Table of Contents
Chronic Myeloid Leukemia
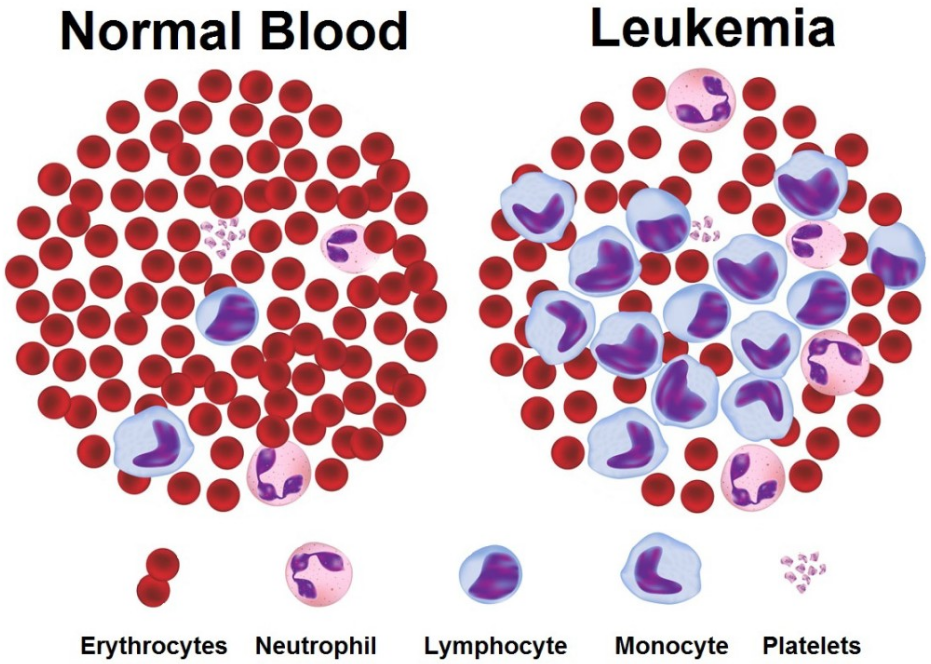
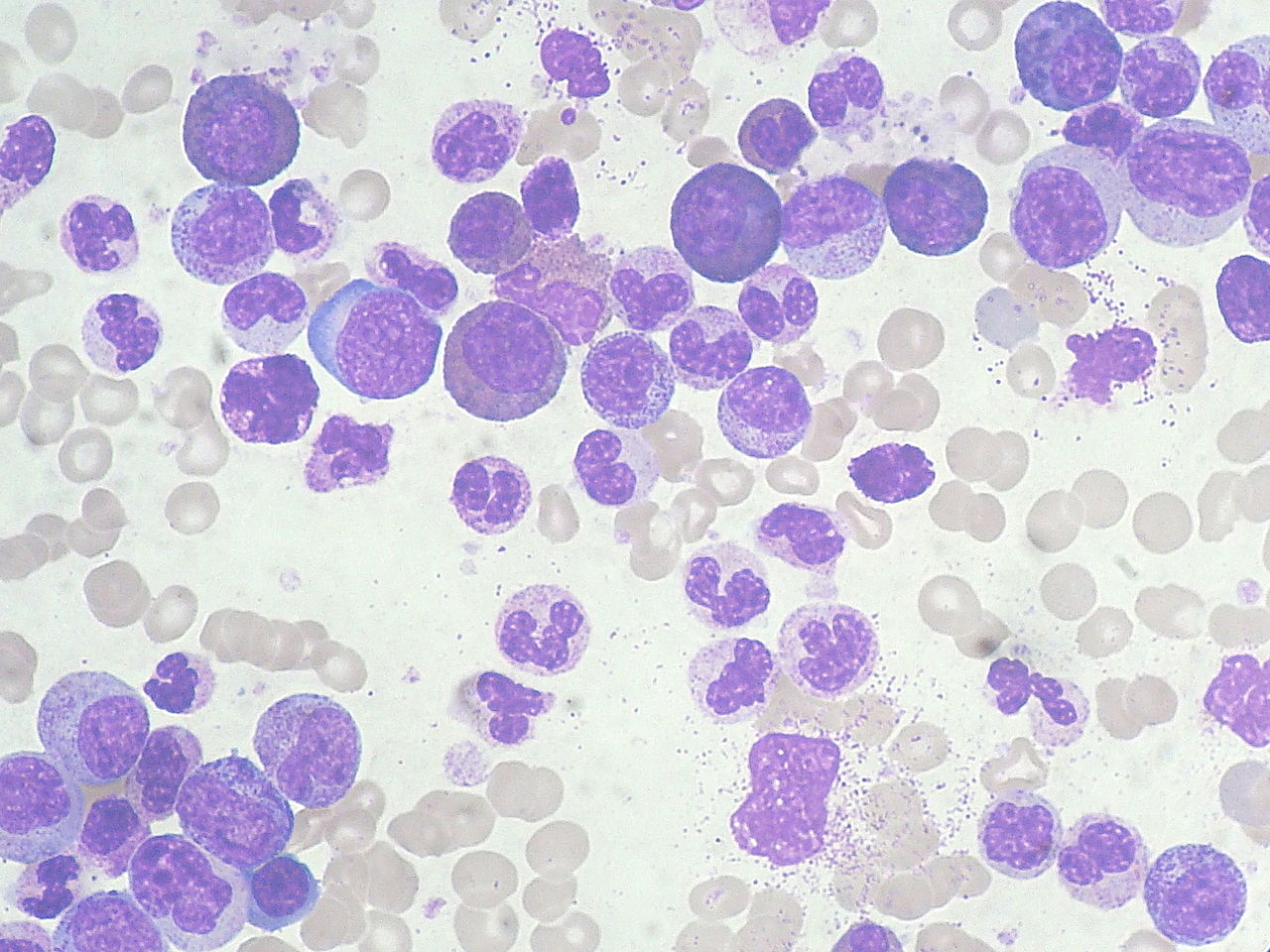
Figure 1: normal blood versus blood of a CML patient.
Link to PPT presentation: https://docs.google.com/a/mcmaster.ca/presentation/d/1yjxDywKLHAQyuMUwxRe52624DxmA9ek-3qR8e3BM-jw/edit?usp=sharing
Introduction
Chronic myeloid leukemia (CML) is a cancer of white blood cells that begins in the bone marrow where blood cells are formed. CML distinguishes itself from other types of leukemia by the abnormal and unregulated growth of myeloid cells – specifically granulocytes. A genetic abnormality, called the Philadelphia chromosome, is consistent with CML; this abnormality involves a reciprocal translocation of the tails of chromosome 9 and 22, which results in a BCR-ABL fusion gene shown to have a significant role in CML occurrence (Goldman, 2010). CML is the only type of myeloproliferative neoplasm that is characterized by being BCR-ABL positive (Vardiman, et al., 2009). Although there is a genetic factor in CML, there is no familial link. According to the Canadian Cancer Society, the only known risk factors that increase the chances of having CML are exposure to intense radiation, old age, and being male. Since the late 1990s to early 2000s, tyrosine kinase inhibitors (TKI) have become the most effective treatment of CML, and their use has shown a significant improvement in CML patient prognosis and quality of life (Druker & Lydon, 2000).
History
CML was the first type of leukemia to be recognized. In the 1840s, Scottish physician David Craigie and pathologist John H. Bennett reported patients with enlarged spleens and abnormally high white blood cell counts; the same was reported by German physician Rudolph Virchow and French physician Alfred Donne (Goldman, 2010). Retrospectively, these patients are suggested of having CML. Virchow and Bennett speculated the disease had to do with hematopoiesis (Virchow, 1845; Goldman, 2010). At the time, arsenic was used to attempt to treat CML, but it only decreased leukocyte counts and did not prolong life (Goldman, 2010). The next significant discovery was that of Ernst Neumann in 1872, who recognized white and red blood cells begin forming in the bone marrow, which led him to believe all blood cells have a common stem cell (Zech, Shkumatov, & Koestenbauer, 2007).
The subsequent major discovery occurred nearly 90 years later, in 1960, by Peter Nowell and David Hungerford, who noted a consistent genetic abnormality of a shortened chromosome 22 in CML patients (Nowell & Hungerford, 1960). This genetic abnormality was named the Philadelphia chromosome, as Philadelphia is where the discovery was made. In 1973, Janet D. Rowley discovered the Philadelphia chromosome abnormality results from a reciprocal translocation of chromosomes 9 and 22 (Goldman & Daley, 2007). In the 1980s, the breakpoint cluster region on chromosome 22 was identified as part of the BCR gene, and it was recognized that the Abelson gene (ABL) from chromosome 9 is translocated to chromosome 22 in CML (Klein, et al., 1986). With this 9:22 translocation known, the BCR-ABL fusion gene was discovered (Goldman, 2010). In 1990, this BCR-ABL fusion gene, specifically P210BCR/ABL gene, was shown to induce CML in mice, thus reinforcing the significant role of the genetic abnormality in the pathogenesis of CML (Daley, Van Etten, & Baltimore, 1990).
Epidemiology
In 2013, the Canadian Cancer Society reported that 675 Canadians were diagnosed with CML (410 men and 265 women) and 119 of them died from CML (72 men and 47 women).
The incidence of CML has been stable with annual average incidences reported between 0.7-1.3/100,000 people, but it has also been reported to be as low as 0.4/100,000 people in Taiwan to as high as 1.75/100,000 people in the USA (Chang, et al. 2011; Chen, et al. 2013; Hoglund, et al. 2016). With age, the incidence of CML increases peaking in the mid-80s (Hoffmann et al. 2015). CML is more common in men, with a male-to-female ratio ranging from 1.2-1.7, and this difference is more distinct in the older populations (Hoglund, et al. 2013; Radivoyevitch, et al. 2014).
The prevalence of CML has less statistical backing, but it is estimated to be around 10-12/100,000 people, and it is increasing slowly due to the increasing survival rates of CML patients and the increase in life expectancy of the general population (Hoglund, et al. 2016). The survival rate has dramatically improved since the introduction of tyrosine kinase inhibitors (TKIs) (Hoglund, et al. 2016). Patients with CML have a similar life expectancy to the general population in countries with a sufficient supply of TKIs, but relative survival in people 70+ years is lower (Bjorkholm, et al. 2016).
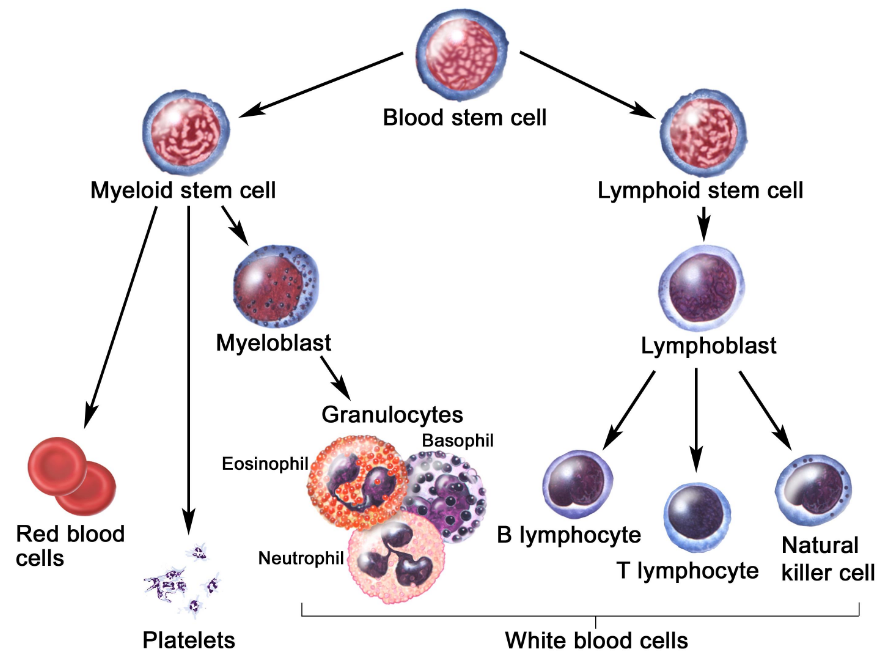
Hierarchy of Blood Cell Development - Hematopoiesis
Hematopoiesis is the process of blood cell development (Smith, Burthem, & Whetton, 2003). Figure 2 illustrates the development of blood cells in a committed, step-wise fashion (Smith, Burthem, & Whetton, 2003). New cells are continually generated by the proliferation of self-renewing hematopoietic stem cells that give rise to non-self-renewing progenitor cells (Smith, Burthem, & Whetton, 2003). Disruption of this molecular mechanism can lead to the abnormal production of blood cell count (Smith, Burthem, & Whetton, 2003). These progenitor cells are committed to giving rise to cells that are more restricted in differentiation potential and perform a variety of functions.
Hematopoietic stem cells (HSCs) are considered multipotent stem cells, which can differentiate into any blood cell type. Specifically, HSCs give rise to the lymphoid and myeloid lineages of blood cells. The myeloid stem cell gives rise to red blood cells, platelets, and granulocytes (i.e., neutrophils, basophils and eosinophils) while the lymphoid stem cells give rise to B lymphocytes, T lymphocytes, and Natural Killer Cells.
Leukemia
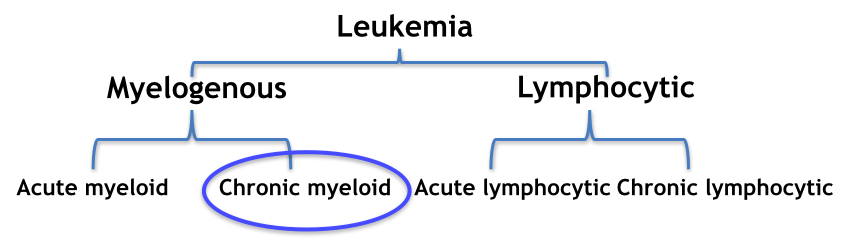
The abnormal presence of white blood cells in the blood is a direct translation of the word “leukemia”. In more general terms, leukemia can be characterized as a cancer of the blood that develops in the bone marrow (Canadian Cancer Society, 2017). There are many different types of leukemia, and they are grouped based on the type of blood stem cell they developed from (Canadian Cancer Society, 2017).
- Lymphocytic Leukemias: develop from abnormal lymphoid stem cells
- Myelogenous Leukemias: develop from abnormal myeloid stem cells
Moreover, the types of leukemia are further grouped based on how quickly the leukemia develops and grows. Acute leukemias start suddenly, developing within days or weeks, and chronic leukemias develop slowly over months or years. The four main types of leukemia are outlined in figure 3 below.
Figure 3: Development of the four main types of leukemia
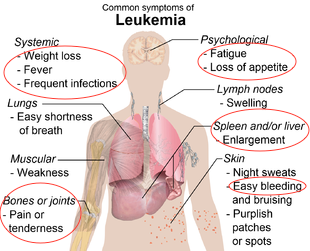
Signs and Symptoms
Symptom onset differs depending on the type of leukemia. In acute myeloid leukemia, symptoms develop rapidly, often initially presenting as flu (Sawyers, 1999). However, in chronic myeloid leukemia symptoms develop more slowly, and severity depends on the production levels of unregulated myeloid cells in the bone marrow. Due to the slow progression of the chronic phase, symptoms often develop slowly and diagnosis results from routine blood tests initially performed to diagnose other hypothesized diseases (Sawyers, 1999). In chronic myeloid leukemia, symptoms result from an increased white blood cell, specifically granulocytes. The typical symptoms include:
- Fatigue
- Due to a relative decrease in RBCs compared to WBCs. The relative reduction of RBCs results in symptoms of anemia resulting from low iron levels.
- Weight loss
- WBCs become localized in the spleen and liver, causing damage to these organs and resulting in enlargement of the liver and spleen.
- Easy bruising
- Due to the high production of WBCs (specifically granulocytes) which outcompetes the production of platelets (responsible for the blood clotting phenomenon).
- Frequent infections
- The overproduced granulocytes are released into the blood from the bone marrow as immature cells that are incapable of their normal, pathogen-fighting mechanisms.
- Bone pain
- Resulting from hyperplasia of the bones. The high turnover rate of myelogenous progenitors leads to pressure buildup in the bones.
Risk Factors
The risk of having CML increases with age and it is more likely to occur in males. The only other risk factor frequently reported to increase the probability of CML is radiation exposure (i.e. from a nuclear reactor accident or an atomic bomb) (Tanaka et al., 1989). A retrospective study of CML patients by Corso et al (1995) found that those with a history of exposure to radiation tend to have fewer tumours, higher frequency of anemia, and longer survival.
The Canadian Cancer Society (CCS) reports potential risk factors which need further research; these include: breathing in formaldehyde, previous radiation therapy, obesity. The CCS also reports no link between family history and CML.
Diagnosis
Chronic Myeloid Leukemia can be diagnosed in the following ways:
Complete Blood Count (CBC)
This is often a routine test showing the number of red blood cells, white blood cells, and platelets in the blood. In a patient with CML, leukocytosis is seen with a remarkable left shift (Quintás-Cardama and Cortes, 2006). This means that there are a high number of immature white blood cells present in the blood, as a result of their early release from the bone marrow. Leukocytosis is most prevalent in basophils and eosinophils. Red blood cells are usually present in less than normal levels, as they are outnumbered by the white blood cells (Sessions, 2007). This usually results in mild anemia. The platelet count may be less than normal, as the WBC production outcompetes it (Sessions, 2007).
Figure 5: CML blood smear showcasing increased WBC count and left shift.
Bone Marrow Biopsy
This test removes a small amount of bone filled with marrow to confirm blood test findings. In CML, the bone marrow is hypercellular with evidence of myeloid hyperplasia (Sessions, 2007). The primary biologic defect is not the unregulated proliferation of leukemic stem cells, but rather a slight delay in myeloid maturation resulting in increased myeloid cell mass (Quintás-Cardama and Cortes, 2006).
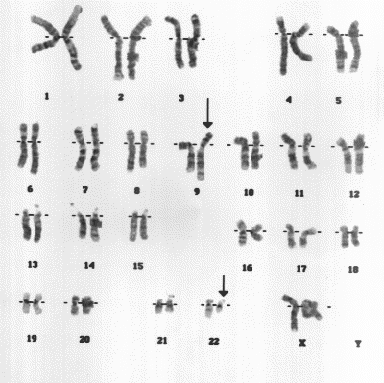
Cytogenetics (karyotyping)
This test is performed on a bone marrow biopsy. Cells are cultured for 1-3 days, allowing the chromosomes to be condensed and enter into the metaphase state (Sessions, 2007). At this point, cells are stained in order to identify the sizes and patterns of the chromosome bands. The sizes and patterns of the bands allow the identification of chromosomal abnormalities such as translocations, amplifications, and deletions ((Sessions, 2007). The Ph chromosome is identified as a result of the translocation of chromosome 9 at band q34.1 that transposes the 3’ segment of the ABL1 gene to the 5’ segment of the BCR gene on chromosome 22 at band q11.21(Quintás-Cardama and Cortes, 2006). The Ph chromosome is detected in 95% of patients with CML and is therefore required for a conclusive diagnosis of CML ((Quintás-Cardama and Cortes, 2006).
Figure 6 : Karyotype showing the translocation of genetic material to produce the Ph chromosome.
Molecular Pathogenesis of Chronic Myeloid Leukemia
In 1960, Peter Nowell and David Hungerford, working in Philadelphia, described a consistent chromosomal abnormality in patients with CML (Druker, 2008). They consistently reported an acrocentric chromosome that was thought to be a chromosomal deletion (Druker, 2008). Through this discovery, CML became known as the first human malignant disease to be linked to an acquired genetic abnormality (Kantarjian, Talpaz, Giles, O’Brien, & Cortes, 2006).
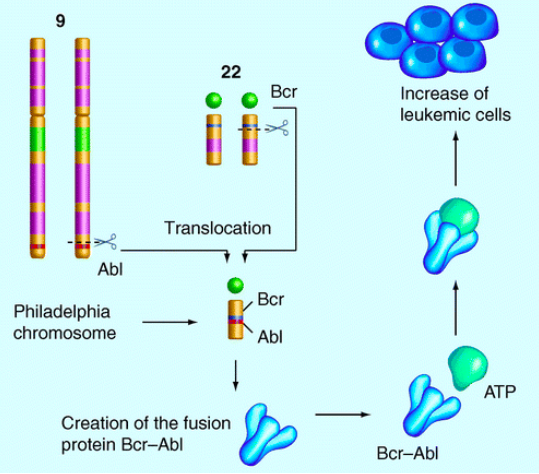
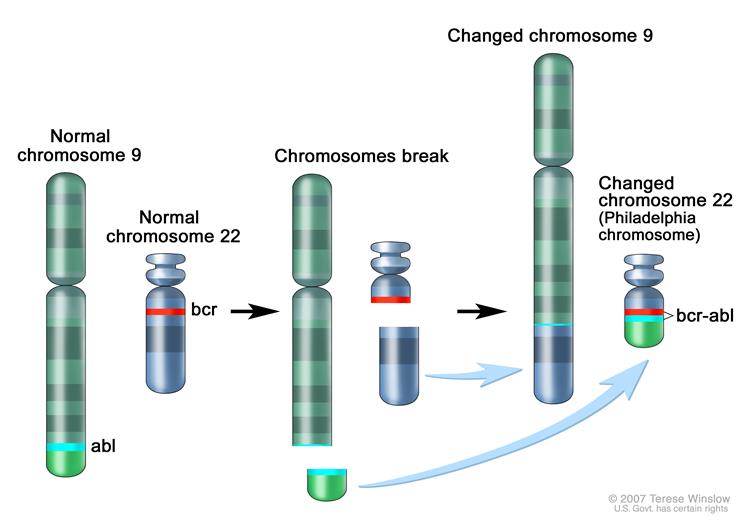
The characteristic genetic abnormality of CML, the Philadelphia chromosome, is present in the bone marrow cells of more than 90% of all patients with CML (Kantarjian, Talpaz, Giles, O’Brien, & Cortes, 2006). The reciprocal chromosomal translocation between the long arms of chromosomes 9 and 22, as illustrated below in figure 7, is the hallmark of CML (Kantarjian, Talpaz, Giles, O’Brien, & Cortes, 2006). This process fuses the Abelson murine Leukemia viral oncogene homolog (ABL) on chromosome 9 with the breakpoint cluster region (BCR) protein on chromosome 22, generating an oncogene that encodes the BCR-ABL protein, a constitutively active cytoplasmic form of the ABL kinase (Kantarjian, Talpaz, Giles, O’Brien, & Cortes, 2006). It is this translocation of the two chromosomes and consequent expression of the BCR-ABL kinase that is considered to be the initiating factor in the pathogenesis of CML (Smith, Burthem, & Whetton, 2003). Furthermore, this reciprocal translocation occurs only in somatic cell lines and ultimately, the BCR-ABL fusion kinase leads to increased and unregulated growth of myeloid cells in the bone marrow.
The "Philadelphia Chromosome"
Normal Physiology of BCR Gene
The normal cellular BCR gene, found on chromosome 22, encodes a 160,000 dalton phosphoprotein associated with serine/threonine kinase activity (Maru & Witte, 1991). This kinase activity enables BCR to phosphorylate proteins containing the amino acids serine and threonine (Maru & Witte, 1991). Furthermore, studies show that the BCR protein activates GTPases such as RAC1, which is involved in cell growth/proliferation, and CDC42, which is involved in cell signaling and cell cycle progression (Genetics Home Reference 2017; GeneCards, 2017).
Normal Physiology of ABL Gene
Non-oncogenic ABL, found on chromosome 9, is a member of the tyrosine kinase family (Warmuth, Danhauser-Riedl, & Hallek, 1999). It is normally regulated by auto-inhibitory mechanisms, involved in cell differentiation and division, and participates in many signaling pathways in the cytoplasm and the nucleus (Hantschel & Superti-Furga, 2004). It has been reported that the SH3 domain negatively regulates the activity of ABL, and the deletion of this domain turns ABL into proto-oncogene (Hantschel & Superti-Furga, 2004).
Figure 8: The reciprocal translocation between chromosome 9 and 22, a hallmark of CML, generating the Philadelphia Chromosome BCR-ABL.
Mechanism of Action of the BCR-ABL Fusion Kinase
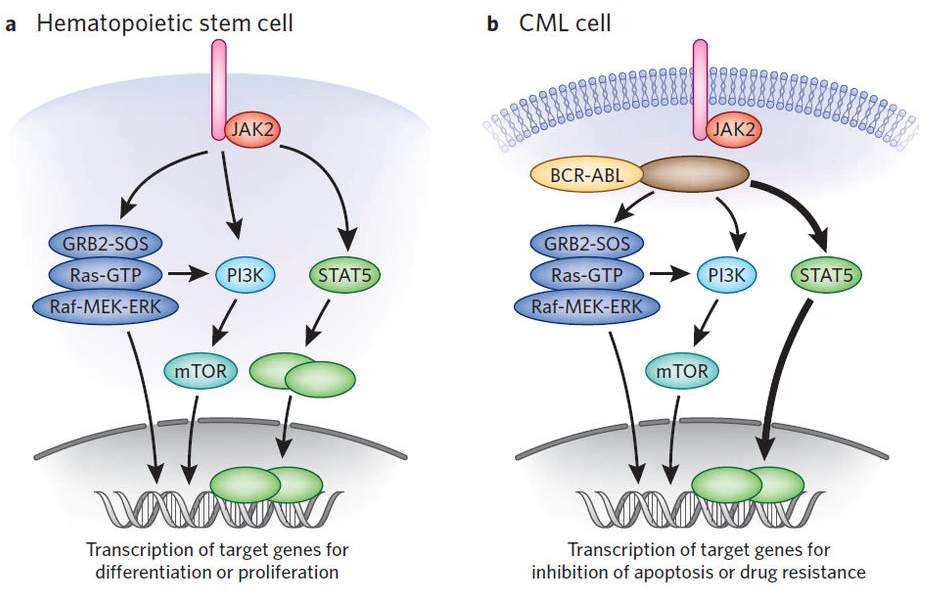
In vitro analysis has shown that BCR-ABL expression transforms lineage committed HSCs to growth factor independence (Smith, Burthem, & Whetton, 2003). Several downstream signaling pathways are influenced by the fusion of BCR-ABL. The kinase activity of the BCR-ABL oncogene appears to be important in the pathogenesis of CML (Sattler & Salgia, 1997). Of the several signaling pathways activated by BCR-ABL, which include the Ras-Raf-ERK, JAK-STAT, PI3K, JNK/SAPK, NF-κB and c-Myc pathways, the JAK-STAT pathway appears to play a critical role in the maintenance of CML (Fabbro, 2012).
Normal physiology of JAK-STAT pathway
In normal hematopoietic stem cells, cytokines (such as Epo or IL-3) bind to target cytokine receptors and lead to the activation the JAK-STAT pathway (Darnell, Kerr, and Stark, 1994; Fabbro, 2012). Cytokines receptors are dimers, which are normally embedded in a cell membrane, with intracellular and extracellular components. Several cytokines will bind to the extracellular components, while JAK2 (Janus Kinase) is embedded in the intracellular components of these receptors (Schindler, Levy, and Decker, 2007). Binding of cytokines to its receptors results in the auto-phosphorylation of two JAK2 kinases being brought in close proximity to each other. As a result, activated JAK2 kinases phosphorylate tyrosine on the cytokine receptors, creating docking sites for STAT5 proteins (Darnell, Kerr, and Stark, 1994). STAT5 (Signal Transducer and Activator of Transcription) proteins are always present in the cytoplasm, waiting to be phosphorylated by JAK2 kinases (Schindler, Levy, & Decker, 2007). Once phosphorylated, the two STAT5 transcription factors dissociate from the receptor and create a STAT5 dimer (Darnell, Kerr, and Stark, 1994). The resulting dimer is an active transcription factor and is able to travel to the nucleus of a cell and activate transcription of target genes (Darnell, Kerr, and Stark, 1994).
JAK-STAT pathway influenced by BCR-ABL
However, in CML patients, the fusion of BCR-ABL results in a constitutively activated intracellular tyrosine kinase which phosphorylates the STAT5 transcription factor independent of JAK kinase activation (Fernandez-Luna, 2000; Steelman et al., 2004; Fabbro, 2012). This oncogenic pathway differs from the normal pathway in several ways. First, the phosphorylation cascade is independent of cytokine binding. Second, BCR-ABL constitutively activates STAT5 transcription factors, independent of JAK kinase activity (Steelman et al., 2004). As a result, STAT5 dimers will continuously activate transcription of genes responsible for the increased proliferation of HSCs and decreased apoptosis of HSCs (Steelman et al., 2004). Therefore, this pathway maintains CML as it gives rise to a high number of immature myelogenous cells that are unable to differentiate properly (Fernandez-Luna, 2000; Steelman et al., 2004; Fabbro, 2012).
Figure 9: Normal JAK-STAT pathway versus JAK-STAT pathway of a CML patient, influenced by BCR-ABL.
Treatment
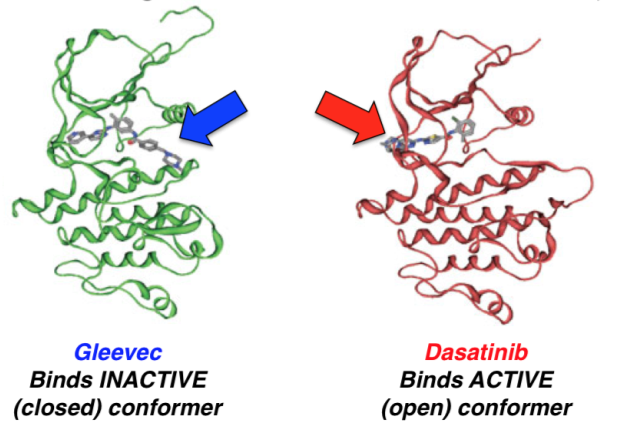
Gleevac (Imatinib)
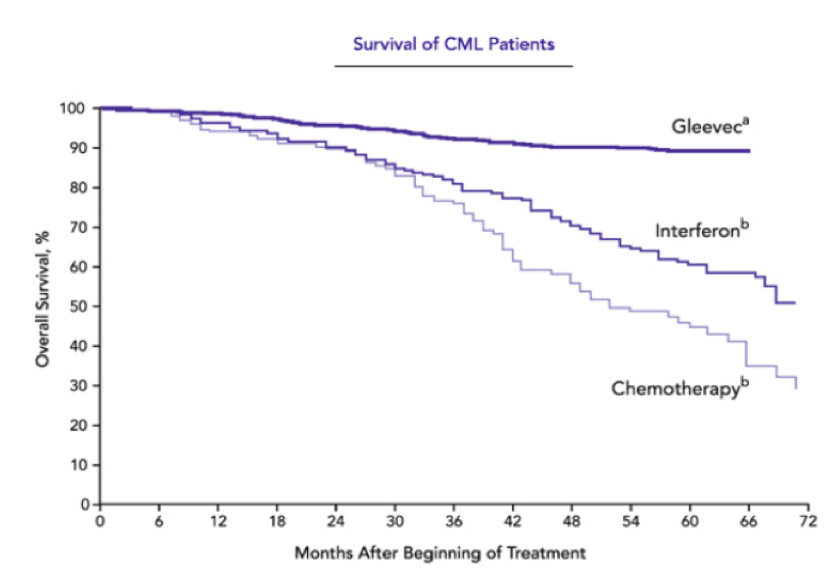
When Gleevec first came out it was called the miracle drug since it seemed as though it was able to cure CML due to its huge success rate. Gleevac works by binding to the closed/inactive conformer of BCR-ABL. This keeps the binding site for ATP closed and thus inhibiting the downstream signaling pathway which contributes to the progression of CML. During one of its first clinical trial conducted by Druker et al (2001), they looked at 54 patients with CML and prescribed them Gleevac of 300mg daily. Out of the 54 patients, 53 had a complete hematologic response within 4 weeks of therapy. A hematologic response is used to measure the recovery process of a CML patients by looking at whether their blood counts have returned back to normal and that there are no more immature cells in the blood. Druker et al (2006) did a 5-year follow-up study and found that after 60 months on Gleevac, 98% of all their patients showed a complete hematologic response. The more outstanding result was that after 60 months the survival rates for patients were 89%. This is a huge improvement since only 30% of patients survived 5 years after being diagnosed with CML. Although within one year of the drug being released, some individuals started showing resistance to the drug due to CML cells having constant exposure to it. However, Gleevac still is the recommended and first line of drug for CML patients.
Dasatinib
After resistance grew on Gleevac, there was a development of another variation of the drug called Dasatinib. Unlike Gleevac, this drug targets the open/active conformer of BCR-ABL by binding to the active site and directly competing with ATP. During Clinical trials it showed it showed promising results. The researchers tested on 670 CML patients and broke them up into 4 groups, which were prescribed varying doses. At the end of their trials, 86-92% of patients saw complete hematologic response (Aguilera & Tsimberidou, 2009). There is research being done on combine drug therapy with Dasatinib and tyrosine kinase inhibitors. They have shown to work synergistically really well in producing growth inhibition and inducing apoptosis in CML cells (Aguilera & Tsimberidou, 2009). Dasatinib is currently used as a form of treatment for CML and is an effective therapy.
Hydrea (Hydroxyurea)
Traditional chemotherapeutics can still be used once TKI’s (Tyrosine Kinase Inhibitors) have stopped working. Hydroxyurea is taken orally in pill form with manageable side effects. The mechanism acts via inhibition of the enzyme ribonucleotide reductase. This enzyme is required to convert ribonucleoside diphosphates into deoxyribonucleoside diphosphates, disrupting the DNA replication process of dividing cancer cells in the body. Thereby preventing cells from leaving the G1/S phase of the cell cycle halting the cell cycle and triggering apoptosis of cancerous cells. (Koç A, Wheeler LJ, Mathews CK, Merrill GF 2004). It can bring the white blood cell count down back to normal levels and can prolong the life of CML patients.
Synribo (Omacetaxine Mepesuccinate)
Synribo is used as a treatment for patients who become resistant to or build up a tolerance to TKIs. It is a protein translation inhibitor, inhibiting protein translation by preventing the initial elongation step of protein synthesis. It specifically interacts with the ribosomal A-site and prevents the correct positioning of AA side chains of incoming aminoacyl-tRNAs. At the molecular level omacetaxine mepesuccinate reduces levels of the Bcr-Abl oncoprotein expression (responsible for the rapid pathogenesis of cancerous lymphocytes) and the Mcl-1 anti-apoptosis oncoprotein (prevents apoptosis). Synribo is effective as it directly interferes with the production of the oncoproteins allowing the abnormal lymphocytes to proliferate uncontrollably (Cortes et al., 2012).
Synribo is administered in liquid form subcutaneously, a standard treatment plan involes an initial dosage followed by maintenance dosing. Initial dosage includes 1.25 mg/m2 administered subcutaneously twice daily for 14 consecutive days every 28 days, over a 28-day cycle. Cycles should be repeated every 28 days until patients achieve a hematologic response (no further increase in WBC). Upon achieving a hematologic response maintenance dosing involves 1.25 mg/m2 administered subcutaneously twice daily for 7 consecutive days every 28 days, over a 28-day cycle. Treatment with synribo should continue as long as patients are hematologically benefitting from therapy.
Future Research
Lonafarnib
A potential emerging chemotherapeutic has demonstrated significant preclinical success. Lonafarnib is a type of farensyl transferase inhibitor (FTI), which target the farensyltransferase (FTase)enzyme. FTase are responsible for the post-translational attachment of prenyl groups to proteins. One of the targets of FTase include the cyseine residue on the Ras protien. The Ras protein is a G protein (dependent on GTP binding for function) that relays a growth signal from a growth factor receptor on the plasma membrane of cells which triggers an internal cascasde leading to protein kinase activation ultimately mobilizing cell proliferation proteins. FTIs such as Lonafarnib are able to block this post-translational modification to the Ras protein and thereby attenuate this cell-proliferation response.
A study published in the American Cancer Society launched a pilot clinical study with lonafarnib in patients in the chronic and accelerated stages of CML who were resistant to imatinib therapy. The small sample size of 13 patients were treated with lonafarnib at a dose of 200mg orally twice daily. Average treatment plan with lonafarnib lasted for about 8 weeks (ranging from 2-41 weeks). (Borthakur et al., 2005)
Out of this small pilot study 2 patients responded. A patient in the accelerated phase returned to the chronic phase (a response which lasted for 3 months) and another patient in the chronic phase, had a lowering of the leukocyte count without the need for hydroxyurea. Overall limited success has been seen with lonafarnib on its own in this small sample size study, currently the possiblity of combination therapy with imatinib is being investigated. Results still remain inconclusive. (Borthakur et al., 2005)
References
Aguilera, Dolly G, and Apostolia M Tsimberidou. “Dasatinib in Chronic Myeloid Leukemia: A Review.” Therapeutics and Clinical Risk Management 5 (2009): 281–89.
BCR Gene - GeneCards | BCR Protein | BCR Antibody (2017) Retrieved November 19, 2017, from http://www.genecards.org/cgi-bin/carddisp.pl?gene=BCR
Bjorkholm, M., Bower, H., Dickman, P. W., Lambert, P. C., Höglund, M., & Andersson, T. M. (2016) Temporal trends in chronic myeloid leukemia outcome using the loss in expectation of life: a Swedish population-based study. Journal of Clinical Oncology, 126(23):2779–2779.
Borthakur, G., Kantarjian, H., Daley, G., Talpaz, M., O'Brien, S., Garcia‐Manero, G., . . . Cortes, J. (2005, December 08). Pilot study of lonafarnib, a farnesyl transferase inhibitor, in patients with chronic myeloid leukemia in the chronic or accelerated phase that is resistant or refractory to imatinib therapy. Retrieved November 21, 2017, from http://onlinelibrary.wiley.com/doi/10.1002/cncr.21590/full
Chang, C. S., Lee, K., Yang, Y. H., Lin, M. T., Hsu, C. N. (2011) Estimation of CML incidence: disagreement between national cancer registry and health claims data system in Taiwan. Leukemia Research, 35(5):e53–e54. doi:10.1016/j.leukres.2010.12.034
Chen, Y., Wang, H., Kantarjian, H., & Cortes, J. (2013) Trends in chronic myeloid leukemia incidence and survival in the United States from 1975 to 2009. Leukemia Lymphoma, 54(7):1411–1417. doi:10.3109/10428194.2012.745525
Corso, A., Lazzarino, M., Morra, E., Merante, S., Astori, C., Bernasconi, P., & Bernasconi, C. (1995). Chronic myelogenous leukemia and exposure to ionizing radiation—a retrospective study of 443 patients. Annals of Hematology, 70(2), 79-82.
Cortes, J., Lipton, J. H., Rea, D., Digumarti, R., Chuah, C., Nanda N, et al. (2012) On behalf of the Omacetaxine 202 Study Group Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood, 120(13), 2573-2580.
Daley, G. Q., Van Etten, R. A., Baltimore, D. (1990). Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science, 247 (1990), 824-830.
Darnell Jr, J. E., Kerr, I. M., & Stark, G. R. (1994). Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science-AAAS-weekly paper edition-including guide to scientific information, 264(5164), 1415-1420.
Druker, B. J. (2008). Translation of the Philadelphia chromosome into therapy for CML. Blood, 112(13), 4808–4817. https://doi.org/10.1182/blood-2008-07-077958
Druker, B. J., François, G., O’Brien, S. G., Gathmann, I., Kantarjian, H., Gattermann, N., et al. (2006). Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. New England Journal of Medicine, 355(23), 2408–17. https://doi.org/10.1056/NEJMoa062867.
Druker, B. J., Lydon, N. B. (2000) Lessons learned from the development of an Abl tyrosine kinase inhibitor for chronic myelogenous leukemia. Journal of Clinical Investigation, 105, 3-7.
Druker, B. J., Talpaz, M., Resta, D. J., Peng, B., Buchdunger, E., Ford, J. M., et al. (2001). Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. The New England Journal of Medicine, 344(14), 1031–37. https://doi.org/10.1056/NEJM200104053441401.
Fabbro, D. (2012). BCR-ABL signaling: A new STATus in CML. Nature Chemical Biology, 8(3), nchembio.900. https://doi.org/10.1038/nchembio.900
Genetics Home Reference (2017) BCR gene. Retrieved November 19, 2017, from https://ghr.nlm.nih.gov/gene/BCR
Genetics Home Reference (2017) CDC42 gene. Retrieved November 19, 2017, from https://ghr.nlm.nih.gov/gene/CDC42
Goldman, J. M. (2010). Chronic myeloid leukemia: a historical perspective. In Seminars in Hematology, 47(4), 302-311.
Goldman, J. M., & Daley, G. Q. (2007). Chronic Myeloid Leukemia—A Brief History. Myeloproliferative Disorders, 1-13.
Hoglund, M., Sandin, F., Hellstrom, K., Bjoreman, M., Bjorkholm, M., Brune, M., et al. (2013) Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood, 122(7):1284–1292. doi:10.1182/blood-2013-04-495598
Hoglund, M., Sandin, F., & Simonsson, B. (2015). Epidemiology of chronic myeloid leukaemia: an update. Ann Hematol, 94(Suppl 2): S241–S247. doi:10.1007/s00277-015-2314-2
Kantarjian, H. M., Talpaz, M., Giles, F., O’Brien, S., & Cortes, J. (2006). New Insights into the Pathophysiology of Chronic Myeloid Leukemia and Imatinib Resistance. Annals of Internal Medicine, 145(12), 913. https://doi.org/10.7326/0003-4819-145-12-200612190-00008
Klein, A., Hagemeijer, A., Bartram, C. R., et al. (1986). Bcr rearrangement and translocation of the c-abl oncogene in Philadelphia positive acute lymphoblastic leukemia. Blood, 68, 1369-1375.
Koç, A., Wheeler, L. J., Mathews, C. K., & Merrill, G. F. (2004). Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. Journal of Biological Chemistry. 279(1).
Maru, Y., & Witte, O. N. (1991). The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell, 67(3), 459–468. https://doi.org/10.1016/0092-8674(91)90521-Y
Nowell, P. C., & Hungerford, O. A. (1960). A minute chromosome in chronic granulocytic leukemia. Science, 132, 1497.
Quintás-Cardama, A., & Cortes, J. E. (2006, July). Chronic myeloid leukemia: diagnosis and treatment. In Mayo Clinic Proceedings (Vol. 81, No. 7, pp. 973-988). Elsevier.
Radivoyevitch, T., Jankovic, G. M., Tiu, R. V., Saunthararajah, Y., Jackson, R. C., Hlatky, L. R., et al. (2014) Sex differences in the incidence of chronic myeloid leukemia. Radiat Environ Biophys 53(1):55–63. doi:10.1007/s00411-013-0507-4
Sattler, M., & Salgia, R. (1997). Activation of hematopoietic growth factor signal transduction pathways by the human oncogene BCR/ABL. Cytokine & Growth Factor Reviews, 8(1), 63–79. https://doi.org/10.1016/S1359-6101(96)00047-0
Sawyers, C. L. (1999). Chronic myeloid leukemia. New England Journal of Medicine, 340(17), 1330-1340.
Schindler, C., Levy, D. E., & Decker, T. (2007). JAK-STAT signaling: from interferons to cytokines. Journal of Biological Chemistry, 282(28), 20059-20063.
Sessions, J. (2007). Chronic myeloid leukemia in 2007. Journal of Managed Care Pharmacy, 13(8 Supp A), 4-7.
Smith, D. L., Burthem, J., & Whetton, A. D. (2003). Molecular pathogenesis of chronic myeloid leukaemia. Expert Reviews in Molecular Medicine, 5(27), 1–27. https://doi.org/10.1017/S1462399403006835
Steelman, L. S., Pohnert, S. C., Shelton, J. G., Franklin, R. A., Bertrand, F. E., & McCubrey, J. A. (2004). JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia, 18(2), 189-218.
Tanaka, K., Takechi, M., Hong, J., Shigeta, C., Oguma, N., Kamada, N., & Kyo, T. (1989). 9; 22 Translocation and BCR rearrangements in chronic myelocytic leukemia patients among atomic bomb survivors. Journal of radiation research, 30(4), 352-358.
Vardiman, J. W., Thiele, J., Arber, D. A., Brunning, R. D., Borowitz, M. J., Porwit, A., et al. (2009). The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 114(5), 937–951. doi:10.1182/blood-2009-03-209262.
Warmuth, M., Danhauser-Riedl, S., & Hallek, M. (1999). Molecular pathogenesis of chronic myeloid leukemia: implications for new therapeutic strategies. Annals of Hematology, 78(2), 49–64. https://doi.org/10.1007/s002770050473
Zech, N. H., Shkumatov, A., & Koestenbauer, S. (2007). The magic behind stem cells. Journal of Assisted Reproduction and Genetics, 24(6), 208-214.