This is an old revision of the document!
Table of Contents
Anorexia
Overview
Introduction
Anorexia is a life threatening mental illness that typically begins around puberty but can occur at any age. It is characterized by persistent behaviours that interfere with maintaining an adequate weight for health, a powerful fear of gaining weight or becoming fat, disturbance in how the person experiences their weight and shape and the individual not fully appreciating the seriousness of their condition. If these characteristics are seen over a period of a least three months an individual can be diagnosed with anorexia (Nedic, 2014).
Persistent behaviours that interfere with maintaining an adequate weight for health includes: restricting food, compensating for food intake through intense exercise, and/or purging through self-induced vomiting or misuse of medications like laxatives, diuretics, enemas, or insulin. In the past anorexia used to be specifically associated with weight loss, making it difficult to recognize in children and adolescents. Weight gain is needed in children and adolescents in order to support healthy growth and development. Therefore, failing to gain weight or grow is just as concerning as weight loss (Nedic, 2014).
The powerful fear of gaining weight or becoming fat is common in individuals with anorexia. Individuals may feel this fear ever if they are maintaining a weight that is too low for their health. Often this fear will result in individuals using a variety of techniques to evaluate their body size or weight - behaviour known as body checking. Frequent weighing, obsessive measuring of body parts and the persistent use of mirrors to check for “fat” are common techniques used to body check (Nedic, 2014).
There is a disturbance in how the person experiences their weight and shape meaning that the person overestimates their body size, they usually evaluate it negatively. Individuals feel that their weight and shape matter more than anything else about them (Nedic, 2014).
Typically, an individual with anorexia does not fully appreciate the seriousness of their condition as it has been linked to cardiac arrest, suicidality, and other causes of death. Anorexia was previously associated with the loss of menstrual periods which made it difficult or impossible to identify in males or in pre-pubescent children or teens, however this is no longer necessary for diagnosis (Nedic, 2014).
History
Progeria is an extremely rare genetic disease of childhood characterized by dramatic, premature aging with death occurring on average at the age of 13, usually from heart attack or stroke (Kashyap et al., 2014). Hutchinson-Gilford progeria syndrome (HGPS) is the most severe form of the disease and the classic type. The disease was named after the doctors who first described it in England; in 1886 by Dr. Jonathan Hutchinson and in 1897 by Dr. Hastings Gilford. The term progeria is derived from the Greek work geras, meaning old age (DeBusk, 1972).
In 1886, the syndrome was first reported by Hutchinson of a 6-year-old boy whose overall appearance was that of an old man. Hutchinson described the case as “congenital absence of hair and its appendages” (DeBusk, 1972). It was a year later that Gilford described a second patient with similar clinical findings. To date, there are only 100 patients with HGPS that have been described in literature (Kashyap et al., 2014). These two boys were further described in 1897 and 1904 by Gildford, who was the one to proposal the term “progeria” and described the post-mortem characteristics (DeBusk, 1971). Little research was done on the disease until the 1990’s due to the rarity of the disease, causing it to be frequently diagnosed erroneously in patients with some of the features such as alopecia and skin of aged appearance (DeBusk, 1972). However, there are three features present in early life; mid-facial cyanosis, skin resembling scleroderma, and glyphic nasal tip, which all facilitate an early diagnosis of HGPS (DeBusk, 1972).
Epidemiology
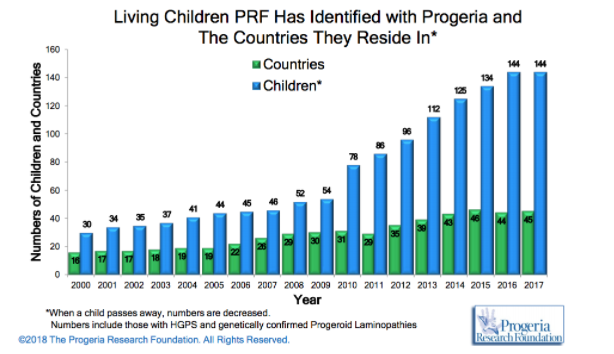
HPGS is an extremely rare genetic disorder affecting about 1 in 4 million live births, if unreported or misdiagnosed cases are taken into account. The reported prevalence rate of the disease is 1 in 8 million births, based on the number of cases (Coppedè, 2013). According to the Progeria Research Foundation database, there are an estimated 350-400 children living with progeria worldwide at any one time. As of January 2018, there are a recorded 114 children living with progeria worldwide with numbers steadily increasing as the years go by (http://www.progeriaresearch.org/prf-by-the-numbersprf.html).
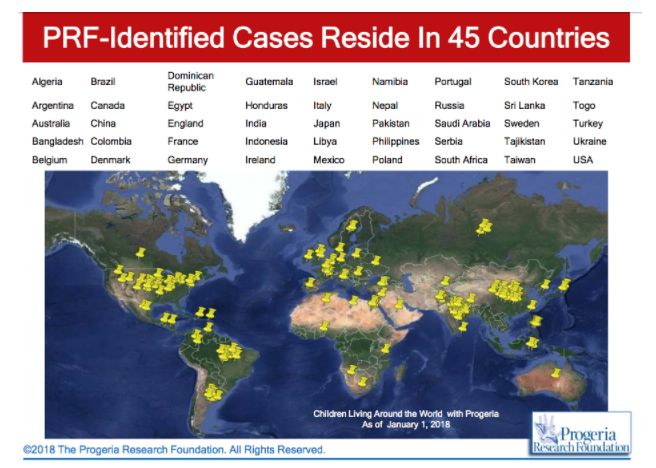
HPGS affects all races; cases of progeria have been discovered in 45 different countries. However, 97% of affected patients are white. Males are affected 1 ½ times more often than females. The disease was thought to be autosomal recessive in the past, however observations made an autosomal recessive inheritance very unlikely and favour a sporadic, dominant mutation. The mutation results in life spans for progeria syndrome to be in the second/third decades of life, with the majority of patients dying of cardiovascular or cerebrovascular disease between 7 and 27 years of age (Sarkar and Shinton, 2001).
Risk Factors
It has been found that there are several risk factors for developing anorexia. These include: perfectionism, negative self-evaluation, obesity, family history of anorexia, affective disorder, substance abuse, obsessive-compulsive disorder and a stress/trigger. It was also found that a lot of these risk factors were correlated to being risk factors of other psychological conditions and that they could have been acquired genetically (Fairburn, Cooper, Doll & Welch, 1999). It should also be mentioned that all of the aforementioned risk factors are not known in detail - their significance is unknown at this point. Social determinants, culture and family also play a role, and vary significantly (“Anorexia Nervosa-What Increases Your Risk”, 2018).
Diagnosis / Symptoms
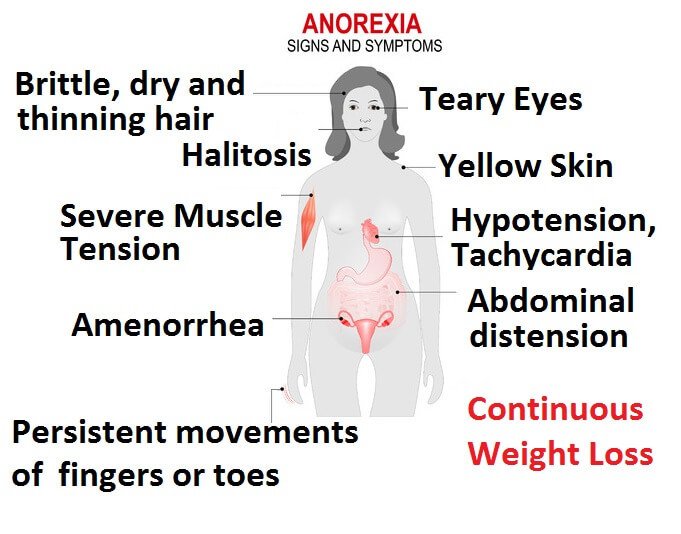
Anorexia is not a straightforward disease to diagnose. It can all be agreed however that a low body weight is the main component for diagnosis. Following such, an individual may also possess fear for gaining weight, as well as have an altered interpretation of healthy weight (Rothenberg, 1988). The first step for diagnosis by the physician is to confirm that the weight loss is not attributed by any other factor. He or she may run several tests to confirm this: a physical exam to determine if the patient’s BMI is under 17.5, and blood tests to measure electrolytes, protein, and thyroid, liver and kidney function. If the tests reveal no abnormalities and the physician believes it is mental, a referral to a mental health professional is commonly seen (“Diagnosis of anorexia nervosa”, 2018). The second step for diagnosis is conducted by a mental health professional, who utilizes the Diagnostic & Statistics of Mental Disorders Manual 5 (DSM-5) as an aid. The professional determines through questionnaires, and evaluations if the patient has fears of gaining weight or has restrictive eating patterns (“Diagnosis of anorexia nervosa”, 2018). Some common symptoms for patients with anorexia are: extreme weight loss, fatigue, insomnia, dizziness or insomnia, constipation and abdominal pain, low blood pressure, and dehydration. Lastly, some patients present with bulimic symptoms - binging and purging (“Anorexia nervosa - Symptoms and causes”, 2018).
Pathophysiology
Background
The nuclear lamina is a filamentous protein layer that provides mechanical support to the inner nuclear membrane (Gonzalez, 2011). The functions of the nuclear lamina include nuclear positioning, chromatin organization, nuclear pore complex organization, nuclear envelope breakdown and reassembly during mitosis, DNA replication, DNA damage response and cell cycle progression, transcriptional control and apoptosis (Gonzalez, 2011). The main components of the nuclear lamina are type V intermediate filaments known as lamins that contain a central α- helical rod surrounded by globular N and C terminal domains; the C terminal region contains the nuclear localization sequences (Gonzalez, 2011). As proteins they form coiled- coil dimers that can associate head to tail. These protofilaments then create the final lamin filaments. Lamins can be classified into two types: A- type and B- type. A -type lamins are basic and type B is acidic. Type A are encoded by the LMNA gene with its two isoforms being Lamin A and C. B type lamins are therefore encoded by the LMNB1 and LMNB2 genes (Gonzalez, 2011). Lamin A is affected in Progeria so an understanding of the normal transcriptional and translational mechanisms of this protein is essential (Gonzalez, 2011). In cells containing the normal LMNA gene, prelamin A undergoes post- translational modifications before it is found in its mature Lamin A form. Firstly, the cysteine in the C – terminal CaaX motif is farnesylated by farnesyltransferase. Rce1, an endoprotease then cleaves the three terminal amino acids. Then the newly- available cysteine is then methylated by carboxyl methyltransferase, ICMT. Lastly to create mature Lamin A, 15 C- terminal residues that include the farnesylated and carbosymethylated C- terminal cysteine are cleaved by another endoprotease, Zmpste24/ FACE-1 (Gonzalez, 2011).
Genetics
Hutchinson- Gilford Progeria syndrome is commonly caused by a single de novo silent mutation in codon 608 of the LMNA gene (Gonzalez, 2011). The mutation in HGPS is a nucleotide substitution from cytosine to thymine at position 1824 (Goldman et al., 2004). This substitution causes for the partial activation of a cryptic splice site (Goldman et al., 2004). It causes deletion of 150 nucleotides in exon 11 (Goldman et al., 2004). This causes for a 50 amino acid deletion near the C- terminus of the protein, which includes the Zmpste24 cleavage site (Pollex & Hegele 2004). This causes for a mutant prelamin A to remain farnesylated throughout the lifespan of the protein (Pollex & Hegele, 2004). The mutant protein that results from this is known as progerin (Pollex & Hegele, 2004). Around 80% of HGPS cases contain this mutation that is known as the LAΔ50 mutation. Evidence has suggested that transfection of progerin or a non-cleavable form of prelamin A causes for nuclear abnormalities (Pollex & Hegele, 2004). During mitosis, the abnormal association of progerin causes a delay in the onset and progression of cytokinesis and impairs the targeting of lamina components to the nucleus of daughter cells (Gonzalez, 2011). In addition, it can change the entry of the cell into S- phase mediated by hyperphosphorylation of the retinoblastoma gene product (pRB) by cyclin D1/cdk4 (Gonzalez, 2011). Progerin accumulation also causes abnormal chromosome segregation and binucleation (Gonzalez, 2011). It also promotes DNA- damage, changes in DNA repair, causes for genomic instability as well as interfering with nuclear architecture. This leads to premature cell death (Gonzalez, 2011).
Additional heterozygous mutations for atypical Progeria patients have also been revealed. R644C affects the C- terminus, E578V also in the C- terminus and T10I within the N- terminal globular domain seen in Seip syndrome (Pollex & Hegele, 2004). The most common hypothesis for the inheritance of this disorder is sporadic autosomal dominant (Pollex & Hegele, 2004).
Genomic Instability
Hutchinson- Gilford Progeria syndrome is commonly caused by a single de novo silent mutation in codon 608 of the LMNA gene (Gonzalez, 2011). The mutation in HGPS is a nucleotide substitution from cytosine to thymine at position 1824 (Goldman et al., 2004). This substitution causes for the partial activation of a cryptic splice site (Goldman et al., 2004). It causes deletion of 150 nucleotides in exon 11 (Goldman et al., 2004). This causes for a 50 amino acid deletion near the C- terminus of the protein, which includes the Zmpste24 cleavage site (Pollex & Hegele 2004). This causes for a mutant prelamin A to remain farnesylated throughout the lifespan of the protein (Pollex & Hegele, 2004). The mutant protein that results from this is known as progerin (Pollex & Hegele, 2004). Around 80% of HGPS cases contain this mutation that is known as the LAΔ50 mutation. Evidence has suggested that transfection of progerin or a non-cleavable form of prelamin A causes for nuclear abnormalities (Pollex & Hegele, 2004). During mitosis, the abnormal association of progerin causes a delay in the onset and progression of cytokinesis and impairs the targeting of lamina components to the nucleus of daughter cells (Gonzalez, 2011). In addition, it can change the entry of the cell into S- phase mediated by hyperphosphorylation of the retinoblastoma gene product (pRB) by cyclin D1/cdk4 (Gonzalez, 2011). Progerin accumulation also causes abnormal chromosome segregation and binucleation (Gonzalez, 2011). It also promotes DNA- damage, changes in DNA repair, causes for genomic instability as well as interfering with nuclear architecture. This leads to premature cell death (Gonzalez, 2011).
Additional heterozygous mutations for atypical Progeria patients have also been revealed. R644C affects the C- terminus, E578V also in the C- terminus and T10I within the N- terminal globular domain seen in Seip syndrome (Pollex & Hegele, 2004). The most common hypothesis for the inheritance of this disorder is sporadic autosomal dominant (Pollex & Hegele, 2004).
Current Treatments
Unfortunately today there are no treatments for Progeria. Physicians and healthcare providers’ main goals right now are to delay or reduce symptoms. This can be achieved by: small doses of aspirin, preventative medications, physical and occupational therapy, nutrition and dental care. Common with Progeria, small doses of Aspirin attempt to reduce the risk of heart attacks and strokes. Preventative medications that can help lower cholesterol, blood pressure, and reduce the chance of getting a blood clot can all be prescribed if the individual seems to be at risk. Physical and occupational therapy can help an individual maintain healthy movement capability and nutrition and dental care for being overall healthy (Mayo Clinic, 2018). Progeria is commonly screened phenotypically or through medical history at the physician’s office. A genetic test for the LMNA mutations can be ordered if the physician deems this appropriate (Sinha, Raghunath & Ghosh, 2018).
For potential future treatments, there are many different angles of approach. Genetics currently is a big area of research for this disease. Anything from early detection capability, to actual cures, genetics is a significant field to target. Additionally, there is also a lot of interest in reducing the severity of symptoms, common with individuals with Progeria. First, heart and blood vessel disease is a target of interest. Farnesyltransferase inhibitors (FTIs), which are drugs for treating cancer, are being investigated to whether they can help vasodilate blood vessels and reduce weight gain (Mayo Clinic, 2018). In 2012, 25 children with Progeria underwent a clinical trial that showed these results (Gordon et al., 2012). FTIs also have shown in mouse models to improve nuclear shape and reduce the negative effects of built up prelamin A. Lonafarnib, an FTI, certainly gives confidence for developing a potential cure to Progeria (Sinha, Raghunath & Ghosh, 2018). (a is Progerin cell, d is a healthy cell - treated with FTI - Capell reference)
Conclusion
The causes of anorexia nervosa are not completely understood by medical and psychological professionals, it is acknowledged that an array of biological, social, genetic, and psychological factors play a role in increasing the risk of its onset. The societies we live in have strong beliefs and attitudes toward just about everything. Different groups within a given society adapt these beliefs, attitudes and behaviours altering our beliefs and attitudes toward ourselves and our bodies. Our feelings and behaviour also affect our physical make-up and health. The first step in the recovery process is realizing that our food- and weight-related behaviours are hurting us, rather than helping us. Once an individual is able to realize this, help can be found or offered in a variety of days. Family members and friends may also benefit from information and help. It is possible to prevent the development of food and weight fixation and eating disorders. It is also possible to prevent existing eating disorders from getting worse. It begins with learning and improving our own self-esteem and body image, to working with others and making positive societal changes.
References
Anorexia nervosa - Symptoms and causes. (2018). Mayo Clinic. Retrieved 26 March 2018, from https://www.mayoclinic.org/diseases-conditions/anorexia/symptoms-causes/syc-20353591
Diagnosis of anorexia nervosa. (2018). Healthdirect.gov.au. Retrieved 26 March 2018, from https://www.healthdirect.gov.au/diagnosis-of-anorexia-nervosa
Rothenberg, A. (1988). Differential diagnosis of anorexia nervosa and depressive illness: A review of 11 studies. Comprehensive Psychiatry, 29(4), 427-432. http://dx.doi.org/10.1016/0010-440x(88)90024-7
Anorexia Nervosa-What Increases Your Risk. (2018). WebMD. Retrieved 26 March 2018, from https://www.webmd.com/mental-health/eating-disorders/anorexia-nervosa/anorexia-nervosa-what-increases-your-risk
Fairburn, C., Cooper, Z., Doll, H., & Welch, S. (1999). Risk Factors for Anorexia Nervosa. Archives Of General Psychiatry, 56(5), 468. http://dx.doi.org/10.1001/archpsyc.56.5.468
Micali, N., Hagberg, K. W., Petersen, I., & Treasure, J. L. (2013). The incidence of eating disorders in the UK in 2000–2009: findings from the General Practice Research Database. BMJ Open, 3(5).
Dell’Osso, L., Abelli, M., Carpita, B., Pini, S., Castellini, G., Carmassi, C., & Ricca, V. (2016). Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive–compulsive spectrum. Neuropsychiatric disease and treatment,12, 1651.
Keski-Rahkonen, A., Hoek, H. W., Susser, E. S., Linna, M. S., Sihvola, E., Raevuori, A., … & Rissanen, A. (2007). Epidemiology and course of anorexia nervosa in the community. American Journal of Psychiatry, 164(8), 1259-1265.
Statistics | National Eating Disorder Information Centre (NEDIC). (2018). Nedic.ca. Retrieved 24 March 2018, from http://nedic.ca/node/24