types of JIA are shown in Figure 2 (Genetics Home Reference, 2015).
Figure 1: This image illustrates a comparison between a normal joint, osteoarthritis and rheumatoid arthritis. Retrieved from: http://www.onhealth.com/content/1/rheumatoid_arthritis_ra
Systemic Juvenile Idiopathic Arthritis
Systemic juvenile idiopathic arthritis causes inflammation in one or more joints, and its symptoms include high daily fevers that can last up to two weeks either preceding or accompanying the arthritis. A skin rash or enlargement of lymph nodes, liver or spleen are symptoms that differentiate this type of juvenile arthritis from other types (Genetics Home Reference, 2015).
Oligoarticular Juvenile Idiopathic Arthritis
Rheumatoid Factor Positive Polyarticular Juvenile Idiopathic Arthritis
Rheumatoid Factor Negative Polyarticular Juvenile Idiopathic Arthritis
Psoriatic Juvenile Idiopathic Arthritis
Enthesitis-related Juvenile Idiopathic Arthritis
Undifferentiated Arthritis
{{}}
Figure 2: This graph shows the percentage at which each subtype of JIA is prevalent. Retrieved from: https://warmsocks.files.wordpress.com/2012/08/jiabreakdown.png
Symptoms and Diagnosis
Flares
{{}}
Figure 3: This shows the location of different body parts with high prevalence of body inflammation Retrieved from: http://www.onhealth.com/content/1/rheumatoid_arthritis_ra
Fever
Morning Stiffness
Rheumatoid Rash
{{}}
Figure 4: This image is showing the rheumatoid rash when present on a child’s face. Retrieved from: http://www.findarthritistreatment.com/wp-content/uploads/2013/12/Skin-rash.jpg
Subcutaneous Nodules
{{}}
Figure 5: This image illustrates what nodules look like on the hands of a child. Retrieved from: http://c.ymcdn.com/sites/www.aocd.org/resource/resmgr/ddb/rheumatoid_nodules_1_low.jpg
Uveitis Eye condition
Blood Tests for JIA
Causes and Risk Factors
{{}}
Figure 6: This image illustrates where rheumatoid arthritis is present on a hand. Retrieved from: https://www.epainassist.com/images/Juvenile-Arthritis.jpg
Epidemiology
{{}}
Figure 7: This image shows the prevalence of the common forms of arthritis in the United States. Retrieved from: https://rheumatoidarthritis.net/wp-content/uploads/2013/07/prevalence_arthritis.png
Pathophysiology
Genetic Susceptibility
JIA is believed to be a complex genetic trait. A complex genetic trait is defined as phenotypes not exhibiting classic mendelian inheritance patterns and therefore, cannot be attributed to variants in a single gene locus. Thus, JIA is a disease which is believed to be determined by a number of genetic and environmental factors (Glass and Giannini, 1999).
HLA and non-HLA Polymorphisms
There are two broad categories for genetic susceptibility genes: human leucocyte antigen (HLA) genes and non HLA-related genes. As a polygenic disease, JIA is mostly affected at the HLA region, while the non-HLA loci exhibit moderate or weak genetic influence on susceptibility (Prahalad and Glass, 2008). JIA is influenced by both HLA class I and HLA class II alleles, which contain certain peptides leading to the activation of certain autoreactive T cells (Wedderburn et al., 2001). Several genetic studies have shown the contribution of polymorphisms in the major histocompatibility complex (MHC). The MHC region is on chromosome 6 and is packed with more than 200 genes, many of which are essential to the immune system. Since MHC class II genes play a great role as a genetic risk factor for JIA, CD4+ T cells play a crucial role in disease development. Associations between HLA polymorphisms and JIA subtypes have been reported in multiple populations (Figure 8) (Prahalad and Glass, 2008).
Figure 8:This figure shows several associations between JIA subtypes and different HLA alleles. Retrieved from (Prahalad and Glass, 2008).
In addition to associations between numerous HLA variants and JIA, non-HLA polymorphisms have also shown to be linked to JIA. For example, genes such as PTPN22, tumor necrosis factor (TNF) alpha (TNFA), MIF, WISP3 and SLC11A6 have been associated with JIA (Figure 9) (Prahalad, 2004; Rosen et al., 2003).
Figure 9:This figure shows non-HLA genetic genes associated with JIA that have been independently confirmed. Retrieved from (Prahalad and Glass, 2008)
Twin Studies
Family studies have provided strong evidence for genetic factors contributing to the susceptibility to JIA. For instance, twin and affected sibling pair (ASP) studies have supported the role for genetic susceptibility to JIA. Specifically, they have shown that monozygotic twin concordance rates for JIA vary between 25 to 40%, a stark contrast to the population prevalence of 1 in 1000 of having the disease (Ansell et al., 1969; Baum and Fink, 1968; Savolainen et al., 2000).
In the largest twin study from the JIA ASP registry conducted by Prahalad and colleagues in 2000, 14 pairs of twins concordant for JIA registered in the National Institute for Arthritis and Musculoskeletal and Skin Diseases were analyzed. Of these 14 pairs of monozygotic twins, 12 were concordant for presence or absence of anti-nuclear antibodies. Prahalad and colleagues found that the first twins to develop JIA did so with an average of 5.5 months before the second twins. This was statistically significant and different compared to the 104 non-twin affected siblings pairs in the registry, who showed a 37 month difference in age at onset between the first and second sibling (Prahalad et al., 2000). Together, these studies solidify evidence for genetic factors in the susceptibility to JIA.
Genetic Variables Underlying Autoimmunity
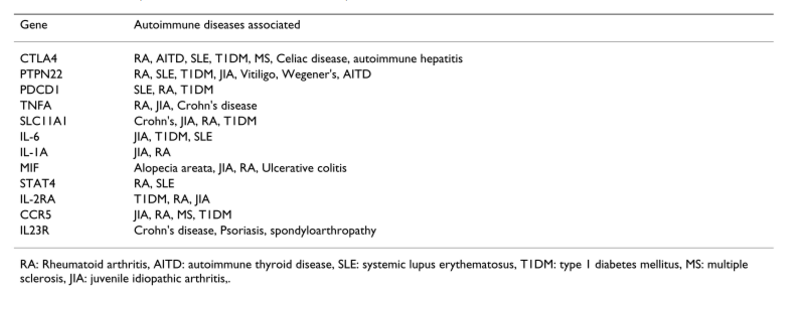
Several studies have shown that clinically distinct autoimmune phenotypes cluster in individuals and families. The data supports the hypothesis that common genetic factors might predispose to clinically distinct autoimmune phenotypes. There are genetic variants which influence susceptibility to multiple clinically distinct autoimmune disorders (Figure 10) (Prahalad and Glass, 2008).
Figure 10:This figure shows different examples of genes associated with multiple autoimmune diseases. Retrieved from (Prahalad and Glass, 2008).
Environmental Factors
The cause of JIA is assumed to be multifactorial, as in the case of most human autoimmune diseases. Unlike JIA, a healthy immune system consists of effector and regulatory mechanisms which are kept in balance. Therefore, the innate and adaptive immune systems closely interact. A genetically susceptible individual might develop a deleterious and uncontrolled response towards a self-antigen on exposure to an unknown environmental trigger. In JIA, it can lead to a self-perpetuating loop of activation of both innate and adaptive immunity, causing tissue damage (Prakhen, Albani, Martini, 2011).
Stress and Psychological Factors
The influence of stressful life events and psychological factors before the onset of JIA as a disease trigger has been studied. In a study by Henoch and colleagues which compared 88 children with JIA to 2952 geographically matched controls, results showed that children whose parents were unmarried due to divorce, separation, or death comprised 28.4% of the JIA population, compared to 10.6% in controls (Henoch, Batson, Baum, 1978).
Stress was also suggested as a contributing factor to the disease. It is a known stimulator to the sympathetic nervous system (SNS) and thus increases the production of interleukin 6, which is one of the most important inflammatory cytokines in JIA (Roupe et al., 2000).
Although psychological factors and stress may play a role for the onset of JIA, more research needs to be conducted since past studies have had several methodological shortcomings (Berkun, Padeh, 2010).
Smoking
Smoking is the strongest known environmental risk factor for developing rheumatoid arthritis (RA) in adults. The risk of RA from smoking has been showed by the number of shared epitope copies, which suggest gene-environment interaction. Specifically, the strongest known genetic risk factor for RA is a specific sequence of amino acids on HLA-DRB1 allele. In addition, the risk of developing RA from gene-environment interactions increases with the intensity of smoking (Liao et al., 2009).
All this suggests that smoke is a stimulator of the immune system and that the exposure to tobacco products during fetal life may influence the developing immune system of the fetus. This can lead to an increased susceptibility to infectious agents and thereafter, trigger arthritis as well as subsequent autoimmune diseases. Jaakkola and Gissler conducted a study assessing the relation between maternal smoking in pregnancy and the risk of inflammatory polyarthropathies, in particular JIA in the first years of life. 58 841 newborn Finnish children until the age of 7 were analyzed. Findings showed that there was a 2-fold higher rate of polyarthropathies during the first 7 years of life in children of smoking mothers, compared to non-smoking ones. In addition, the maternal smoking effect was found prevalent to girls, who experienced 6-fold greater likelihood for JIA compared to unexposed boys (Jaakkola and Gissler, 2005).
Infectious agents and JIA
Infectious agents are believed to be important environmental factors in the development of autoimmunity. An infectious agent may induce a cross-reactive immune response, thereby leading to inflammation provoked by antigen-presenting cells (APCs). This can lead to increased immunogenicity, priming of T cells and subsequent autoimmunity (Berkun and Padeh, 2009). A study by Carlens and colleagues showed that infections during the first year of life and factors related to size and timing of birth were associated with increased risk of developing JIA (Carlens et al., 2009) . However, more research needs to be conducted to understand the role of environmental factors on JIA.
Immunology of JIA
Auto-Immunity
Antibodies to Citrullinated Protein Antigens (ACPA)
Figure 11:This figure shows the process of citrullination. Retrived from: https://www.hopkinsarthritis.org/wp-content/uploads/2012/08/round4-slide-12.jpg
Rheumatoid Factor (RF)
Inflammation
Figure 12:This figure shows a summary of the pathogenic pathways in JIA. Retrived from: https://www.researchgate.net/profile/Abdullah_Nahian/publication/263585727/figure/fig1/AS:202832654409738@1425370481906/Figure-illustrates-the-pathogenic-pathways-of-rheumatoid-arthritis-following-31-38.png
Bone Destruction
Treatments
Physical Therapy
Heat or Cold
Exercise or Stretches
Physiotherapy
Occupational Therapy
Assistive Devices
Drug Therapy
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Disease-modifying antirheumatic drugs (DMARDs)
Figure 13:This figure shows a graph of the percentage of patients that improved over the study span of six months. Retrieved from (Giannini et al., 1990).
Biological Response Modifiers
Corticosteroid Joint Injections
Figure 14:This figure depicts the process of injecting corticosteroids into affected joints in individuals with juvenile idiopathic arthritis. (Retrieved from http://www.aboutkidshealth.ca/En/ResourceCentres/JuvenileIdiopathicArthritis/TreatmentofJIA/MedicationsforJIA/Pages/CorticosteroidJointInjections.aspx)
Surgical Treatment
Figure 15:This image shows the view before and after total knee replacement. Retrieved from (Abdel & Figgie, 2014).
Future Directions
The majority of children are treated adequately with a combination of treatments such as NSAIDs, corticosteroids and DMARDs available today. Yet, there are still some children, who are intolerant or resistant to these therapies, hence suffer from joint damage. For children who fall under this category, autologous stem cell transplantation seems to be a potential option (Brinkman et al., 2007). Autologous stem cell transplantation is a procedure where a person’s own stem cells to replace damaged cells (Autologous Transplants,n.d.). This method of treatment has only been tried on a few as thirty children. First, Wulffraat et al., reported positive results of autologous stem cell transplantation in four children (Brinkman et al., 2007).
References
Abdel, M. P., & Figgie, M. P. (2014). Surgical Management of the Juvenile Idiopathic Arthritis Patient with Multiple Joint Involvement. Orthopedic Clinics of North America, 45(4), 435-442. doi:10.1016/j.ocl.2014.06.002
AboutKidsHealth, and About.kidshealth@sickkids.ca. “Blood Tests and JIA.” Blood Tests and Juvenile Idiopathic Arthritis (JIA) - AboutKidsHealth. AboutKidsHealth, 16 Oct. 2012. Web. 02 Mar. 2017.
AboutKidsHealth -JIA Treatment of JIA. (2017). Retrieved February 20, 2017 from http://www.aboutkidshealth.ca/En/ResourceCentres/JuvenileIdiopathicArthritis/TreatmentofJIA/Pages/default.aspx
Ansell, B. M., Bywaters, E. G., & Lawrence, J. S. (1969). Familial aggregation and twin studies in Still's disease. Juvenile chronic polyarthritis. Rheumatology, 2, 37.
Autologous (Self) Transplants. (n.d.). Retrieved March 03, 2017, from http://www.leukaemia.org.au/treatments/stem-cell-transplants/autologous-self-transplants
Baum, J., & Fink, C. (1968). Juvenile rheumatoid arthritis in monozygotic twins: a case report and review of the literature. Arthritis & Rheumatology, 11(1), 33-036.
Berkun, Y., & Padeh, S. (2010). Environmental factors and the geoepidemiology of juvenile idiopathic arthritis. Autoimmunity reviews, 9(5), A319-A324.
Brescia, A. C. (Ed.). (2016, April). Juvenile Idiopathic Arthritis. Retrieved February 19, 2017, from http://kidshealth.org/en/parents/jra.html#
Brinkman, D. M., Kleer, I. M., Cate, R. T., Rossum, M. A., Bekkering, W. P., Fasth, A., . . . Vossen, J. M. (2007). Autologous stem cell transplantation in children with severe progressive systemic or polyarticular juvenile idiopathic arthritis: Long-term followup of a prospective clinical trial. Arthritis & Rheumatism, 56(7), 2410-2421. doi:10.1002/art.22656
Carlens, C., Jacobsson, L., Brandt, L., Cnattingius, S., Stephansson, O., & Askling, J. (2009). Perinatal characteristics, early life infections and later risk of rheumatoid arthritis and juvenile idiopathic arthritis. Annals of the Rheumatic Diseases, 68(7), 1159-1164.
Demoruelle, M. & Deane, K. (2011). Antibodies to Citrullinated Protein Antigens (ACPAs): Clinical and Pathophysiologic Significance. Current Rheumatology Reports, 13(5), 421-430. doi:10.1007/s11926-011-0193-7 Giannini, E. H., Brewer, E. J., & Kuzmina, N. (1990). Arthritis. Journal of Pediatric Orthopaedics, 10(5), 697. doi:10.1097/01241398-199009000-00034
Gibson, W. M., Grokoest, A. W., & Larson, C. B. (1962). Juvenile rheumatoid arthritis. Arthritis & Rheumatology, 5(2), 211-217.
Ginn, L. R., Lin, J. P., Plotz, P. H., Bale, S. J., Wilder, R. L., Mbauya, A., & Miller, F. W. (1998). Familial autoimmunity in pedigrees of idiopathic inflammatory myopathy patients suggests common genetic risk factors for many autoimmune diseases. Arthritis & Rheumatology, 41(3), 400-405.
Glass, D. N., & Giannini, E. H. (1999). Juvenile rheumatoid arthritis as a complex genetic trait. Arthritis & Rheumatism, 42(11), 2261-2268.
Goldstein, D. A., Horsley, M., & Ulanski, L. J. (2013). II, Tessler HH. Complications of uveitis and their management. Duane's Ophthalmology.
Grossman, B. J., & Mukhopadhyay, D. (1975). Juvenile rheumatoid arthritis. Current problems in pediatrics, 5(12), CO11-65.
Harris, J. G., Kessler, E. A., & Verbsky, J. W. (2013). Update on the Treatment of Juvenile Idiopathic Arthritis. Current Allergy and Asthma Reports, 13(4), 337–346. http://doi.org/10.1007/s11882-013-0351-2
Henoch, M. J., Batson, J. W., & Baum, J. (1978). Psychosocial factors in juvenile rheumatoid arthritis. Arthritis & Rheumatism, 21(2), 229-233.
Jaakkola, J. J., & Gissler, M. (2005). Maternal smoking in pregnancy as a determinant of rheumatoid arthritis and other inflammatory polyarthropathies during the first 7 years of life. International journal of epidemiology, 34(3), 664-671.
Juvenile idiopathic arthritis - Genetics Home Reference. (2015, February 14). Retrieved February 20, 2017, from https://ghr.nlm.nih.gov/condition/juvenile-idiopathic-arthritis#diagnosis
Juvenile Idiopathic Arthritis: Soft Tissue Release of Contracture - Topic Overview. (n.d.). Retrieved February 13, 2017, from http://www.webmd.com/rheumatoid-arthritis/tc/juvenile-idiopathic-arthritis-soft-tissue-release-of-contracture-topic
Juvenile Idiopathic Arthritis - Treatment Overview. (n.d.). Retrieved February 13, 2017, from http://www.webmd.com/rheumatoid-arthritis/tc/juvenile-rheumatoid-arthritis-treatment-overview#1
Kim, K. N. (2010). Treatment of juvenile rheumatoid arthritis. Korean Journal of Pediatrics, 53(11), 936–941. http://doi.org/10.3345/kjp.2010.53.11.936
Liao, K. P., Alfredsson, L., & Karlson, E. W. (2009). Environmental influences on risk for rheumatoid arthritis. Current opinion in rheumatology, 21(3), 279.
Malmström, V., Catrina, A., & Klareskog, L. (2016). The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nature Reviews Immunology, 17(1), 60-75. doi:10.1038/nri.2016.124 McInnes, I. & Schett, G. (2011). The Pathogenesis of Rheumatoid Arthritis. New England Journal Of Medicine, 365(23), 2205-2219. doi:10.1056/nejmra1004965 Miller, M. L. (1994). Juvenile rheumatoid arthritis. Current problems in pediatrics, 24(6), 190-198.
Nath Maini, R. (2010). Rheumatoid arthritis. Oxford Textbook Of Medicine, 3579-3602. doi:10.1093/med/9780199204854.003.1905_update_001 Nelson, W. E., & Kliegman, R. M. (2016). Nelson Textbook of pediatrics. Philadelphia: Elsevier.
Prahalad, S. (2004). Genetics of juvenile idiopathic arthritis: an update. Current opinion in rheumatology, 16(5), 588-594.
Prahalad, S., & Glass, D. N. (2008). A comprehensive review of the genetics of juvenile idiopathic arthritis. Pediatric Rheumatology, 6(1), 11.
Prahalad, S., Ryan, M. H., Shear, E. S., Thompson, S. D., Glass, D. N., & Giannini, E. H. (2000). Twins concordant for juvenile rheumatoid arthritis. Arthritis & Rheumatism, 43(11), 2611-2612.
Prakken, B., Albani, S., & Martini, A. (2011). Juvenile idiopathic arthritis. The Lancet, 377(9783), 2138-2149.
Ramanan, A. V. (2003). Use of methotrexate in juvenile idiopathic arthritis. Archives of Disease in Childhood, 88(3), 197-200. doi:10.1136/adc.88.3.197
Ravelli, A., & Martini, A. (2007). Juvenile idiopathic arthritis. The Lancet, 369(3), 767-778. doi:10.1016/S0140-6736(07)60363-8
Rosen, P., Thompson, S., & Glass, D. (2002). Non-HLA gene polymorphisms in juvenile rheumatoid arthritis. Clinical and experimental rheumatology, 21(5), 650-656.
“Rheumatoid Factor | ANA Test | Lab Tests | Arthritis Today.” Rheumatoid Factor | ANA Test | Lab Tests | Arthritis Today. N.p., n.d. Web. 02 Mar. 2017.
Saurenmann, R. K., Rose, J. B., Tyrrell, P., Feldman, B. M., Laxer, R. M., Schneider, R., & Silverman, E. D. (2007). Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis & Rheumatism, 56(6), 1974-1984.
Savolainen, A., Saila, H., Kotaniemi, K., KAIPIAINEN-SEPPAN, O., Leirisalo-Repo, M., & Aho, K. (2000). Magnitude of the genetic component in juvenile idiopathic arthritis. Annals of the rheumatic diseases, 59(12), 1001.
Schaller, J. G. (1997). Juvenile rheumatoid arthritis. Pediatrics in review, 18, 337-350.
Symmons, D. (2005). Commentary: Juvenile idiopathic arthritis—issues of definition and causation. International journal of epidemiology, 34(3), 671-672.
Ungar, W.J., Costa, V., Burnett, H.F., Feldman, B.F., & Laxer, R.M. (2013). The use of biologic response modifiers in polyarticular-course juvenile idiopathic arthritis: A systematic review. Seminars in Arthritis and Rheumatism, 42(6), 597-618.
Van der Voort, C. R., Heijnen, C. J., Wulffraat, N., Kuis, W., & Kavelaars, A. (2000). Stress induces increases in IL-6 production by leucocytes of patients with the chronic inflammatory disease juvenile rheumatoid arthritis: a putative role for α 1-adrenergic receptors. Journal of neuroimmunology, 110(1), 223-229.
Wedderburn, L. R., Patel, A., Varsani, H., & Woo, P. (2001). Divergence in the degree of clonal expansions in inflammatory T cell subpopulations mirrors HLA-associated risk alleles in genetically and clinically distinct subtypes of childhood arthritis. International immunology, 13(12), 1541-1550.
William C. Shiel Jr., MD, FACP, FACR. (n.d.). Rheumatoid Arthritis (RA) Symptoms & Treatment. Retrieved February 20, 2017, from http://www.onhealth.com/content/1/rheumatoid_arthritis_ra