Table of Contents
Alzheimer's Disease
Introduction
So what is Alzheimer’s exactly? The dictionary definition of this disease is characterized as the following; “a progressive mental deterioration that can occur in middle and old age, due to generalized degeneration of the brain” (Oxford Dictionary, 2018). Because this disease isn’t restricted to old aged individuals, the focus of this presentation will aim to cover different risk factors backed up with their mechanism for infection. Treatments and prevention management techniques will also be discussed consequently looking at future research.
History
In 1907, Alois Alzheimer put together a diagnosis of old individuals that gradually lost their intellectual ability as they aged; the term for this was pre-senile dementia (Armstrong, 2013). When putting this together with cognitive impairment, senile plaques and neurofibrillary tangles (NFT), this became the marker for defining clinical features of Alzheimer’s Disease (Armstrong, 2013). 1910 is when the use of this word came into play by Emil Kraepelin based on two of Alois original cases of this disease. The problem when comparing two of these cases was that both has a significant amount of SPs but only one of the two showed a significant ratio representing NFT, thus making it difficult to establish a single theory for the disease. Many theories have been proposed but 8 categories into which the ‘most likely to be involved’ theories have been grouped and they are as follows: 1. Acceleration of aging 2. Degeneration of anatomical pathways 3. Environmental factors (ie. aluminum exposure, head injury, malnutrition) 4. Genetic factors (ie. mutations in important precursor proteins and genes) 5. Metabolic disorder as a result of mitochondrial dysfunction 6. Vascular factors (compromised blood brain barrier) 7. Immune system dysfunction 8. Infectious agents (Armstrong, 2013).
Epidemiology
Based off of 2005 data, about 24 million people had been diagnosed with dementia. Of these 24 million, 70 percent of them were linked to Alzheimer's Disease. In terms of regional statistics, the highest prevalence of is shown in North America, affected 6.4% of the population. This followed by Western Europe, Latin America, and China. (5.4 percent, 4.9 percent and 4.0 percent respectively). As age increases, one can expect to see these numbers increase exponentially with the peak occurring with individuals in theirs 70s and 80s. (Reitz, Brayne, and Mayeux, 2011). In regards to the future of dementia, the amount of people affected are expected to double every couple decades until 2040 (Reitz, Brayne, and Mayeux, 2011).
Risk Factors
For clarity, it is important to note that AD falls under the umbrella term for dementia. This is important when mentioning some of the risk factors associated with AD as some may be more broad than others. Some cardiovascular risk factors include: diabetes, hypertension, and high cholesterol levels (Imtiaz, et al., 2014). High BMI’s in midlife has also been linked to having a AD later on. Smoking and drinking alcohol have been linked to increased risk of AD. Genetics risk factors include individuals that are carriers for the APOE epsilon 4 allele which coincides with the cerebral amyloid pathology that will be later on discussed (Imtiaz, et al., 2014). A few factors have also been used as protective mechanisms to reduce one's risk of getting AD (ie. healthy eating, high social networking, high education, involvement in cognitively challenging tasks, and being in a relationship)(Imtiaz, et al., 2014).
Signs and Symptoms
There are various psychiatric symptoms that are used to recognize core features of Alzheimer’s disease. These include depression, apathy, aggression, and even psychosis. The severity of these symptoms is known to be helpful in predicting rate of cognitive decline, and length of survival. Depression is also a common disease that coincides in Alzheimer’s disease, and it is expected that patients will show symptoms of clinical depression within 5 years, making it an indicator of Alzheimer’s (Li et al., 2014). Apathy is the loss of motivation towards goal directed cognition, action, and emotion. Similar to the other signs of the disease, apathy is useful in predicting a raid cognitive decline. Aggression and agitation tend to be observed more in male patients, and tend to predict similar trends as the other symptoms. Psychosis is characterized by the perception of delusions and hallucinations and are one of the most serious and less frequent symptoms of Alzheimer’s, especially during the early stages (Li et al., 2014).
There are different stages that characterize Alzheimer’s and have been broadly categorized into the mild, moderate, and severe stage. In the mild form of the disease, a person can seem healthy but continues to have trouble in making sense of the world. The realization of the problem is typically noticed over time to both the person and family members. Signs in this stage of the disease vary and include memory loss, poor judgement, getting lost, mood and personality changes and decreased efficiency of completing daily tasks. This is typically the stage at which the disease is actually diagnosed (Alzheimer’s Association, 2018).
As the disease progresses to the moderate stage, more care is needed. This stage of the disease is characterized by an increase in memory loss and confusion, difficulty with basic problems involving reading and writing, signs of psychosis, restlessness and more.
When it comes to the last severe stage of Alzheimer’s, patients lose the ability to communicate and become dependent on others for care. They tend to be in bed for most of the time, and the symptoms in this stage include; weight loss, seizures, difficulty swallowing, and loss of bowel and bladder control. Patients in this stage may die due to aspiration pneumonia which is when the patient can’t swallow and uptakes food and liquid into the lungs instead of air (National Institute on Aging, 2017).
Diagnosis
In terms of diagnosing Alzheimer’s disease, there are two main time points the disease can arise. If the disease develops before a person is 65 years old, then this is known as early onset Alzheimer’s disease. If after 65, the disease is late onset, and is the most common. One tool used to differentiate the diagnosis of Alzheimer’s from other diseases such as frontotemporal dementia is the neuropsychiatric inventory (NPI). It is a validated clinical rating instrument to evaluate neurological symptoms in demented patients (Kaufer et al., 1998).
There are a wide variety of other factors that clinicians consider when diagnosing this disease. For starters, the history of the patient is looked at. This includes typical questions about family history of dementia, changes in behaviour, and possible harmful medications being taken. Next, standard physical tests may be used to measure things such as blood pressure and other vital signs. They may look for cell counts, and even specific important proteins associated with the disease such as amyloid beta (Aggarwal et al., 2015). Checking levels of various chemicals and hormones can help rule out other possible causes of symptoms. Genetic tests may be used to help determine if a person is already at risk for Alzheimer’s as there are possible gene defects.
Lastly, one other main way of diagnosing Alzheimer’s disease is by imaging and brain scans. These tests can help identify if a stroke or tumor or other problem is causing dementia. It can help visualize changes in the brain structure and the tests include; Computed tomography (CT), Magnetic resonance imaging (MRI), and Positron emission tomography (PET). PET scans for instance can detect levels of glucose metabolism and determine if there is a decline such as those associated with decreasing cognitive function. This can be very helpful in detecting early signs of the disease (Landau et al., 2011).
Pathophysiology
Beta-Amyloid Plaque:
Amyloid plaques are extracellular deposits of Beta- amyloid in the brain. In an healthy individual, the transmembrane Amyloid Precursor Protein (APP) undergoes an alpha cleavage leading to sAPPa (soluble Amyloid Precursor Protein alpha) to be sent to the extracellular region and a gamma cleavage releasing AICD (APP Intracellular Domain) into the cell. While the in-between portion becomes P3 - with no known biological functions (Jouanne & Voisin-Chiret, 2017). However, in patients with late-onset Alzheimer's dieases why undergo beta cleavage that cleaves to form sAPPb (soluble APP beta) and gamma cleavage to form AICD (BioLegend, 2018). However this time the in-between portion becomes 42 Beta-amyloid and accumulate to form the plaque that will cause disturb long-term potentiation, which over time leads to dysfunctional synapse and neuron deaths (Oddo et al., 2003).
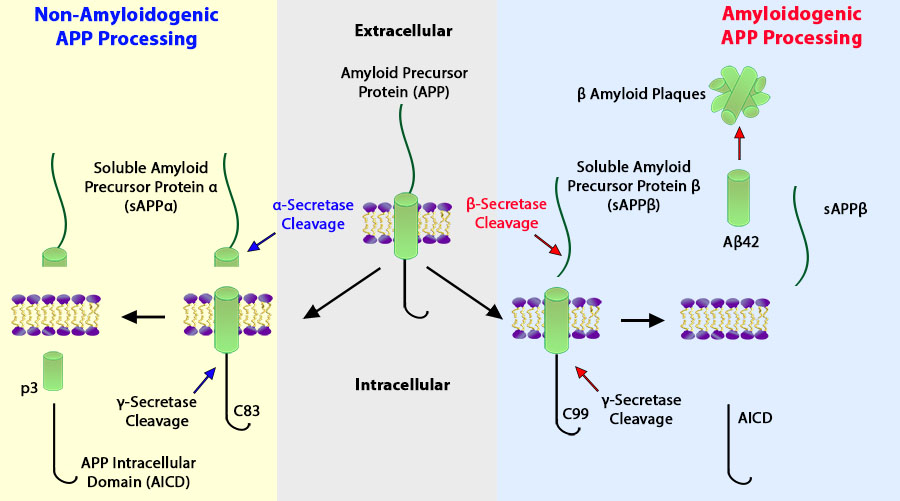 Figure 1: The APP process in healthy individual (left) verses in late-onset Alzheimer's patient (right)
Figure 1: The APP process in healthy individual (left) verses in late-onset Alzheimer's patient (right)
Tau Protein and Neurofibrillary Tangle:
Another hallmark of Alzheimer's disease is characterized by the abnormality in the Tau protein. In healthy person the Tau protein act as a regulator for of tubulin which controls the stabilization of microtubules (Wang et al., 2013). This allows for many cellular dynamic activities such structuring, transporting materials and DNA replication. However, Tau protein is hyperphosphorylated by the overexpression of Tau kinase in Alzheimer's patients (Jouanne & Voisin-Chiret, 2017). This causes these proteins to misfold and aggregate to form neurofibrillary tangles that leads to the loss of neurons in the patient's brain, however the specific mechanism behind this is still unknown.
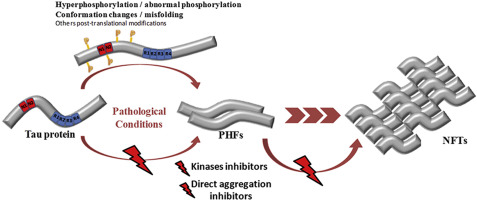 Figure 2: The pathway which Tau protein is hyperphosphorylated leading to neurofibrillary tangles. The red lighting represent possible therapeutic targets in treating Alzheimer's disease.
Figure 2: The pathway which Tau protein is hyperphosphorylated leading to neurofibrillary tangles. The red lighting represent possible therapeutic targets in treating Alzheimer's disease.
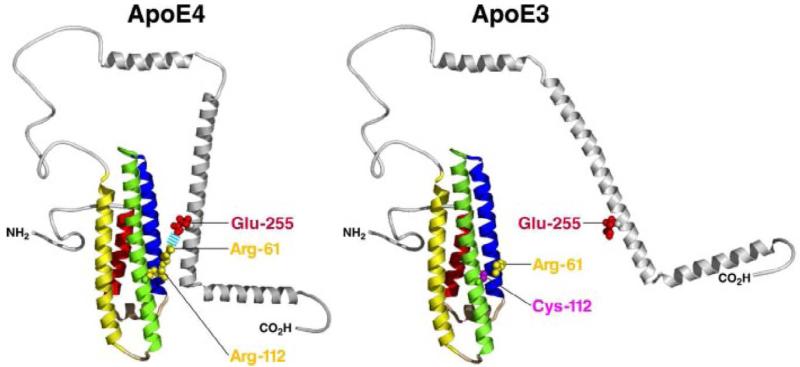
APO-E, Genetic Promotor:
Another pathogenic gene mutation is also identified to be responsible for in Alzheimer's patients. APO-E or apolipoprotein-e in its wildtype (E3) act as a ligand that regulates the cholesterol level in the cell (Czyzewski et al., 2003). However, this gene is mutated to the E4 form in Alzheimer's patient that ultimately bind with the beta-amyloid to accelerate their aggregation and promote the formation of plaque (Wisniewski et al., 1994). This is important as it describes the genetic connection of the inheritability of the disease and people who may be at higher risk of this neuro-degenerative disease.
Drugs and Medication
Current Treatments
There has yet to be an effective cure for Alzheimer's Disease. According to the Alzheimer's Association, pharmaceutical intervention treatment strategies slow down the progression of the disease, and alleviate symptoms such as memory loss, but do so generally in mild to moderate cases. The pathophysiology of the neurodegenerative disease disrupts the process by which neurons in the brain communicate at synapses by destroying the synapse and damaging or killing the neurons themselves (Alzheimer's Association, 2018). The current treatments for Alzheimer's Disease focus on improving the communication between nerve cells through the manipulation of neurotransmitters in order to repair the brain's communication network (Alzheimer's Association, 2018).
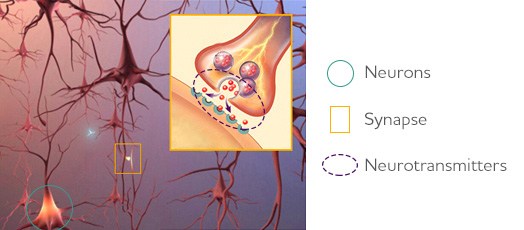 Figure 4: Image that represent the relation between synapse, neurons and neurotransmitters.
Figure 4: Image that represent the relation between synapse, neurons and neurotransmitters.
Cholinesterase inhibitors:
One of the most prominent treatment measures for this disease is the administration of cholinesterase inhibitors (Alzheimer's Association, 2018). This is often considered the gold standard of treatment in mild to moderate cases. Acetylcholine is an integral neurotransmitter for various cognitive functions including learning and memory (Alzheimer's Association, 2018). Individuals with Alzheimer’s disease have lower levels of acetylcholine in their brain and thus a common therapeutic treatment is the use of cholinesterase inhibitors. Cholinesterases are enzymes which target acetylcholine for breakdown (Alzheimer's Association, 2018). By inhibiting the action of the enzymes, more acetylcholine can be available for cell signalling. Common prescribed cholinesterase inhibitors include: Razadyne® (galantamine), Exelon® (rivastigmine), and Aricept® (donepezil) (Alzheimer's Association, 2018). Common side effects of these drugs may include nausea or vomiting.
N-methyl-D-aspartate (NMDA) antagonists:
N-methyl-D-aspartate receptor is a glutamate receptor found in nerve cells with an important function in controlling memory and information processing through regulation of the neurotransmitter, glutamate (Alzheimer's Association, 2018). NMDA receptors are also critical for excitotoxicity, the process that results in the death of nerve cells, through over stimulation by neurotransmitters such as glutamate (Dong et al., 2009). Glutamate is often overproduced in individuals with Alzheimer’s leading to damaged nerve cells. Excitotoxicity is linked to several neurodegenerative diseases such as Alzheimer’s and Huntington's disease, as well as strokes and epileptic episodes (Dong et al., 2009). One known treatment for Alzheimer’s is the use of MDA antagonists, such as memantine or Namenda®, to block glutamatergic receptors (Alzheimer's Association, 2018). Common side effects of this drug may include constipation, confusion, headaches, and dizziness (Alzheimer's Association, 2018).
Combination Therapy:
Presently, acetylcholinesterase inhibitors are the first-line drugs in the treatment of Alzheimer's disease. These options are now complemented by memantine (NMDA antagonists). As NMDA antagonists work differently from cholinesterase inhibitors, the two types of drugs can be prescribed in combination (Alzheimer's Association, 2018). Clinical studies have shown evidence in favour of combination therapy, including delay or prevention of disease progression in AD patients (Schmitt et al., 2004).
4 Pillars of Alzheimer's Prevention
The Alzheimer’s Research and Prevention Foundation has established 4 pillars to prevent Alzheimer’s. This foundation is known for researching medical approaches, educating others, and facilitating communication with health care professionals for 20 years. These 4 pillars fall into the category of diet and supplements, physical and mental exercise, yoga and meditation, as well as psychological well-being (Alzheimer’s Research & Prevention Foundation, 2018).
 Figure 5: The Alzheimer's Research and Prevention Foundation
Figure 5: The Alzheimer's Research and Prevention Foundation
Pillar 1: Diet and Supplements
Maintaining a healthy diet by avoiding foods with high concentration of trans-fat and saturated fats can be beneficial for those with Alzheimer’s. Since these foods can cause inflammation it may product free radicals, damaging the brain cells (Alzheimer’s Research & Prevention Foundation, 2018). Studies have shown that those who choose to eat less processed foods and eat whole food options have better memory and less decline in mental health (Khalsa and Perry, 2017). The areas that a healthy diet can affect may enhance cognitive performance with glucose utilization, mitochondrial function, as well as reducing oxidative stress. Furthermore, the Mediterranean diet includes foods such as fruits, nuts, olive oil, and vegetables (Khalsa and Perry, 2017). These whole foods indicate that those who followed this diet had lower levels of amyloid-beta plaques as well as fewer tau aggregates. Taking supplements may also help the prevention of Alzheimer’s such as high-potency multi-mineral supplements including folic acid. Supplements such as omega-3 oils, coenzyme Q10, and resveratrol is also recommended for the diet (Khalsa and Perry, 2017).
Pillar 2: Physical and Mental Exercise
Physical exercise may be beneficial for those who have AD although there is no optimal physical activity that individuals can do (Chen, Zhange, and Huang 2016). However, studies have shown that regular walking and strength training does show more effectiveness for motor function as this improves muscle strength which is shown is affected in those with AD. Furthermore, in optimal environmental conditions with good nutrition and light exposure, there is effective training result (Chen, Zhange, and Huang 2016). A study conducted by Columbia University showed that older individuals that exercised on a treadmill for 30 minutes four times a week demonstrated new cells in the dentate gyrus. This is an area of the brain that is related to memory as well (Khalsa and Perry, 2017).
Mental exercise is also important for AD prevention and having the individual to mentally be aware such as think and engage in everyday activities and outside everyday activities is also important. For example, reading, listening to music, or focusing on brain-aerobic activities could help (Khalsa and Perry, 2017). Furthermore, doing simple calculations, storytelling, and reading aloud from books could be beneficial for those who are in the later stages of Alzheimer’s. It is important to choose activities that are not beyond the individual’s capacity as this may lead for them to become frustrated (Dementia Australia, 2006).
Pillar 3: Yoga/Meditation
Stress is shown to be a major risk factor for AD, and meditation or yoga could help prevent memory loss as well as anxiety, which is an indication of AD. An effective yoga/meditation technique called Kirtan Kriya, also known as KK (Khalsa and Perry, 2017). This exercise utilizes primal sounds and allows the individual to concentrate, focus, and improve memory. These sounds consist of chanting repeatedly with the order of Saa, Taa, Naa, and Maa. Furthermore, with this meditation technique, motor skills are also used as well (Alzheimer’s Research & Prevention Foundation, 2018). KK has been shown to reduce stress level as well as increase blood flow to the brain. Furthermore, KK activates the anterior cingulate gyrus which controls stress balance and emotional control (Khalsa and Perry, 2017). A study at the University of Pennsylvania showed the positive effects of this meditation technique where KK enhanced the brain connectivity and the benefits lasted in a follow up period as well (Khalsa and Perry, 2017).
Pillar 4: Psychological Well-Being
Psychological well-being, also known as PWB promotes the acceptance of self, self-confidence, and the reduction of negativity. By promoting positive attributes on an individual, this can reduce cholesterol and inflammation as well (Khalsa and Perry, 2017). Furthermore, when an individual is stressed, chemicals such as adrenaline and cortisol is released an in excess of cortisol, this can damage the cells in the brain (Alzheimer’s Research & Prevention Foundation, 2018). The Alzheimer’s research & prevention foundation states that high levels of cortisol also prevents the ability to learn and retain new information which will affect the overall health of an individual’s brain (Alzheimer’s Research & Prevention Foundation, 2018).
Management
An individual who has AD requires attention from health professionals as patients who have severe AD may experience more behavioural and psychological symptoms. For example, monitoring can include assessing the function, cognition, and nutritional status from frequent visits to family physicians (Herrmann and Gauthier, 2008). Patients with AD may have increased agitation, aggression, and motor restlessness. It is important that caregivers to recognize further distress and provide quality life for the patient, maintain optimal function, and provide maximum comfort (Herrmann and Gauthier, 2008.) Furthermore, evaluating treatment and providing continuous and guidance to the patient as well as the caregiver is extremely important. (Waldemar et al., 2007). Evaluating medication effects and treatments requires both the cooperation from the caregiver as well as physician (Waldemar et al., 2007).
Future Therapeutics
Aducanumab Targeting Beta-amyloid Protein
According to the Alzheimer’s Association, there are currently many targets and studies being conducted to prevent AD. Beta-amyloid is present in those who have Alzheimer’s by the two enzymes beta-secretase and gamma-secretase which ultimately forms the beta-amyloid protein (Alzheimer’s Association, 2018). Aducanumab, also known as BIIB037, tries to target the aggregated forms of beta-amyloid that can eventually develop into the amyloid plaque. This antibody was derived by a biotech company in Switzerland and has passed its phase 1, 2, and is currently in phase 3 (Alzforum, 2018). A one-year data for the 6 mg/kg dose of aducanumab showed significant reduction in brain amyloid levels and therefore a study consisting of the phase 3 trials objective is to evaluate how effective doses of aducanumab is in individuals with AD (Alzforum, 2018). This is currently conducted within around 1605 participants with a dosages of monthly intravenous infusions. Furthermore, this drug is still in the research and is not released to the public. This study has started in September 30, 2015, and is expected to be completed by April 30, 2022 (National Library of Medicine, 2018).
AADvac1 Targeting Tau Protein
The function of Tau protein helps to maintain the structure of the neuron. However, within individuals that have AD, the tau protein is abnormal and therefore collapses which destabilizes the structure of neruons (Alzheimer’s Association, 2018). A current drug in research is AADvac1, which is a vaccine that promotes the body’s immune system to help attack these abnormal forms of tau protein. This vaccine has synthetic peptide which is derived from the amino acids 294 to 305 (Alzforum, 2018). This vaccine is currently in the phase 2 safety trial by Axon Neuroscience where they are currently evaluating the safety and efficacy of AADvac1 with patients who have mild AD (National Library of Medicine, 2018).
Conclusion
Although there currently is not a definite cure for Alzheimer's disease, current research such as Aducanumab and AADvac1 shows promising progression with research. Furthermore, other research focusing on the inflammation of the brain with the disease is also being researched and will hopefully pass the trials so patients are able to use the drug. There are also many preventive ways described within the 4 pillars of Alzheimer's Prevention and the management of the patient is just as important.
References
Armstrong, R. A. (2013). What causes Alzheimer’s disease?. Folia Neuropathologica, 51(3), 169-188.
Aggarwal, N. T., Shah, R. C., & Bennett, D. A. (2015). Alzheimer's disease: Unique markers for diagnosis & new treatment modalities. The Indian journal of medical research, 142(4), 369.
Alzheimer's Association. (2018). Latest Medication for Memory Loss. Retrieved September 21, 2016, from http://www.alz.org/alzheimers_disease_standard_prescriptions.asp)
Alzheimer's Disease. (n.d.). Retrieved from https://www.saintlukeskc.org/condition/alzheimers-disease
Alzheimer's Disease: Symptoms and Care | Emed Multi Specialty Group. (2018, May 07). Retrieved from https://emedmultispecialtygroup.com/2018/03/20/alzheimers-disease-symptoms-care/
Alzheimer's Prevention. (2018). Alzheimer's Prevention through Diet and Supplements, Stress Management, Exercise, and Spiritual Fitness. [online] Available at: http://alzheimersprevention.org/4-pillars-of-prevention/ [Accessed 30 Oct. 2018].
Alzheimer's Prevention. (2018). Home - Alzheimer's Prevention. [online] Available at: http://alzheimersprevention.org/ [Accessed 27 Oct. 2018].
Alzheimer's Prevention. (2018). Kirtan Kriya Yoga Singing Exercise - Alzheimer's Prevention. [online] Available at: http://alzheimersprevention.org/research/12-minute-memory-exercise/ [Accessed 27 Oct. 2018].
Alzheimer's Prevention. (2018). Prevent Alzheimer's Disease - Pillar 1: Diet and Supplements. [online] Available at: http://alzheimersprevention.org/4-pillars-of-prevention/pillar-1-diet-supplements/ [Accessed 27 Oct. 2018].
Alzheimer's Prevention. (2018). Prevent Alzheimer's Disease - Pillar 2: Stress Management. [online] Available at: http://alzheimersprevention.org/4-pillars-of-prevention/pillar-2-stress-management/ [Accessed 27 Oct. 2018].
Alzheimer's Disease and Dementia. (2018). Treatment Horizon. [online] Available at: https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon [Accessed 28 Oct. 2018].
Alzforum.org. (2018). Aducanumab | ALZFORUM. [online] Available at: https://www.alzforum.org/therapeutics/aducanumab [Accessed 28 Oct. 2018].
Alzforum.org. (2018). AADvac-1 | ALZFORUM. [online] Available at: https://www.alzforum.org/therapeutics/aadvac-1 [Accessed 28 Oct. 2018].
BioLegend. (2018, June 1). BioLegend Amyloid Precursor Protein and Aβ. Retrieved October 30, 2018, from https://www.biolegend.com/amyloid_precursor_protein Caffeine, mental exercise benefit brain. (n.d.). Retrieved from https://www.iadvanceseniorcare.com/article/caffeine-mental-exercise-benefit-brain
Chen, W. W., Zhang, X., & Huang, W. J. (2016). Role of physical exercise in Alzheimer's disease. Biomedical reports, 4(4), 403-407.
Clinicaltrials.gov. (2018). 221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer's Disease - Full Text View - ClinicalTrials.gov. [online] Available at: https://clinicaltrials.gov/ct2/show/NCT02484547?term=BIIB037&rank=5 [Accessed 28 Oct. 2018].
Clinicaltrials.gov. (2018). 24 Months Safety and Efficacy Study of AADvac1 in Patients With Mild Alzheimer's Disease - Full Text View - ClinicalTrials.gov. [online] Available at: https://clinicaltrials.gov/ct2/show/NCT02579252?term=AADvac1&rank=4 [Accessed 28 Oct. 2018].
Czyzewski, K., Pfeffer, A., & Barcikowska, M. (1998). Apolipoprotein E function in the nervous system. Neurologia i neurochirurgia polska, 32(1), pp.125-132.
Doctor and Clinic Management System Software. (n.d.). Retrieved from http://www.summittech.com.sg/products/doctor-and-clinic-management-system-software-singapore/
Dong, X. X., Wang, Y., & Qin, Z. H. (2009). Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacologica Sinica, 30(4), 379.
English Dictionary, Thesaurus, & grammar help | Oxford Dictionaries. (n.d.). Retrieved from https://en.oxforddictionaries.com/definition/alzheimer's
Herrmann, N. and Gauthier, S. (2008). Diagnosis and treatment of dementia: 6. Management of severe Alzheimer disease. Canadian Medical Association Journal, 179(12), pp.1279-1287.
Imtiaz, B., Tolppanen, A. M., Kivipelto, M., & Soininen, H. (2014). Future directions in Alzheimer's disease from risk factors to prevention. Biochemical pharmacology, 88(4), 661-670.
Jiang, H (2017). Life Sci 3AA3 Essay - Apolipoprotein E (APO-E) isoforms and Alzheimer’s disease (unpublished).
Jouanne, M., Rault, S., & Voisin-Chiret, A. S. (2017). Tau protein aggregation in Alzheimer's disease: An attractive target for the development of novel therapeutic agents. European journal of medicinal chemistry, 139, 153-167.
Khalsa, D. S., & Perry, G. (2017). The Four Pillars of Alzheimer's Prevention. Cerebrum : the Dana forum on brain science, 2017, cer-03-17. Kirtan Kriya Yoga Singing Exercise. Retrieved from http://alzheimersprevention.org/research/12-minute-memory-exercise/
Kaufer, D. I., Cummings, J. L., Christine, D., Bray, T., Castellon, S., Masterman, D., … & DeKosky, S. T. (1998). Assessing the impact of neuropsychiatric symptoms in Alzheimer's disease: the Neuropsychiatric Inventory Caregiver Distress Scale. Journal of the American Geriatrics Society, 46(2), 210-215.
Landau, S. M., Harvey, D., Madison, C. M., Koeppe, R. A., Reiman, E. M., Foster, N. L., … & Alzheimer's Disease Neuroimaging Initiative. (2011). Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiology of aging, 32(7), 1207-1218.
Li, X. L., Hu, N., Tan, M. S., Yu, J. T., & Tan, L. (2014). Behavioral and psychological symptoms in Alzheimer’s disease. BioMed research international, 2014.
Oddo, S., Caccamo, A., Shepherd, J. D., Murphy, M. P., Golde, T. E., Kayed, R., … & LaFerla, F. M. (2003). Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron, 39(3), 409-421.
Optometry, F. (2018). Physical Exercise Is Good For Your Eyes – Fresno CA | Fig Garden Optometry. [online] Figgardenoptometry.com. Available at: https://figgardenoptometry.com/2017/03/01/physical-exercise-is-good-for-your-eyes/ [Accessed 30 Oct. 2018].
Puglielli, L., Tanzi, R. and Kovacs, D. (2003). Alzheimer's disease: the cholesterol connection. Nature Neuroscience, 6(4), pp.345-351. Schmitt, B., Bernhardt, T., Moeller, H. J., Heuser, I., & Frölich, L. (2004). Combination therapy in Alzheimer’s disease. CNS drugs, 18(13), 827-844.
Reitz, C., Brayne, C., & Mayeux, R. (2011). Epidemiology of Alzheimer disease. Nature Reviews Neurology, 7(3), 137.
Wang, J. Z., Xia, Y. Y., Grundke-Iqbal, I., & Iqbal, K. (2013). Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. Journal of Alzheimer's Disease, 33(s1), S123-S139.
Wisniewski, T., Castano, E. M., Golabek, A., Vogel, T., & Frangione, B. (1994). Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. The American journal of pathology, 145(5), 1030.