Table of Contents
Breast Cancer
Powerpoint file: a-look-inside-breast-cancer-1.pptx
Introduction
Breast cancer is the cancer associated with an uncontrollable division of cells in the breast tissues. It is attributed by abnormal cell growth in beginning the ducts and lobules. Although both males and females can be diagnosed, breast cancer is more prevalent in women on a much larger scale. 1 in 8 women will be diagnosed in their lifetime therefore both breast cancer research is vital for treatment of the diseases. Breast cancer is attributed to four different stages. These stages are defined by both the size of the tumour and the spread of the cancer to tissues, muscles, and organs. Symptoms of the disease can include a lump in the breast, skin dimpling, and red patches on the skin.
Etiology
Breast Cancer Aetiology
There are a large number of risk factors associated with the incidence of breast cancer, a disease that sees over 1 million new cases each year[15]. This section will be analyzing 13 different factors that have correlated either positively or negatively with onset of breast cancer[25].
Age: Incidence is relatively low before the age of 30, and increase until the age of 80. Proof from a study by Surveilance, Epidimiology and End Results show that for women under 50, the incidence was 0.000428% whereas for those over 50, it was 0.003355%[25].
Ethnicity: Highest standardized incidence rates were found in USA and Northern Europe, with 50-100/100,000 compared to the lowest rates found in Asia at 10-30/100,000. Migration studies also showed that immigrants from Asia to USA (throughout numerous generations) showed similar results to Caucasians who were born in USA, alluding to environmental/cultural factors playing a key role in breast cancer[25].
Genetic Factors/Family History: Indeed, a predisposition to breast cancer via family history increases prevalence of the disease, and 5-10% of all cases are attributable to family history. Mutations in genes such as BRCA1/2 and p53 are also associated with a high risk of breast cancer[47].
Age at Menarche/Menopause: It was found that if a female had her first occurrence of ovulation at a later age, then there was a 10-24% decreased chance of developing breast cancer, every year menarche was delayed[3]. In contrast, if there was a later onset of menopause, the chances to develop breast cancer increased by 3% each year[25].
Endogenous Hormones: With increased concentrations of sex hormones, such as estradiol and testosterone and progesterone, the risk of breast cancer increased[25].
Diet: As of date, there is no consistently strong correlation between specific diets and breast cancer, however there is slight proof that a high fat diet may increase risk of breast cancer, while high soy, fruit and vegetable intake may decrease the risk[25].
Alcohol Consumption: It was proven that with a 10 g/day intake of alcohol in pre- and postmenopausal women, there was a 7% increase in breast cancer prevalence[10]. Some possible explanations include that alcohol decreases DNA repair efficiency, or that it increases metabolism of carcinogenic acetaldehyde[43].
Obesity: Obesity as a factor depends on the menopause status of the woman. Premenopausal obese women have a decreased risk of breast cancer, whereas the opposite occurs for postmenopausal women. A proposed mechanism is that obesity may protect women pre-menopause by creating more frequent anovulatory cycles, which in turn decreases progesterone and estradiol[25].
Physical Activity: There is some association between physical activity and reduced breast cancer, even though the results are not consistent and the mechanism as to why is not known[25].
Age at First Birth/ Breastfeeding: Women with their first birth before or at the age of 20 had a decreased chance of breast cancer development, whereas those 35 and older had a 40% increased risk of developing the disease[14]. Also, women who breastfed longer had a higher degree of protection from breast cancer, with a 4.3% decrease in risk after every 12 months of breastfeeding[10].
Epidemiology
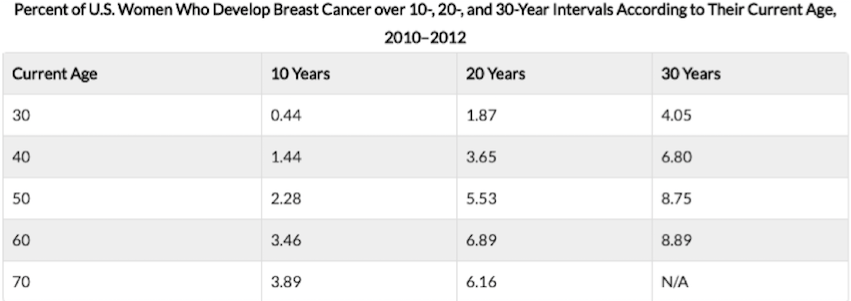 Figure 1: This chart shows the risk of developing breast cancer at different ages divided in ten year intervals. Risk of breast cancer increases by age
Figure 1: This chart shows the risk of developing breast cancer at different ages divided in ten year intervals. Risk of breast cancer increases by age
Incidence:
The greatest incidence is found in European countries, followed by America and Canada. Rates of breast cancer are significantly lower in African countries. According to WHO, there were over 508 000 deaths around the globe in 2011 [4].
Distribution
Breast cancer affects women around the globe. Death rates are lower in developed countries as more treatment options are available in these regions. The number of cases is steadily increasing in developing countries where treatment and cancer tests are not as readily available [24]. According the Canadian Cancer society, it is projected that there will be 1.6 million new cases of breast cancer in the world [6].
Control of disease
There are a variety of methods to treat breast cancer (see more in the treatment section) [4]. They can generally be divided into invasive and non-invasive [4]. Moreover, continuous research is done by different organizations such as the Breast Cancer organization [4].
Survival rates
Although survival rates are increasing, low-income countries are still suffering from lack of available resources to diagnose and treat cancer. Survivability rates have reached over 80% in North America but still remain below 40% in developing and low income countries [5].
Detection and treatment of breast cancer in earlier stages show an increase in success rates. In 2014, over 236, 968 women were affected in the US and 2141 men and led to 41, 211 deaths in women and 456 deaths in men [5].
Predisposition
Predisposition to breast cancer can be broken up into 3 categories[37].
- Familial - accounts for 15-20% of all cases of breast cancer, in which family history of breast cancer is present but these individuals may be negative for BRCA
- Hereditary - accounts for 5-10% of all caases of breast cancer, in which the individual tests for BRCA1 or BRCA2 positive
- Sporadic - accounts for 70-80% of all cases of breast cancer, in which individuals present no family history of the disease.
Although only 5-10% of cases are hereditary, once the mutation is inherited there is a 65-85% chance of developing the disease by age 70[37]. The genetic predisposition to breast cancer is commonly caused by the abnormalities in the BRCA1 and BRCA2 genes. Under normal bodily function, these genes produce tumor suppressor proteins that help repair and regulate damaged DNA[37]. Mutated copies of these genes do not repair DNA properly.
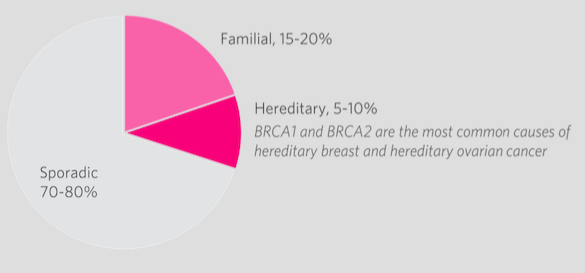 Figure 2: Percentage of breast cancer diagnoses that are a result of hereditary, familial or sporadic factors.
Figure 2: Percentage of breast cancer diagnoses that are a result of hereditary, familial or sporadic factors.
Symptoms
The symptoms of breast cancer include[29]:
- The formation of a new lump or thickening in the breast or armpit area
- Change in nipple (i.e. inverted or retracted nipple)
- Change in skin of the breast, areola or nipple (i.e. colour, dimpling, etc.)
- discharge from the nipple(s)
- The presence of a rash or redness of skin
- Change in breast shape or size
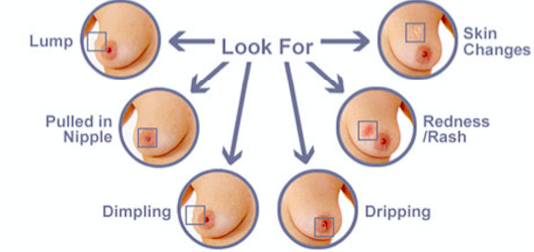 Figure 3: Various symptoms that are observed with breast cancer.
Figure 3: Various symptoms that are observed with breast cancer.
Screening
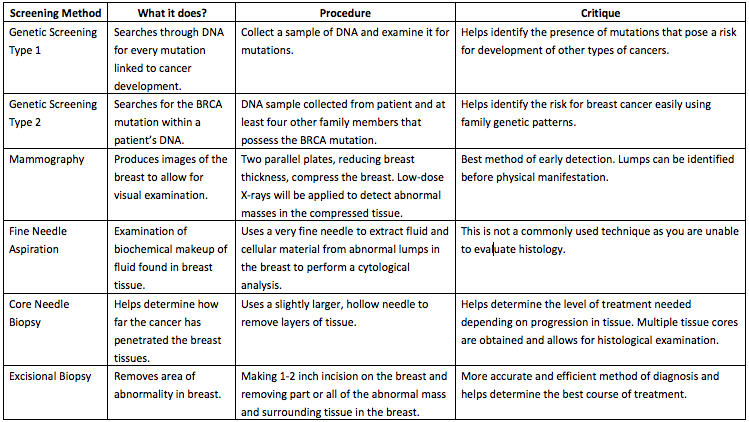
Screening for breast cancer involves testing healthy women for the presence of abnormalities to achieve an early diagnosis and result in lower mortality rates. This can be performed via breast exams, mammograms, genetic screening, ultrasounds and MRIs. In Ontario, it is recommended that women aged 50-74 conduct mammograms every 2 years[18]. Some detected cancers are benign and may not progress at all. At the same time, not all cancers found by screening can be cured.
 [18][48]
Table 1: Comparison of screening methods with their functions, procedure and critique of effectiveness.
[18][48]
Table 1: Comparison of screening methods with their functions, procedure and critique of effectiveness.
Abnormalities in Screening
Abnormalities detected through initial screening procedures require follow up tests to determine whether the abnormality is cancerous. This may be achieved through a follow up mammogram, ultrasound or MRI. A tissue biopsy will also be conducted to check for the presence of cancerous tissue.[20] There are noncancerous abnormalities that may also be present in the breast as a result of abnormal cell growth. These conditions are commonly categorized as fibrocystic breast disease (FBD) and result from non-proliferative and proliferative lesions in the breast.[20]
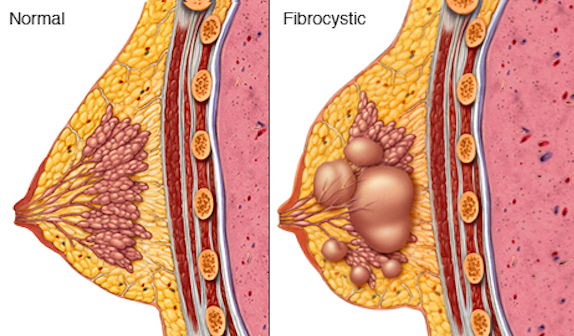 Figure 4: A normally functioning female breast compared to a female breast affected by FBD.
Figure 4: A normally functioning female breast compared to a female breast affected by FBD.
Non-proliferative lesions are lesions that occur due to trauma, irregularities in secretion, and clogging of ducts. This may form cysts, ductal ectasia (clogging of lactiferous duct), periductal fibrosis and columnar cell change. The is no elevated risk of breast cancer associated with non-proliferative lesions as the relative risk is at 1.[13]
Proliferative lesions are a result of rapid growth of cells. A proliferative lesion without atypia is the growth of normal cells. In this case, ducts and lobules are growing quickly but appear to be normal. This incldues the formation of ductal hyperplasia (overgrowth of cells), radial scars, and intraductal papilloma (tumor found in milk duct). There is an associated increased relative risk of developing breast cancer, ranging from 1.3 – 1.9.[13]
Proliferative lesions with atypia refer to the excessive growth of ducts and lobules that are growing rapidly and appear to be abnormal. Possible conditions include atypical ductal and lobular hyperplasia. There is an increased relative risk of 2.9 – 12.0 associated with development of breast cancer.[13]
Anatomy and Physiology
Breasts are organs that have a specialized function that is the production of milk for lactation[34]. The breast is made up of lobes, ducts, fat, connective tissues, and glands. Female breasts have specialization as both lobes and ducts are connected to allow for secretion of nutrients[34]. The male breasts however only have the ducts that have a blunt end, and the ducts do not connect to lobes[34]. Both female and male breasts sit atop the pectoralis muscle[34].
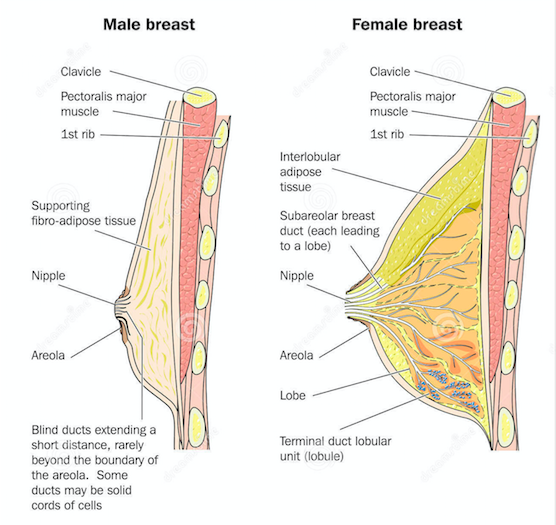 Figure 5: Compares the male breast anatomy and the female breast anatomy.
Figure 5: Compares the male breast anatomy and the female breast anatomy.
The breasts are connected to the blood and lymph vessels. The lymph vessels collect and move the lymph fluid away from the breast into lymphatic tissue (lymph nodes) around the breast[6]. The lymph vessels and lymph nodes compose the lymphatic system of the breast[6]. The axillary lymph nodes are under the arm, and are divided into three levels include[6]:
- Level I is low axilla, which is along the outer border of the pectoralis minor
- Level II is mid axilla, beneath the pectorals minor
- Level III is high axilla, along the inner border of pectorals minor
When breast cancer spreads, it spreads to level I lymph nodes first, followed by level II, and level III[6].
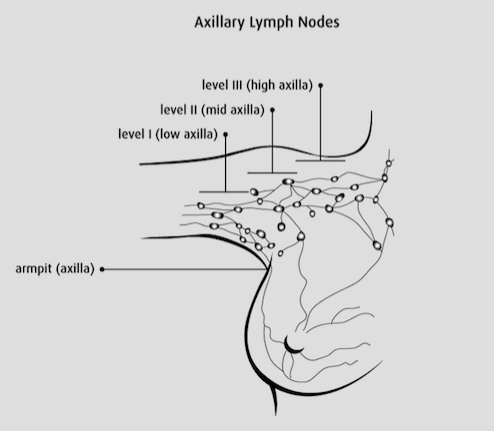 Figure 6: Illustrates the axillary lymph nodes : level I, level II, and level III.
Figure 6: Illustrates the axillary lymph nodes : level I, level II, and level III.
Stages of Breast Cancer
The stage of breast cancer is determined from testing the tumour tissue, lymph nodes and organs surrounding the breast. Non-invasive techniques such as image testing and invasive techniques such as biopsies can help determine the spread of the cancer and help define the stage the cancer is in.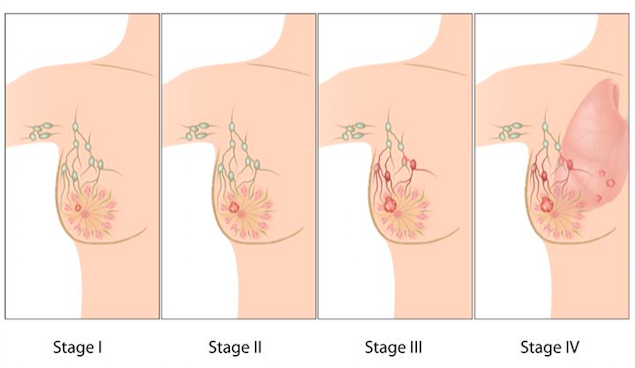 Figure 7:
Figure 7:
TNM System
The Tumour, Lymph Node, and Metastasis (TNM) system is widely recognized when labeling the stages of breast cancer.
Stage 0: Carcinoma In Situ Stage
There is abnormal but non-invasive cellular growth in the ducts, lobule, or nipple region [44]. Generally clinical breast exams or mammograms are recommended followed by a tamoxifen hormone therapy [44]
Stage 1
The cancer is found and contained in the regions where abnormal cells began to grow [44]. If the cancer is stage 1a, the tumour is less than 2cm in size and has not spread to lymph nodes [44]. If the cancer is in stage 1b, the cancer has moved to the lymph nodes but is less than 0.2-2mm in size [44].
Stage 2
The cancer continues to grow but remains either in the breast or has extended a little beyond the lymph nodes [44]. Stage 2a means the tumour is either less than 2cm but has crossed less than 4 auxiliary lymph nodes or the tumour is between 2-5cm in size but has not spread towards the lymph nodes [44]. Stage 2b can be characterized by the size of the cancer being between 2-5cm in size spreading into the lymph nodes or the tumour being about 5cm but has remaining stationary [44].
Stage 3
The breast cancer has now spread beyond the region of the original tumour growth and is beginning to affect muscles and lymph nodes beyond the original site [44]. In stage 3a the tumour is growing beyond 5cm in size. There are also small clusters of cancerous cells found in the lymph node region. Otherwise, the tumour is larger than 5cm and spreading to lymph nodes and nearby regions like the breastbone or under the arm [44]. In stage 3b, the tumour can grow to any size as it spreads and invades of the skin near the breast and chest walls [44].
Stage 4
This is considered the most dangerous stage, as there is no treatment to cure the cancer [44]. The cancer has spread to other regions around the body and has metastasized into regions of the brain, bones, liver, and lungs [44]. Treatment can only prolong the individual’s lifespan.
Pathophysiology
BCRA Gene
The breast cancer susceptibility gene 1 (BRCA1) and breast cancer susceptibility gene 2 (BRCA2) are tumour suppressor genes, and translational protein products expressed in all humans [39]. The specific locality of BRCA1 and BRCA2 gene is on chromosome 13 and 17, respectively [32][40]. Moreover, the significance of such genes is predominantly due to the associated lifetime risk for breast cancer (BC) when mutations are present. This is evidently shown through a prospective cohort study by Kuchenbaecker et al. which revealed the cumulative BC risk to age 80 years to be 72% for BRCA1 and 69% for BRCA2. Additionally, further data has shown a rapid increase in BC incidence from adulthood until ages 30-40 for BRCA1, and until ages 40-50 for BRCA2. However, past the upper-limit for the peak-incidence of each respective gene, the frequency of BC presentation remains constant at 20-30 per 1000 persons until age 80 [27]. When the BRCA gene is translated into its BRCA protein product, it plays a role in the repairing double DNA breaks such as non-homologous end-joining and homologous recombination DNA repair [2] [36]. It also functions to facilitates cellular responses to DNA damage through blockage of cell proliferation and induction of apoptosis [11]. Thereby, a genetic mutation on BRCA1 and BRCA2 affecting in the functional capability of its protein products would ultimately lead to the susceptibility in mutated-BRCA carriers for BC.
Evidence that BRCA Involved in DNA Repair
There are several studies which evidently validate the evidence of BRCA1 and BRCA2 in DNA repair. Firstly, a study conducted by Foray et al. reveals the inability of irradiated cells to repair DNA double-strand breaks due dysfunctional BRCA1 and BRCA 2 [17]. Secondly, an additional study has demonstrated the impairment of chromosomal-break repair by homologous recombination in BRCA1- and BRCA2-mutant cell-lines. It is further explained in the study that BRCA proteins conjugate with Rad51 recombinase to effectively repair DNA damage, and thus allowing for chromosomal stability [33]. Lastly, Chen et al. reveals the coexistence of BRCA1 and BRCA2 in a biochemical complex which co-localize at the DNA replication sites post-application of hydroxyurea or UV radiation to cause double DNA breaks [8][42].
Alterations in the Cell Cycle
Apart from the susceptibility of BC due to the inability of mutant-BRCA to repair DNA breakages; there are additional genes, when unregulated, results in cancerous-cell phenotypes of the breast tissue [1]. These genes are primarily responsible for regulation of the cell cycle which is an important mechanism for normal cell growth, survivability and replication. Therefore, such disruption in cell-cycle regulatory factors can ultimately lead to sustained proliferative signaling, evasion of growth suppressors, replicative immortality, activation of invasion and metastasis, induction of angiogenesis, and resistance to cell death [12]. In breast cancer, the overexpression of cyclin D1 and E, down-regulation of cyclindependent kinase inhibitors, or the activation of tumour suppressor proteins, retinoblastoma and p55 are the known altered-regulatory cell-cycle proteins that result in the development of cell-malignancy [28].
Up/Down-Regulation of Breast Cancer Associated Genes
Although the mechanism that malignant tumour cells use to migrate, invade and induce angiogenesis is very well studied, the preliminary cell alterations in which genomic modifications are made are not well known. However, a study by Patsialou et al. in 2012, illustrated that most up-regulated gene networks of migratory great tumour cells included those involved in DNA replication and repair, embryonic and tissue development and cellular movement. Likewise, the most down regulated genes included those involved with nervous system development and function and cell death/cell cycle genes[35].
Metastatic Process of Breast Cancer
Epithelial-to-mesenchymal transition (EMT) is the start of breast cancer metastasis. Malignant cells which originate from the primary tumour site invade local tissue and then intravasate into the circulatory system or the lymphatic system respectfully, thereby prompting cell cycle arrest and promoting angiogenesis at secondary tumour sites[41]. Subsequently, polarized epithelial cells go through biochemical changes due to the EMT process which ultimately allows for the gain of mesenchymal phenotype. With the gain in phenotype comes extra migratory and motility features[30].
To begin invasion, cancer cells need to manipulate cell-to-cell adhesion through the use of cadherins— to cross the ECM (extra-cellular matrix)[41]. Under normal conditions E-cadherin maintains cell-cell junctions while N-cadherins assist with EMT and thus progresses cancer by inducing ECM degrading enzymes such as MMPs[49]. EMT is a two-fold mechanism which not only guides invasion and intravasation into the bloodstream, but also up-regulates proteases involved in the degradation of the ECM. Evidence for such was observed in a study that replaced N-cadherin with E-cadherin, which in turn caused inflammation in mammary tissues eventually leading to breast cancer tumour in mice[26]. After the malignant cells have passed the mammary tissue to spread— they must adhere to the ECM of peripheral host cells which is induced via integrins. Passing through the extra cellular matrix (ECM) involves integrins[41]. Integrin are generally found on basic ECM structures such as collagen, fibrinogen and fibronectin to name a few. Integrins are transmembrane receptors that also are associated with up-regulating ECM-degrading enzymes[41]. For example, one study done on mice showed that integrin α5β1 upregulated matrix matelloproteinase-9 (MMP-9) as cells migrated from the tumor[38]. MMPs specifically mediate the ECM degradation at the invadopodial front of invasive breast cancer cells[41]. Studies have also shown that over-expression of epidermal growth factor receptor (EGFR) and its STP (signal transduction pathway) causes EMT, migration and invasion by increasing the amount of MMP made in the malignant cell[31]. Another way the ECM is broken down is by using heparanase to target the heparan sulfate proteoglycan, significant for its role in the integrity of the ECM as well cell matrix adhesion and growth factor receptor interactions[41]. An in vitro and in vivo study by Cohen et al. in 2006 showed that over expression of heparanase in breast cancer cells increased cell replication and infiltration, thus promoting metastasis.
Tumour cells migrate either on their own or coordinately, however coordinate travel requires the presence of intercellular junctions. Single tumour cells have two pathways to migrate, either protease-dependent mesenchymal movement or protease-independent amoeboid movement[41]. The former pathway has already been covered, as EMT (epithelial-to-mesenchymal transition) which degrades the ECM and uses MMPs to exit and migrate. However, the latter pathway includes round cells which protrude from pores in the matrix via causing structural deformations in the ECM[50]. The force in the amoeboid movement is generated by actin/myosin muscular contractions and cortical actin through a signal transduction pathway (STP) such as RhoA/Rho kinase (ROCK)[50]. The motility of cancer cells to peripheral bodily areas also involves increasing diffusion past pre-existing blood vessels and screening against host immune cells[19]. Moreover, breast cancer cells also promote vascular endothelial growth factor-C (VEGF-C). An increase in VEGF-C allows for more lymphatic networking permitting entry into, and dilation of lymphatic vessels for further spread[21].
Current Treatments
Surgical Interventions
Surgery depends on the size and location of tumour, and if the cancer has spread to the lymph nodes[16]. Before surgery, neoadjuvant chemotherapy is commonly used to shrink the tumour so it is relatively easier to remove[16].
Lumpectomy is one of the surgical interventions that is the least invasive and is also referred to breast-conserving surgery (BCS), and is always followed up by radiation therapy [16]. BCS involves the removal of a tumour that is small without removing the entire breast tissue [16]. The surgeon makes small incision near the abnormal area, and removes the abnormal mass along with a margin of healthy tissue[16]. BCS is very effective at the early stages of breast cancer when the tumours are locally invasive[16].
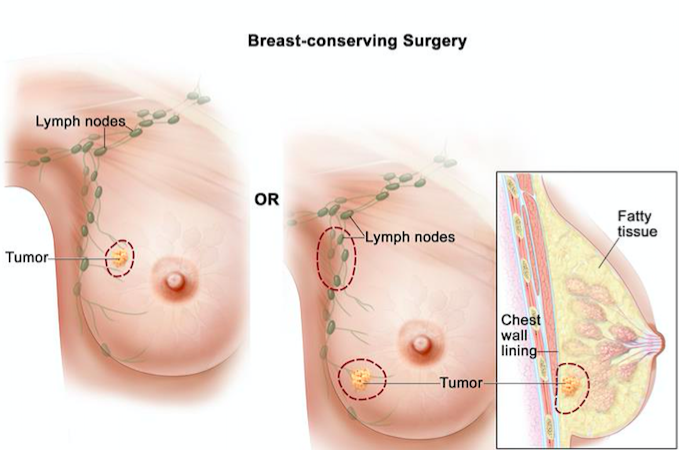 Figure 8: Illustrates the removal of a small tumour without removing entire breast tissue using lumpectomy.
Figure 8: Illustrates the removal of a small tumour without removing entire breast tissue using lumpectomy.
Mastectomy is another surgical intervention that is used to treat large tumours that have spread to more than one area in the breast[16]. Mastectomy involves the complete removal of the breast tissue, and the removal of the fascia over the pectoralis muscle [16]. After the removal, the surgeon places tubes where removal has occurred, and these tubes function to remove blood and lymph fluid that collect during the healing process [16].
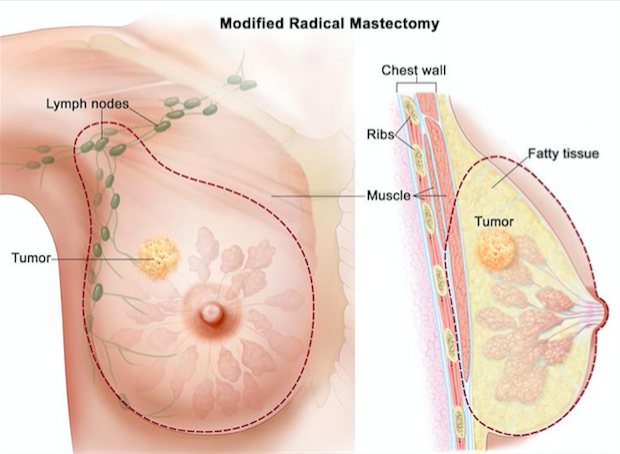 Figure 9: Illustrates the complete removal of the breast tissue using mastectomy.
Figure 9: Illustrates the complete removal of the breast tissue using mastectomy.
Radiation Therapy
Radiation therapy uses high-energy rays to destroy cancer cells, and prevent cancer cells from recurring after surgery[34]. It is also used to shrink tumour size before surgery[34]. The therapy damages the genetic material of the cancer cells and stops growth[34]. The high-energy rays can also damage the normal cells, however the normal cells have the ability to repair themselves after the radiation therapy is applied[34].
Chemotherapy
Chemotherapy is a treatment that is used to kill rapidly diving cells, and is highly invasive. There are two types of chemotherapy: neoadjuvant and adjuvant therapy [34]. Neoadjuvant therapy is used before surgery, and targets to shrink the large tumour to increase the chance of patient to have a breast-conserving surgery [34]. The patient is treated with high risk of metastasis without delay, and the therapy can also show if the tumour is responding to the treatment, making the removal of the tumour safer. Adjuvant therapy is used after surgery for patients who have no evidence of cancer. The therapy targets to destroy any cancer cells that may be left behind or cells that have metastasized[34]. Taxol chemotherapy is another specific branch of chemotherapy where taxol binds to the tubulin subunits and stabilizes the microtubules against depolymerisation[34]. This results in the cell to remain in metaphase-anaphase stage, and leads to cell apoptosis than cell replication[34]. Chemotherapy is usually offered to treat early stage breast cancer with a high risk of recurrence or locally advanced breast cancer[34]. Chemotherapy can also cause side effects depending on the type of drug used, the dosage, and overall health[34]. Some common side effects include low blood cell count, hair loss, infection, nausea and vomiting, nervous system damage, and loss of appetite[34].
Hormonal Therapy
Hormonal therapy is used to treat breast cancer by adding, blocking, or removing hormones. Hormone therapy is used for breast cancer tissue tested for hormone receptor positive, which means that the cancer cells have receptors for estrogen, progesterone, or both [6]. With these receptors present on the cancer cell, the hormones can attach to the receptors, and help these cells to grow[6].
The two common therapies to treat hormone receptor-positive breast cancer are anti-estrogen drugs, and aromatase inhibitors. Tamoxifen is most commonly used as anti-estrogen drug, which blocks the estrogen receptors on cancer cells so cancer cells are not able to use estrogen[6]. Aromatase is an enzyme that the body uses to make estrogen in areas of the body other than the ovaries, such as fat tissues and adrenal glands[6]. Aromatase inhibitors block the action of aromatase enzyme, which decreases levels of estrogen in the body[6]. A decrease in estrogen levels is beneficial because very little estrogen can be used by the cancer cells, minimizing growth of the tumour cell[6].
Future Treatments
Immunotherapy
Current research has progressively contributed to the advancement of breast cancer treatment. The investigations have been especial towards engineered-viral therapy to provide additional modalities of treatment associated with enhanced efficacy. Presently, numerous oncolytic viruses for both monotherapy, and combination therapy are undergoing clinical trials to evaluate the safety and efficacy of treatments. One recent study conducted by Hemminki et al. reveals the impact of the oncolytic adenovirus Ad5/3-E2F- delta24-GMSF in inducing oncolysis and initiating a potent anticancer immune response towards solid non-metastatic tumours. The results showed a radiological disease control rate of 83%, and accumulate of immunological cells to tumours in 9/12 patients [22]. However, the monotherapeutic delivery of oncolytic viruses may not serve optimal success for metastatic and advanced stages of cancer. Thereby, oncolytic viruses can be combined with other anticancer remedies to maximized the efficacy of therapeutics in a synergetic manner. A study conducted by Cerullo et al. demonstrates the immunological effects of cyclophosphamide (CP) in combination with oncolytic adenovirus on cancer patients. The results show that CP-adenoviral treatments had higher rates of disease control than that of the virus alone, as well as overall patient-survivability [7].
Apart from oncolytic therapies, natural killer (NK) cells have also been a significant focus in the field of immunology due to its role in cancer immunosurveillance and potential to eradicate cancer cells. A recent study conducted by Shenouda et al. demonstrates ex-vivo expansion of activated NK-cells in providing highly effective cytotoxicity against breast cancer cell lines [44]. The ability to accumulate personalized activated NK-cells would enable patients to restore or boost NK-cell levels to optimally combat cancerous cells. Ultimately, these findings may offer various modalities of management for more individualized approaches to breast cancer patients, as well as bridging the next step of innovation towards cancer treatment.
References
- Ahmad, A. (2013). Breast Cancer Metastasis and Drug Resistance. In Breast Cancer Metastasis and Drug Resistance(pp. 143–159). https://doi.org/10.1007/978-1-4614-5647-6
- Bau, D. T., Mau, Y. C., & Shen, C. Y. (2006, August 18). The role of BRCA1 in non-homologous end-joining. Cancer Letters. https://doi.org/10.1016/j.canlet.2005.08.003
- Bernstein, L. (2002). Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 7, 3–15.
- Breast cancer: prevention and control. (n.d.). Retrieved October 03, 2017, from http://www.who.int/cancer/detection/breastcancer/en/
- Breast Cancer. (2017, June 07). Retrieved October 03, 2017, from https://www.cdc.gov/cancer/breast/statistics/index.htm
- Canadian Cancer Society. (2017). Retrieved September 30, 2017, from
- Cerullo, V., Diaconu, I., Kangasniemi, L., Rajecki, M., Escutenaire, S., Koski, A., … Hemminki, A. (2011). Immunological Effects of Low-dose Cyclophosphamide in Cancer Patients Treated With Oncolytic Adenovirus. Molecular Therapy, 19(9), 1737–1746. https://doi.org/10.1038/mt.2011.113
- Chen, J., Silver, D. P., Walpita, D., Cantor, S. B., Gazdar, A. F., Tomlinson, G., … Scully, R. (1998). Stable Interaction between the Products of the BRCA1 and BRCA2 Tumor Suppressor Genes in Mitotic and Meiotic Cells. Molecular Cell, 2(3), 317–328. https://doi.org/10.1016/S1097-2765(00)80276-2
- Cohen, I., Pappo, O., Elkin, M., San, T., Bar-Shavit, R., Hazan, R., Peretz, T., Vlodavsky, I, and Abramovitch, R. (2006). Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int J Cancer. 118, 1609-1617.
- Collaborative Group on Hormonal Factors in Breast Cancer. (2002). Alcohol, tobacco and breast cancer – collaborative reanalysis of individual data from 53 epidemiological studies, including 58 515 women with breast cancer and 95 067 women without the disease. British J Cancer. 11, 1234–1245. http://doi.org/10.1038/sj.bjc.6600596
- Deng, C. X. (2006). BRCA1: Cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Research, 34(5), 1416–1426. https://doi.org/10.1093/nar/gkl010
- Diederichs, S., & Gutschner, T. (2012). The hallmarks of cancer A long non-coding RNA point of view. RNA Biology, 96, 703–719.
- Dupont, W. D., Parl, F. F., Hartmann, W. H., Brinton, L. A., Winfield, A. C., Worrell, J. A., … & Plummer, W. D. (1993). Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. CANCER-PHILADELPHIA-, 71, 1258-1258.
- Ewertz, M., Duffy, S.W., Adami, H.O., Kvale, G., Lund E, Meirik O, Mellemgaard A, Soini I, Tulinius H. (1990). Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 46, 597–603.
- Ferlay, J., Bray, F., Pisani, P., and Parkin, D.M. (2002). Globocan: cancer incidence, mortality and prevalence worldwide. IARC CancerBase No 5, version 2.0. Lyon: IARC Press; 2004.
- Fisher, B., Anderson, S., Bryant, J., Margolese, R. G., Deutsch, M., Fisher, E. R., … & Wolmark, N. (2002). Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine, 347(16), 1233-1241.
- Foray, N., Randrianarison, V., Marot, D., Perricaudet, M., Lenoir, G., & Feunteun, J. (1999). Gamma-rays-induced death of human cells carrying mutations of BRCA1 or BRCA2. Oncogene, 18(51), 7334–7342. https://doi.org/10.1038/sj.onc.1203165 from the laboratory. Nature reviews. Cancer, 3(11), 821.
- Gagnon, J., Lévesque, E., The Clinical Advisory Committee on Breast Cancer Screening and Prevention, Borduas, F., Chiquette, J., Diorio, C., … Simard, J. (2016). Recommendations on breast cancer screening and prevention in the context of implementing risk stratification: impending changes to current policies. Current Oncology, 23(6), e615–e625. http://doi.org/10.3747/co.23.2961
- Gupta, G. P., and Massagué. (2006). Cancer metastasis: building a framework. Cell. 127, 679-695.
- Guray, M., & Sahin, A. A. (2006). Benign breast diseases: classification, diagnosis, and management. The oncologist, 11(5), 435-449.
- He, Y., Rajantie, I., Pajusola, K., Jeltsch, M., Holopainen, T., Yla-Herttuala, S.,… Alitalo, K. (2005). Vascular endothelial cell growth factor receptor 3–mediated activation of lymphatic endothelium is crucial for tumour cell entry and spread via lymphatic vessels. Cancer Res. 65, 4739-4746.
- Hemminki, O., Parviainen, S., Juhila, J., Turkki, R., Linder, N., Lundin, J., … Hemminki, A. (2015). Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget, 6(6), 4467. https://doi.org/10.18632/oncotarget.2901 http://www.cancer.ca/en/?region=on
- Johnston, S. R., & Dowsett, M. (2003). Aromatase inhibitors for breast cancer: lessons
- Key, T. J., Verkasalo, P. K., & Banks, E. (2001). Epidemiology of breast cancer. The Lancet Oncology, 2(3), 133-140. doi:10.1016/s1470-2045(00)00254-0
- Kluttig, A., & Schmidt-Pokrzywniak, A. (2009). Established and suspected risk factors in breast cancer aetiology. Breast Care, 4(2), 82–87. http://doi.org/10.1159/000211368
- Kotb, A. M., Hierholzer, A., and Kemler, R. (2011). Replacement of E-cadherin by N-cadherin in the mammary gland leads to fibrocystic changes and tumor formation. Breast Cancer Res. 13,104, 2011.
- Kuchenbaecker, K. B., Hopper, J. L., Barnes, D. R., Phillips, K.-A., Mooij, T. M., Roos-Blom, M.-J., … Olsson, H. (2017). Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. Jama, 317(23), 2402–2416. https://doi.org/10.1001/jama.2017.7112
- LANDBERG, G., & ROOS, G. (1997). The cell cycle in breast cancer. APMIS, 105(7–12), 575–589. https://doi.org/10.1111/j.1699-0463.1997.tb05056.x
- Marcu, A., Black, G., Vedsted, P., Lyratzopoulos, G., & Whitaker, K. L. (2017). Educational differences in responses to breast cancer symptoms: A qualitative comparative study. British journal of health psychology, 22(1), 26-41
- Martin, T.A., Ye, L., Sanders, A.J., Lane, J., and Jiang, W. G. (2013). Cancer invasion and metastasis: Molecular and cellular perspective. Austin (TX): Landes Bioscience.
- Masuda, H., Zhang, D., Bartholomeusz, C., Doihara, H., Hortobagyi, G. N., & Ueno, N. T. (2012). Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast Cancer Research and Treatment, 136(2), 10.1007/s10549–012–2289–9. http://doi.org/10.1007/s10549-012-2289-9
- Miki, Y., Swensen, J., Shattuck-eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S., … Skolnick, M. H. (1994). Strong Candidate for the Breast and Ovarian Cancer Susceptibility Gene BRCA1. Science, 266, 66–71. https://doi.org/10.1126/science.7545954
- Moynahan, M. E., Pierce, A. J., & Jasin, M. (2001). BRCA2 is required for homology-directed repair of chromosomal breaks. Molecular Cell, 7(2), 263–272. https://doi.org/10.1016/S1097-2765(01)00174-5
- National Cancer Institute. (2015, July 8). Breast Cancer Treatment for Health
- Patsialou, A., Wang, Y., Lin, J., Whitney, K., Goswami, S., Kenny, P. A., and Condeelis, J. S. (2012). Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast Cancer Research. 14, 1-19.
- Powell, S., & Kachnic, L. (2003). Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene, 22(37), 5784–5791. https://doi.org/doi: 10.1038/sj.onc.1206678 Professionals (PDQ).
- Radford, D. M., & Zehnbauer, B. A. (1996). Inherited breast cancer. Surgical Clinics of North America, 76(2), 205-220
- Rolli, M., Fransvea, E., Pilch, J., Saven, A., and Felding-Habermann, B. (2003). Activated integrin αvβ3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 100, 9482-9487.
- Rosen, E. M., & Pishvaian, M. J. (2014). Targeting the BRCA1/2 tumor suppressors. Current Drug Targets, 15(1), 17–31. https://doi.org/10.2174/1389450114666140106095432
- Salgado, J., Aramendía, J. M., Gutierrez, C., Gil, C., Robles, M., & García-Foncillas, J. (1995). Identification of the breast cancer susceptibility gene BRCA2. Nature, 378(6559), 789–792. https://doi.org/10.1038/378789a0
- Scully, O. J., Bay, B., Yip, G., and Yu, Y. (2012). Breast cancer metastasis. Cancer Genomics Proteomics. 9, 311-320.
- Scully, R., Chen, J., Plug, A., Xiao, Y., Weaver, D., Feunteun, J., … Livingston, D. M. (1997). Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell, 88(2), 265–275. https://doi.org/10.1016/S0092-8674(00)81847-4
- Seitz, H.K., & Becker, P. Alcohol metabolism and cancer risk. (2007). Alcohol Res Health. 30, 38–41, 44-47.
- Shenouda, M. M., Gillgrass, A., Nham, T., Hogg, R., Lee, A. J., Chew, M. V., … Ashkar, A. A. (2017). Ex vivo expanded natural killer cells from breast cancer patients and healthy donors are highly cytotoxic against breast cancer cell lines and patient-derived tumours. Breast Cancer Research, 19(1), 76. https://doi.org/10.1186/s13058-017-0867-9
- Sölétormos, G., Nielsen, D., Schiøler, V., Mouridsen, H., & Dombernowsky, P. (2004). Monitoring different stages of breast cancer using tumour markers CA 15-3, CEA and TPA. European Journal of Cancer, 40(4), 481-486. doi:10.1016/j.ejca.2003.10.015
- Thigpen, J. (2012). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Yearbook of Medicine,2012, 111-112. doi:10.1016/s0084-3873(12)00182-4
- Turnbull, C., & Rahman, N. (2008). Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 9, 321–345.
- Warner, E., Plewes, D. B., Shumak, R. S., Catzavelos, G. C., Di Prospero, L. S., Yaffe, M. J., … & Taylor, G. A. (2001). Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. Journal of Clinical Oncology, 19(15), 3524-3531
- Wendt, M. K., Taylor, M. A., Schiemann, B. J., and Schiemann, W. P. (2011). Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 22, 2423-2235.
- Yilmaz, M., and Christofori, G. (2010). Mechanisms of motility in metastasizing cells. Mol Cancer Res. 8, 629-642.