This is an old revision of the document!
Table of Contents
Anterior Cruciate Ligament Injury
<style justify> Multiple Sclerosis (MS) is an autoimmune disease affecting the central nervous system. The disease specifically affects the myelin and as a result inflammation occurs and the myelin is damaged. Myelin performs the crucial role of nerve impulse through nerve fibers. If myelin is damaged then there will be a decrease in the transmission of nerve impulses. When the damage is significant then scar tissue will replace the myelin. The symptoms of MS vary for each individual, some include fatigue, pain, bladder problems, walking problems, cognitive difficulties and optic neuritis. Canada currently has the highest rate of MS with approximately 100,000 Canadians currently diagnosed (Multiple Sclerosis Society of Canada, 2016). </style>
<br> <br> <br>
Causes
There currently is no known cause for the affliction of MS; however the general consensus is that which follows both environmental as well as genetic factors contributing to the emergence of the disease (Dyment et al. 2004). There is a variance in the amount of environmental factors thought to influence the presence of MS. Many theories attempt to analyze different combinations of risk factors with hopes of coming to a conclusion which explains the emergence of MS; however none have been successful (Dyment et al. 2004).
Some of the most prominent environmental risk factors include an array of microbes (Ascherio & Munger, 2007). Microbes vary depending on geographic region and it is thought that a change in exposure to a set of microbes native to a specific location is what increases the risk of developing MS (Ascherio & Munger, 2007). A theory which further investigates this ideology is that of the hygiene hypothesis. It states that in early life contact with infections are beneficial to the individual, with later exposure to high risk microbes resulting in the development of MS further along in life (Compston & Coles, 2008). Therefore an individual who has recently moved to an area with high risk microbes is more likely to acquire MS due to its novelty to their immune system. In addition to microbes there is evidence in support of a virus as a candidate for causing MS (Ascherio & Munger, 2007). An example of this would be the human herpes virus (hhv), more specifically hhv-4, which is one of the 8 different known strains of hhv (Gildan, 2005). The majority of the human population has been exposed to at least one strain of the hhv, with one of the most common being the Varicella zoster virus which causes chickenpox or shingles (Gildan, 2005). Studies show that individuals who have not previously been infected with hhv-4, otherwise known as the Epstein-Barr virus are at less of a risk of acquiring MS compared to those who have previously contracted it (Gildan, 2005). Furthermore according to the hygiene hypothesis individuals exposed to the Epstein-Barr virus as youths have a reduced risk of being affected by MS as opposed to the higher risk experienced by adults since it is their first interaction with the causative virus in a relatively later stage in life which acts as a trigger (Gildan, 2005).
While believed by many, MS is not categorized as a hereditary disease (Dyment et al. 2004). Instead its connection with genetics lies within the chance of genetic variation leading to increase risk in developing the disease (Ascherio & Munger, 2007). Due to the similarity of genetic expression found among related individuals, the probability of developing MS is increased when comparing with an afflicted member of the family versus the general public (Ascherio & Munger, 2007). In addition, MS is more prevalent in certain ethnic groups than others due to the genetic expression variance (Ascherio & Munger, 2007). More specifically there are a few genes which when mutated are more likely to be linked with MS, they are found in the human leukocyte antigen (HLA) system (Ascherio & Munger, 2007). Mutations in this system have been shown to be susceptible to the progression of various autoimmune diseases including type-1 diabetes (Ascherio & Munger, 2007). In general research show that changes in the HLA system are responsible for between 20 to 60 percent of the genetic predisposition to MS (Ascherio & Munger, 2007).
<br>
Symptoms
Symptoms of a severe and acute ACL injury include:
• Feeling or hearing a “pop” in the knee at the time of injury.
• Sudden instability in the knee. This may happen after a jump or change in direction or after a direct blow to the side of the knee.
• Pain on the outside and back of the knee.
• Knee swelling within the first few hours of the injury. This may be a sign of bleeding inside the joint. Swelling that occurs suddenly is usually a sign of a serious knee injury.
• Limited knee movement because of swelling and/or pain.
Following an acute injury, the patient will almost always have to stop whatever activity they are doing, however, they may still be able to walk.
If the ACL injury is chronic (long-lasting and recurrent), the knee buckles or gives out, sometimes with pain and swelling. This occurs more frequently over time. It is important to note that not everyone with an ACL injury develops a chronic ACL deficiency.
<br>
Diagnosis
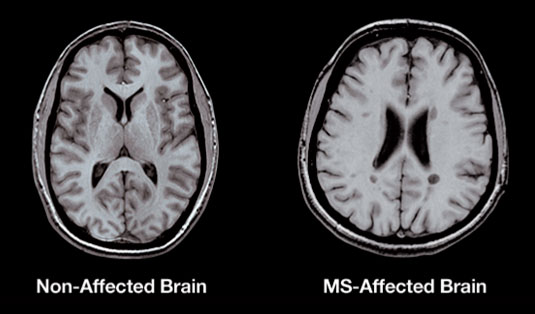
Figure 1: A T1-weighted MRI demonstrating permanent lesions in a MS patient. The dark spots
in the scan are the lesions. (Source: Spinms, 2016)
</style>
<style justify> The diagnosis of Multiple Sclerosis (MS) can be detected through MRI, lumbar puncture and electroencephalography. MS is difficult to diagnose due to the multiple symptoms associated with the disease, which can vary from person to person. Magnetic resonance imaging (MRI) can be used to reveal areas of lesions. However, MRI has disadvantages as it lacks specificity and many conditions have similar conditions of MS and as a result many false positives occur. It has been determined that 90% of MS patients will display an abnormal MRI scan and thus MRI should be the first diagnostic tool to be used. However, 5% of individuals show no sign of lesions in the brain while using MRI (Rolak, 2003).
When there are abnormalities in the cerebrospinal fluid, it can be used to diagnose MS. In the Cerebral spinal fluid (CSF), the white blood cell and spinal fluid proteins are slightly elevated. An elevated Immunoglobin G level in CSF is the most significant in detecting MS. The Immunoglobin G reflects an autoimmune activation and appears as oligoclonal bands on the electrophoresis performed on the CSF. Oligoclonal bands indicate the presence of immunoglobins, which indicate inflammation in the central nervous system. The oligoclonal bands vary from MS patient, but 90% of MS patients present these bands. A limitation to this method is other diseases produce these bands as well and can lead to misdiagnosis. To obtain the CSF it requires undergoing lumbar puncture, which many patients are unsure of doing (Rolak, 2003).
Another method that could be used is evoked potential, which is used to measure conduction rates in the CNS pathway though the recording of electroencephalographic response to sensory stimulation. Slow conduction rates indicate inflammation and demyelination, presenting an MS lesion (Rolak, 2003).
Prognosis and progression
There are four different patterns of MS,
- Clinically Isolated Syndrome (CIS)
- Relapsing and remitting (RRMS)
- Secondary progressive (SPMS)
- Primary progressive (PPMS)
Clinically Isolated Syndrome refers to a first episode where there is inflammatory demyelination in the CNS. It is not yet considered MS, but could become MS if further activity occurs. In the relapse and remitting stage this indicates good health followed by an immediate change in symptoms. Secondary progressive MS occurs after the relapse and remitting stage. At this point there are more symptoms that are progressively getting worse. Primary progressive MS is the steady development of symptoms that will eventually become worse as the diseases progresses. RRMS, SPMS, and PPMS can be active or not active depending on if there is evidence of relapse or disease activity present (Hedley, 2012). </style>
<br>
Pathophysiology
<style justify> Three characteristics that may be present in Multiple Sclerosis are lesion/plaque formation, inflammation and destruction of myelin sheaths. The exact mechanism of T cells becoming autoreactive is widely unknown, however they have been able to propose molecular mimicry as a possible reason for the autoimmune process involved in MS (Wu and Alvarez, 2011). Molecular mimicry concept proposes that the Myelin Basic Protein (MBP) sequence from 85-99 resembles some pathogens or viruses antigens and could be involved in initiating autoimmunity. Some of these viruses can include the herpes virus family (EBV, herpes simplex, and cytomegalovirus), influenza viruses, and papillomaviruses (Wucherpfennig and Strominger, 1995). After the penetration of the Blood-brain barrier, the inflammatory process causes the demyelination of neurons and therefore leading to the formation of lesions. The disease process also consists of loss of myelin and disappearance of oligodendrocytes, which is known for the synthesis of myelin protein (Wu and Alvarez, 2011). </style>
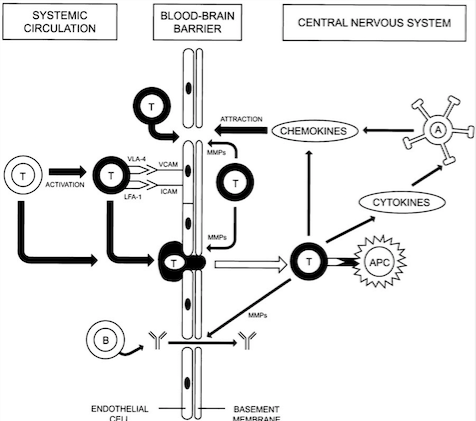
<style justify> Blood-brain barrier Penetration: Initially, for the T cells to cause lesion formation, they need to penetrate the Blood-brain barrier. The Blood-brain barrier consists of endothelial cells, tight junctions and basement membrane (Löscher and Potschka, 2005). The immune system cells travels through blood vessels which also contain endothelial cells. The Blood-brain barrier has selective permeability and prevents the immune system cells from entering the brain (Wingerchuk et al., 2001). However, when triggered by a virus such as Epstein Barr Virus (EBV), the Blood-brain barrier can be penetrated by T cells that can be autoreactive or be programmed to attack myelin protein present on the axons of the neurons (Wu and Alvarez, 2011).
Inflammation in the area due to a virus or foreign substances causes more T cells and macrophages to be recruited into the Blood-brain barrier due to the help of increased expression of adhesion molecules (Wingerchuk et al., 2001). Then, activated and non-activated T cells can secrete Matrix metalloproteinases (MMPs) which cause the degradation of the extracellular matrix, therefore weakening the Blood-brain barrier and allowing for other leukocytes and macrophages to enter, as highlighted in Figure 2 (Wingerchuk et al., 2001). Inside the Central Nervous System, T cells can be activated by Antigen Presenting Cells and antibodies, therefore releasing an increased amount of chemokines and cytokines, which can recruit a much larger inflammatory response and lead to initiation of lesion formation (Wingerchuk et al., 2001). </style>
Figure 2: Illustrates the mechanism used by T cells to penetrate the Blood-brain.
Barrier (Source: Wingerchuk et al., 2001).
</style>
<style justify> Lesions: In Multiple Sclerosis, lesions will be formed in the white matter of the brain due to demyelination of neurons in the Central Nervous System. Demyelination or lesion formation can occur in any area, but most often occurs in the periventricular regions, optic nerves, brainstem, cerebellum and spinal cord (Wingerchuk et al., 2001). Demyelination usually occurs due to an autoimmune response by T cells towards myelin protein and oligodendrocytes, which are responsible for synthesizing myelin protein (Wu and Alvarez, 2011).
Different type of lesions: (Wu and Alvarez, 2011) There are four different types of patterns of lesions and each one of them varies in composition. (Most common forms are III and IV).
- Pattern I: Contains T cells and macrophages
- Pattern II: Contains T cell and macrophages, as well as immunoglobulin and myelin degradation products
- Pattern III: Loss of Oligodendrocytes and myelin glycoprotein
- Pattern IV: Oligodendrocytes dystrophy and absence of remyelination
</style>
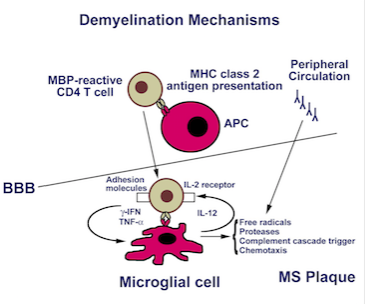
Figure 3: Illustrates the inflammatory response towards myelin
protein, thus causing formation of lesions (Source: Luzzio, (n.d.)). </style>
<style justify> Lesion Formation Mechanism: (Brosnan) One of the proposed mechanisms of lesion formation was further examined by Brosnan and Raine (1996) and is illustrated below in Figure 3. They proposed that CD4 T cells were activated outside the Blood-brain barrier by Antigen presenting cells to either myelin protein or oligodendrocytes. The CD4 T cells would enter the Blood-brain barrier and the activated CD4 T cells would recognize antigens presented by microglial cells, which are the macrophages in the Blood-brain barrier or B cells antibodies (Brosnan and Raine, 1996). Once the MHC class 2 receptor recognition and costimulation occur, the CD4 T cells can now release cytokines such as TNF-alpha, gamma-IFN and IL-17. Then the activated microglial cell releases free radicals, nitric oxide, proteases which can cause destruction of tissue in Central Nervous System and recruit more of an inflammation response. This can ultimately cause irreversible tissue and axonal damage and therefore leading to the formation of lesions (Brosnan and Raine, 1996). </style>
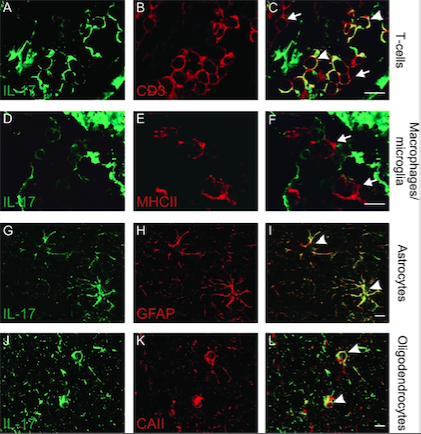
Figure 4: Demonstrates the location and as well as the immune cells involved
in the production of IL-17 (Source: Tzartos et al., 2008). </style>
<style justify> Inflammation: The attacks by the T-cells on myelin cause inflammatory processes, triggering other immune cells, cytokines and antibodies. The Blood-brain barrier starts to swell as well as macrophages are activated along with cytokines and other destructive proteins (Wu and Alvarez, 2011).
</style>
Effect of Demyelination: After demyelination, the nerve tissue is damaged and the axons are not able to conduct action potentials effectively due to loss of myelin, which ultimately acts as an insulator. This prevents the body to communicate with the Central Nervous System effectively, therefore causing numbness in certain areas of the body (Smith and McDonald, 1999).
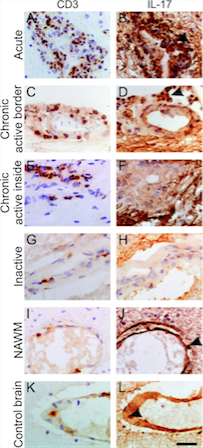
Figure 5: Illustrates the expression
of CD3 T cells and as well as the
activity of the CD3 T cells based on
IL-17 production in patients with
different stages of MS.
(Source: Tzartos et al., 2008) </style>
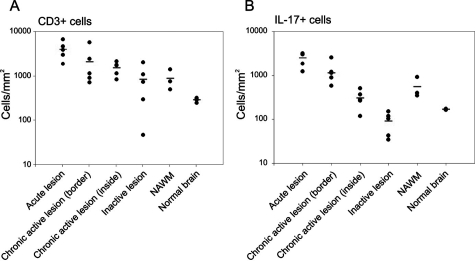
<style justify> Evidence Supporting Immune-mediated Pathophysiology: In an experiment conducted by Tzartos et al. (2008), they wanted to explore specifically which immune system factors and cells were involved in demyelination as well as the role of Interleukin-17 in demyelination (Tzartos et al., 2008). Initially, they wanted to determine whether T cells, astrocytes, microglia/macrophages, oligodendrocytes were able to produce IL-17, since there were earlier reports that IL-17 is present in higher concentrations in MS tissues. They used in situ hybridization and immunofluorescent-labelled IL-17 mRNA (Tzartos et al., 2008). In addition, they also labelled the interested cells and used an overlap imaging technique to show where IL-17 was present and therefore indicating its production . Astrocytes, T cells, oligodendrocytes all overlapped with IL-17 expression while microglia/macrophages did not, as shown in Figure 4 (Tzartos et al., 2008). In another experiment, they then wanted to see whether there was an increase of activity of CD3 T cell involved ( Th17 cells) in lesions and compare this to normal nerve tissue. They also looked at IL-17 to assess the activity of the activated Th17 cells (Tzartos et al., 2008). Using tissue samples from the perivascular areas of acute lesions, active borders of chronic active lesions, inactive lesions, inactive areas of chronic active lesions and as well as NAWM in MS patients(normal appearing white matter), they observed that there was increased amount of IL-17 cytokine and CD3 T cells in MS patient tissues samples compared to the non MS brain (control variable), highlighted in Figure 5 below. This indicated that IL-17 is largely involved in the inflammatory response in MS (Tzartos et al., 2008). In addition, they looked at alternative method to quantify the presence of IL-17 in MS patient brain sample. They compared the densities from Figure 6 and were able to conclude the similar results as previously reported. Therefore, demonstrating that IL-17 and CD3 T cells were involved in carrying out an inflammatory response leading to demyelination of neurons (Tzartos et al., 2008). </style>
Figure 6: Illustrates the observed expression as a density for both CD3 T cells and
IL-17 based on the different stages of MS (Source: Tzartos et al., 2008). </style>
<style justify> Non-immunological Roles of Demyelination: The immune-mediated destruction of myelin protein on neurons is not the only to cause demyelination. Other known non-immunological causes of demyelination can be soluble substances such as perforin, calpain (Calcium activated proteases involved in myelin protein degradation) and also reactive oxygen and nitrogen species such as nitric oxide (Brosnan and Raine, 1996).</style>
<br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br> <br>
Treatment
Nonsurgical Treatment: Torn ACL are not able to healed without surgery, however surgery may not always be a viable option for elderly patients or patients with low activity levels. In this situation, non-surgical treatment methods would be more beneficial. These methods are only recommended if the overall stability of the knee is intact. Some of these non-surgical treatment methods can include (UCSF, 2016):
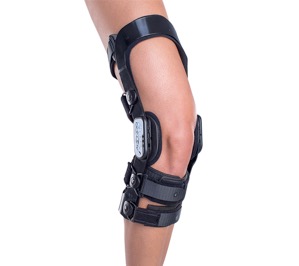
Use of Knee Braces: This can be recommended by your doctor. Knee braces help support and protect the knee and the ACL from further damage. Furthermore, crutches may be implemented to transfer your weight away from your injured knee. This further protects and prevents more damage.
Figure X: Illustrates what a typical knee brace looks like and how it is applied to the knee. (Source: Better Braces, 2016 ).
</style>
Physical Therapy: This is usually recommended by doctors when swelling and inflammation of the knee go down. This program involves a series of exercises that will help improve function of the knee joint and strength surrounding muscles. Some of these exercises focus on training the Gluteus Maximus and Gluteus Medius in non-weight scenarios, followed by weight bearing scenarios. This helps to improve the control of the hip movements. In addition, exercises that focus on strengthening the Quadriceps muscles are also implemented. This is because this helps with improved bending of the knee. These exercises are also being implemented in ACL prevention programmes (Physioroom, 2016).
ACL Reconstructive Surgery: ACL reconstruction is recommended for those:
- Who are young and active
- Who suffer from persistent knee pain
- Who suffer from pain during routine activities (ex- Walking)
- Who are aspiring athletes
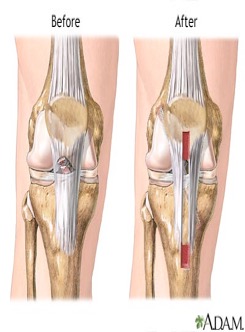
Surgical Treatment: The most common method to repair a torn ACL is through ACL reconstructive surgery. This surgery is required to replace the torn anterior cruciate ligament with either another ligament from your body or from a tissue sample from a cadaver. This tissue graft will help with the growth of a new ligament. Ultimately, this surgery helps restore knee function and stability (UCSF, 2016).
Figure X: Illustrates how the ACL looks before the injury and how the ACL looks after surgery. (Source:A.D.A.M, 2013).
</style>
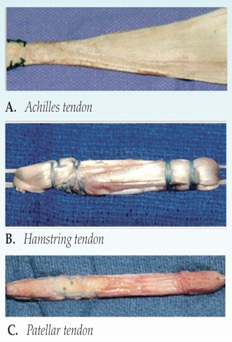
Tissue grafts can be retrieved from many sources. Some of these sources can include (Krans, 2016):
- Patellar tendon: Graft can be taken from the tendon that attaches from the bottom of the patella to your tibia.
- Hamstring: The graft can be taken from the tendon that connects the hamstring muscle to back of the knee.
- Quadriceps: A graft from the tendon that connects the quadriceps muscle.
- Cadaver: It is retrieved from a corpse, and is called an allograft.
Allografts: Risks and Benefits: An allograft is when a tissue graft is retrieved from a member of the same species but are not genetically identical to the recipient. The increased in the development of new techniques and research are one of the main reasons for the rise in the use of allografts in surgery. Some of the advantages of using allografts are very beneficial in cases requiring multiple ligament reconstruction surgeries and they have a very low rate of donor morbidity. However, this type of tissue graft does have some disadvantages. Primarily, this method is very costly and takes a longer time to treat and prepare for the procedure. Furthermore, the sterilization process involves radiation, which can potentially alter the biomechanical properties of the graft. Most importantly, this type of tissue graft has been associated with elongation and rupturing after surgery. A study was conducted on 120 young active adults that were cadets at the U.S. Military Academy at West Point. This study observed that an allograft ACL surgery was approximately 7 times more likely to fail compared to an autograft ACL reconstruction (Mayo Clinic, 2016).
Figure X: Demonstrates the different types of allografts. (Source: Mayo Foundation for Medical Education and Research, 2016).
</style>
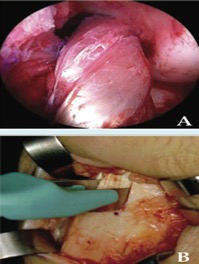
Autografts: Risks and Benefits: An autograft is when a tissue graft is retrieved from one part of the recipient’s body and placed in another part. Some common autograft sites can include patellar, hamstring and quadriceps tendons. Some of the associated advantages with this type of graft are minimal contamination, disease transmission and less structural alteration due to not irradiating the graft. The disadvantage associated with this type of graft is there is an increased post-surgery pain and recent research has indicated that if the hamstring tendon is used as a graft and the diameter is less than 8 millimetres, then there is increased risk of failure of the graft (Mayo Clinic, 2016).
Figure X: Demonstrates the extraction and retrieval of an autograft sample. (Source: Mayo Foundation for Medical Education and Research, 2016).
</style>
How to Prepare for ACL reconstruction: Throughout this whole process, you will always be meeting with various health practitioners to help prepare you for this procedure. Before the surgery, the doctor and surgeon will discuss treatment options as well advising on medical and personal decisions required. Also, using medical imaging techniques several knee examinations occur before and after the surgery. During the day of the surgery, it is recommended you fast for 12 hours and refrain from aspirin and other blood thinning medications, as this may cause complications during the surgery. It is also recommended to have someone else with you so they can support you and help with post-surgery instructions to follow. The most important advice would be to ask questions and for advice from your health practitioner, as this may reduce some of the burden associated with the procedure (Krans, 2016).
How ACL Reconstruction is Performed: This procedure involves the use of intravenous (IV) lines, which are used to administer and inject medication as well as sedatives. Next, the allograft or autograft is prepared to be implanted into the knee. The tendon is prepared with bone plugs, which can anchor the tendon to the knee. Next, an incision is made into the front of the knee to allow for a thin tube that can allow for a fiber optic camera and surgical tools to pass through. Furthermore, the surgeon will remove the torn ACL and remove debris from the area. The surgeon will then drill small holes into your tibia and femur so the bone plugs can be attached with posts, screws, staples, or washers. After, the attachment of the new ligament, the surgeon will assess the knee’s motion and will ensure that the graft is secure. In addition, after the knee is assessed, the surgeons will suture the wound up. They will then place a brace on your knee to stabilize it. This procedure length can vary as there are many various techniques and versions of this procedure. It all depends on the various factors involved (Krans, 2016).
Risks of ACL Reconstruction: There are some risks associated with ACL reconstruction surgery. These can include: - Excessive Bleeding and blood clotting - Persistent Knee Pain - Possible Infections - Loss of Knee motion - Rejection of tissue graft, causing inflammation There are other risks that are also associated with this procedure. If someone is a young growing child, they can have potential growth plate injuries, preventing the growth of their bones in affected areas. One way to prevent this is that doctors advise children to wait until they are older to have this procedure. Although there are risks associated with this procedure, this procedure has been labelled a gold standard solution to persistent knee injuries. This procedure has approximately 82 to 90% success rate (Krans, 2016).
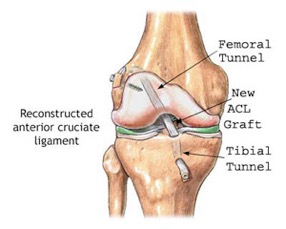
Post ACL Reconstruction: The road to recovery from surgery involves rehabilitation. Also, to deal with some of the other symptoms such as excessive pain, pain suppression medications are prescribed. Furthermore, to prevent infections to the wound, it is suggested that you clean the wound and keep it clean. In addition, to alleviate pain and reduce inflammation to the knee, it is recommended that you ice this area, as advised by the doctor. After a few weeks, the patient should be able to regain range of motion in the knee. Athletes typically return to full fitness within 6 to 12 months. Finally, this is all followed by physical therapy and rehabilitation, which is very vital to the success of recovering (Krans, 2016).
Figure X: Illustrates how the ACL is reconstructed and where the new graft is placed. (Source: Tower Orthopaedics, 2016).
</style>
Conclusion
Multiple Sclerosis is an inflammatory, autoimmune disease which disrupts the neurons’ ability to trigger action potentials. As of today, the pathophysiology of this disease is not fully understood, however, considerable advances have been made in understanding the factors behind this disease. It is evident that further research needs to be conducted to figure out the precise mechanism of the demyelination and remyelination process. The findings of this research will lead to more efficient treatment mechanisms and may even lead to a cure for Multiple Sclerosis.
<br>
References
Amato, M. P. & Portaccio, E. (2012). Management options in multiple sclerosis-associated fatigue. Expert Opinion on Pharmacotherapy, 13(2), 207-216
Ascherio A, Munger KL (April 2007). “Environmental risk factors for multiple sclerosis. Part I: the role of infection”. Annals of Neurology. 61 (4): 288–99.
Brosnan, C. F., & Raine, C. S. (1996). Mechanisms of immune injury in multiple sclerosis. Brain Pathology, 6(3), 243-257.
Compston A, Coles A (October 2008). “Multiple sclerosis”. Lancet. 372 (9648): 1502–17
de la Fuente AG et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol. 2015; 211(5):975-85. Multiple Sclerosis Clinical Presentation. (2016). Retrieved September 18, 2016, from http://emedicine.medscape.com/article/1146199-clinical
Dyment DA, Ebers GC, Sadovnick AD (February 2004). “Genetics of multiple sclerosis”.Lancet Neurol. 3 (92): 104–10
Fowler, C. J., Panicker, J. N., Drake, N., Harris, C., Harrison, S. C. W., Kirby, M., Lucas, M., Macleod, N., Mangnall, J., North, A., et al. (2009). A UK consensus on the management of the bladder in multiple sclerosis. Postgraduate Medical Journal. 85, 552-559.
Gilden DH (March 2005). “Infectious causes of multiple sclerosis”. The Lancet Neurology. 4 (3): 195–202
Hedley, L. (2012). Multiple sclerosis treatment options. The Pharmaceutical Journal. 288, 247-250.
Hillman, A. & Khorassani, F. (2014). Multiple sclerosis management. Pharmacy Times. Retrieved from http://www.pharmacytimes.com/publications/health-system-edition/2014/march2014/multiple-sclerosis-management
Löscher, W., & Potschka, H. (2005). Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx, 2(1), 86-98.
Multiple Sclerosis Clinical Presentation. (2016). Retrieved September 18, 2016, from http://emedicine.medscape.com/article/1146199-clinical
Multiple Sclerosis Society of Canada. (2016). Retrieved September 18, 2016, from https://mssociety.ca/about-ms/what-is-ms
Rolak, L. A. (2003). Multiple Sclerosis: It's Not The Disease You Thought It Was.Clinical Medicine & Research, 1(1), 57-60. doi:10.3121/cmr.1.1.57
Smith, K. J., & McDonald, W. I. (1999). The pathophysiology of multiple sclerosis⋮ the mechanisms underlying the production of symptoms and the natural history of the disease. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 354(1390), 1649-1673.
Tzartos, J. S., Friese, M. A., Craner, M. J., Palace, J., Newcombe, J., Esiri, M. M., & Fugger, L. (2008). Interleukin-17 Production in Central Nervous System-Infiltrating T Cells and Glial Cells Is Associated with Active Disease in Multiple Sclerosis. The American Journal of Pathology, 172(1), 146–155.
Wingerchuk, D. M., Lucchinetti, C. F., & Noseworthy, J. H. (2001). Multiple sclerosis: current pathophysiological concepts. Laboratory investigation, 81(3), 263-281.
Wu, G. F., & Alvarez, E. (2011). The immuno-pathophysiology of multiple sclerosis. Neurologic Clinics, 29(2), 257–278.
Wucherpfennig, K. W., & Strominger, J. L. (1995). Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell, 80(5), 695-705.