This is an old revision of the document!
Table of Contents
Tay Sachs Disease
Epidemiology
Tay-Sachs Disease is a very rare disease in the general population. Although this disease can occur in any ethnic group, the genetic mutations that are responsible for Tay Sachs are more frequent among the Ashkenazi Jews compared to other Jews (Sephardic and Oriental) and non-Jews (Koeslag 1984). According to a study done in New York City by Kozinn et al. (1957) over 12 years, it was determined that Tay Sachs occurred in 1 per 8300 Jewish people and in 1 per 450, 000 non-Jewish people. The estimated frequency of the heterozygous carrier according to this study was found to by 1 in 50 for Jews and 1 in 300 for non-Jews (Myrianthopoulos 1962). Although less frequent, Tay Sachs is also found in French Canadians, Pennsylvania Amish and Louisiana Cajuns. There are different possible reasons that explain the frequency of Tay Sachs among Ashkenazi Jews and other populations. For one, there may be a conferred adaptive advantage of Tay Sachs. Being a carrier of the gene that is mutated in Tay Sachs provides resistance against tuberculosis (Withrock 2015). Therefore, this gene has a heterozygote advantage (Frisch 2004). As well, there may have been the effect of random genetic drift. Random genetic drift occurs when there is a change in allele frequencies due to chance. Since most of the populations that have Tay Sachs are isolated, genetic drift has a greater effect in changing allele frequencies. Further, the founder effect may have impacted allele frequencies in the population. For example, if the initial population of individuals had the genetic mutation, those individuals would pass on the mutation to their descendants. As the population increases, the number of individuals with the mutation would increase in high frequency as well. It is possible that there may have been a high incidence of the mutation in the founder population. (Chakravarti 1978). Another theory known as reproductive compensation states that parents of infants affected by Tay-Sachs may continue having more children, which maintains the mutated gene in populations in high frequencies (Koeslag 1984).
Genetic Risk Factors
Individuals of the following ethnicities are at a higher risk for being affected by Tay-Sachs or being heterozygous for the mutated allele:
- Ashkenazi (eastern and central European) Jewish
- French-Canadians of Quebec
- Old Order Amish in Pennsylvania
- Cajuns of Louisiana
- Family history of condition
Genetic Inheritance
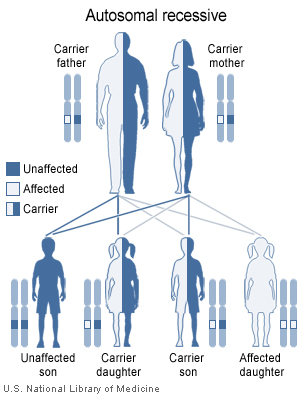
Tay-Sachs disease is caused by mutations in the HEXA gene, which contains information on how to make enzyme beta-hexosaminidase A. This enzyme is important for the central nervous system (brain and spinal cord) and also helps with breaking down GM2 ganglioside. GM2 ganglioside is unable to breakdown properly when there is a mutation in the HEXA gene. This causes a buildup of toxins in the neurons, which results in the observable symptoms of Tay-Sachs disease. This mutation is inherited in an autosomal recessive pattern. For example, if both parents are unaffected carriers of the mutated HEXA gene and they both pass on the mutated gene to their child, the child will inherit both mutated genes to produce a non-functional HEXA gene and will develop Tay-Sachs. If both parents are carriers of the mutated gene, there is a 1 in 4 chance of having a child who is affected with Tay-Sachs, 2 in 4 chance of having a child who is a carrier and 1 in 4 chance of having a child who is an unaffected non-carrier (GHR 2017).
<style center>
</style>
Diagnosis
Tay - Sachs disease (TSD) can be diagnosed through the use of tests and clinical evaluation. In a blood test screening, levels of hexosaminidase A are assessed using an enzyme assay (NORDs, n.a). Individuals with Tay-Sachs have reduced levels to almost absent amounts of this enzyme. Another way to diagnose Tay-Sachs is through the use of molecular genetic testing (NORDs, n.a). This method can find mutations in the HEXA gene, which is known to cause the disease. However, this diagnostic test is only offered at certain labs. Tay- Sachs can also be diagnosed prenatally through the use of screening such as chorionic villus sampling (CVS) and amniocentesis. CVS involves removing tissue samples from a part of the placenta and can be conducted around the 11th week of pregnancy (National Human Genome Research Institute, 2011). While in amniocentesis, it is done around the 16th week of pregnancy and involves the testing of a fluid sample surrounding the baby that is removed through the use of a needle National Human Genome Research Institute, 2011).
The Tay-Sachs disease carrier screening program was first started in Baltimore Maryland, in 1970 (Lew et al., 2014). Screening programs are important as it has been found that there is about a 90% decrease in the incidence of TSD cases in communities where these programs have been offered (Lew et al., 2014). A study by Gason et al. (2005), studied the efficacy of carrier screening programs in 2 Jewish schools in Melbourne students (ages 14-17) over a two year period. One of the components of the study involved assessing the compliance rate for blood sampling for the enzyme and genetic testing versus using a cheek brush method for solely genetic sampling. Until 2002, Jewish high school students were screened for TSD via an enzyme analysis, along with a mutation detection test. The cheek brush test method was implemented in 2003 and consisted of mutation analysis of the three common mutations found in the Ashkenazi Jewish populations. The study found that was a higher proportion of students who were tested for TSD carrier status via the cheek brush method (96%, N=214) compared to those who took a blood sample (84.9%, N=163). Researchers also saw that receiving a blood test caused more anxiety among the group 8.2% compared to those who received the cheek brush method 20.9%. Student attitudes were considered a powerful motivator for testing as there was a higher level of discomfort linked to blood testing.
In a review article by Lew et al (2014), there are several barriers to diagnosing TSD that are highlighted. On the clinician’s side, some of these include a lack of clinical education regarding the disease and failure to identify the genetic risk during their medical history. Other barriers include unplanned pregnancies and expenses incurred during testing for diagnosing (Lew et al., 2014). In Sydney Australia, having a test done for TSD costs a patient around $A100 (Lew et al., 2012). However even when obstacles such as cost and blood testing are removed, there is not always full maximization for carrier screening which makes diagnosis of TSD more difficult. In the study by Gason et al (2005), there were students at the high schools who simply did not feel that they wanted to know if they were carriers at this point in their life.

References
Chakravarti, A. and Chakraborty R. (1978). Elevated frequency of Tay-Sachs disease among Ashkenazic Jews unlikely by genetic drift alone. Am J Hum Genet, 30(3): 256-261. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1685578/
Frisch, A. et. al. (2004). Origin and spread of the 1278insTATC mutation causing Tay-Sachs disease in Ashkenazi Jews: genetic drift as a robust and parsimonious hypothesis. Human Genetics, 114 (4): 366-376.Doi 10.1007/s00439-003-1072-8 http://link.springer.com/article/10.1007%2Fs00439-003-1072-8
Gason, A. A., Metcalfe, S. A., Delatycki, M. B., Petrou, V., Sheffield, E., Bankier, A., & Aitken, M. (2005). Tay Sachs disease carrier screening in schools: educational alternatives and cheekbrush sampling. Genetics in Medicine, 7(9), 626-632.
Koeslag, J. H. and Schach, S. R. (1984), Tay–Sachs disease and the role of reproductive compensation in the maintenance of ethnic variations in the incidence of autosomal recessive disease. Annals of Human Genetics, 48: 275–281. doi:10.1111/j.1469-1809.1984.tb01025.x
Lew, R. M., Burnett, L., Proos, A. L., Barlow‐Stewart, K., Delatycki, M. B., Bankier, A., … & Fietz, M. (2015). Ashkenazi Jewish population screening for Tay–Sachs disease: The International and Australian experience. Journal of paediatrics and child health, 51(3), 271-279.
Tay Sachs disease (2017). Genetics Home Reference. https://ghr.nlm.nih.gov/condition/tay-sachs-disease
Withrock, I. C. et. al. (2015). Genetic diseases conferring resistance to infectious diseases. Genes & Diseases, 2(3): 247-254. doi: 10.1016/j.gendis.2015.02.008 http://www.sciencedirect.com/science/article/pii/S2352304215000239