Table of Contents
What is Yellow Fever?
Yellow Fever is a disease caused by the Yellow Fever virus. This virus originates from primates in the forests of Africa where they were transmitted to other animals and humans via a vector: the Aedes aegypti Mosquito - therefore making Yellow Fever a Zoonotic Virus (CDC, 2015). In fact, Yellow fever was the first proven human-pathogenic virus in 1927.
It is responsible for deaths and distress in regions ranging from Africa to South America (where it has grown to become an endemic problem). Perhaps most troublesome is that as the climate continues to get warmer the Aedes aegypti mosquito is able to nest in increasingly northern regions and thus spreading this disease to regions previously immune to this virus.
Epidemiology
There are roughly 200,000 new cases of yellow fever every year, of which 90% occur in Africa. It leads to 30,000 deaths annually, with the largest incidence rate being in Nigeria (Monath, 2001). The virus persists in tropical and subtropical areas of Africa and South America but in recent years has been very prevalent in sub-Saharan regions of Africa and South America. Even with the presence of the vector mosquito in some regions and continents such as Asia and Australia, the virus has not affected individuals living there. The high prevalence of the virus in Africa is reported to be directly correlated with the high population of mosquitos living in close proximity to unvaccinated people. Due to the higher number of vaccinated people in South America, the transmission rate is much lower. Generally, obtaining reputable and accurate information regarding the transmission rate of the virus is difficult due to unreported cases, limited surveillance and recording techniques. (Barnett, 2007)
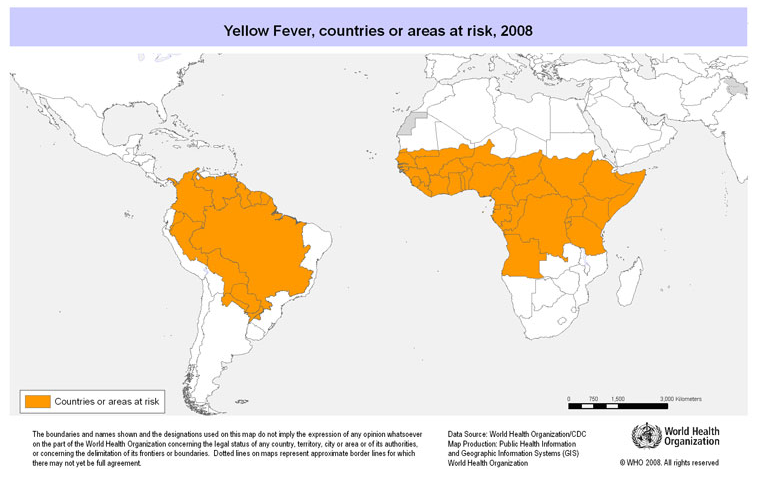
Figure #01: This image from the Public Health Agency of Canada shows the areas that are currently affected by the Yellow Fever Virus. Taken from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/acs-11/index-eng.php
Transmission
As mentioned above, the main vector of transmission of Yellow Fever is the Aedes aegypti mosquito but can also be carried by other types of mosquitoes such as Aedes albopictus & Aedes hemocaucos (Fontenille et al., 1997). A depiction of the Aedes aegypti mosquito is shown below in Figure #02.
Figure #02: A visual depiction of the Aedes aegypti mosquito (Fontenille et al., 1997).
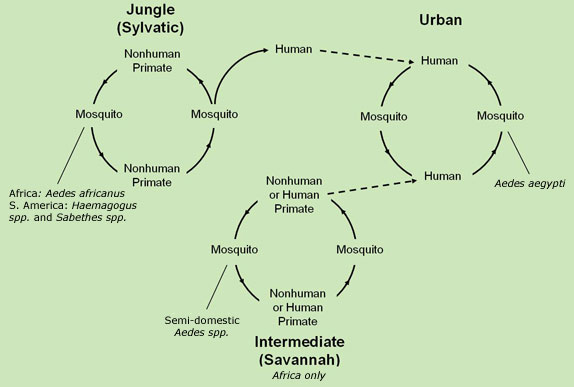
Female mosquitoes obtain the virus by drinking the blood of primates or humans infected with the virus. It is then transmitted to non-infected humans / animals, via a mosquito bite (CDC, 2015). There are three transmission stages (all depicted below in Figure #03: sylvatic, intermediate and urban. The first stage known as sylvatic is based in tropical rainforests where monkeys are infected as a result of vector mosquitos. These mosquitos may infect humans entering the forest. The second stage is intermediate and is based in humid or semi-arid areas of Africa where mosquitos can infect both humans and primates. This stage is the most common in Africa and has the potential to grow into an occasional epidemic. The third stage is known as urban and occurs in highly populated areas where the potential for an epidemic to occur is the greatest (WHO, 2014). Note that the Yellow Fever is not directly passed from affected host to unaffected individual - it must be passed via a vector.
Figure #03: Diagram depicting the Sylvatic, Intermediate & Urban Transmission stages of the Yellow Fever Virus (CDC, 2015).
Symptoms and Prognosis
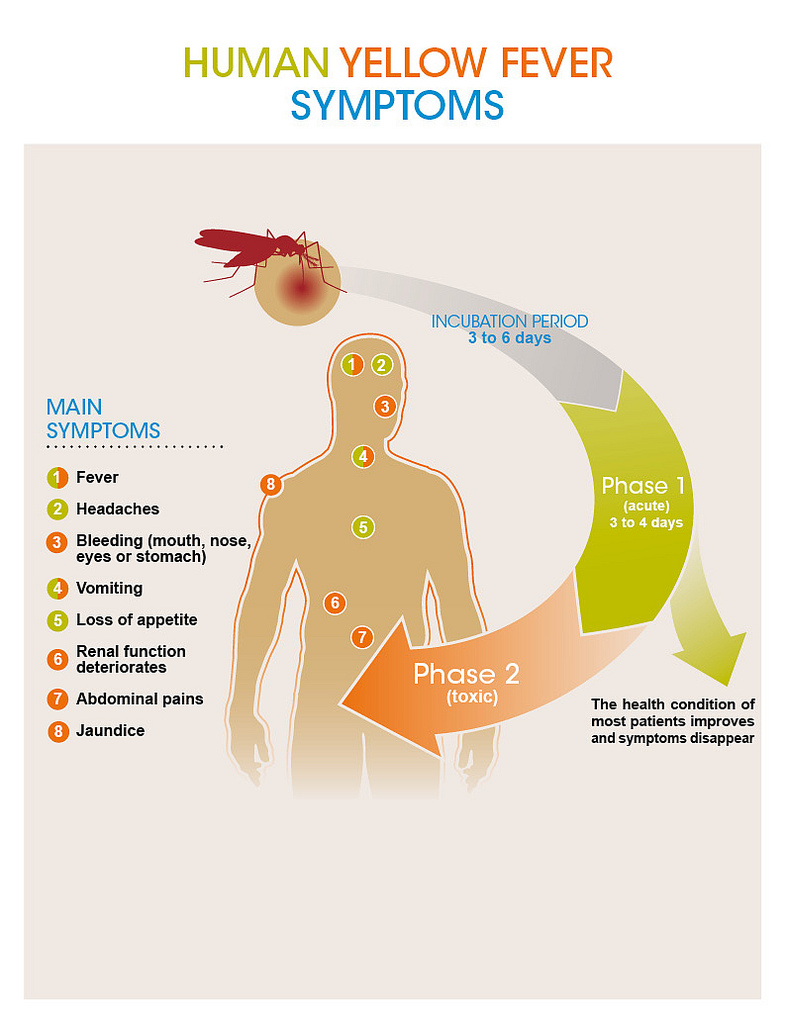
Yellow fever has an incubation rate of 3-6 days typically and will usually present itself with mild or no symptoms at all. (CDC, 2015) Infection begins with the “acute” phase where symptoms can include fever, chills, muscle pain, shivers, headache, loss of appetite, nausea and vomiting as depicted below in Figure #04. Roughly 85% of people infected with the virus improve in a matter of days, however the remaining affected will enter a more severe phase, usually a day after the onset of the acute phase. Symptoms for this include high fever, rapid jaundice, vomiting and abdominal pain - again these are depicted in Figure #04. Mortality occurs in half of these patients within 10-14 days from the onset. (WHO, 2014).
Figure #04: Diagram depicting the different phases of the virus and the symptoms associated with each phase (CDC, 2015).
Yellow Fever Virus
As mentioned before, the Yellow Fever is caused when an individual is infected with the Yellow Fever Virus (YFV).
Classification: Flavivirus
The YFV is part of the family of viruses named Flaviviruses. These viruses are classified as Group IV Viruses (this classification is based on the replicatory processes of the genome of the viruses as classified under the Baltimore Classification of Viruses(Stock et al., 2013)).
Flaviviruses are a group of disease-causing viruses consisting of approximately 70 human or veterinary pathogens and are mostly spread through contact with a mosquito or a tick (Stock et al., 2013). Other prominent viruses that are part of the flavivirus family include the West Nile virus, Dengue virus, Tick-borne Encephalitis virus and also the more recent Zika virus. Originating in Africa, the first case of flaviviruses in America was in the 17th century. Flaviviruses are all similar in size, with diameters ranging from 40-65 nm (Stock et al., 2013). This family of viruses also have relatively the same genome length which is consistently between 9500 and 12500 nucleotides. Full-length genome sequencing of all lineages of the virus examined over the span of decades showed little nucleotide variability, suggesting that the virus has a slow evolution rate and is genetically stable (Stock et al., 2013).
Structure of Yellow Fever Virus
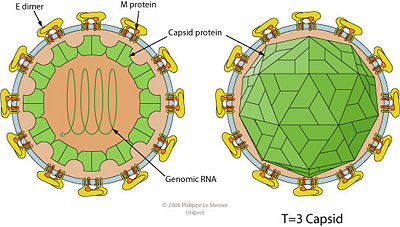
The YFV is spherical in structure and is ~50 nm in diameter. It's structure is based on 3 major types of strucutral proteins that the virus codes for: Envelope (E) Proteins, Membrane (M) Proteins & Capsid (C) Proteins (Barnett, 2007). The structure is depicted in Figure #05. Note that the genome is housed in an icosahedral shape.
Figure #05: Structure of the Yellow Fever Virus. Note the relationship between the structural proteins (E, M & C) as well as the spherical structure of the virus (Barnett, 2007).
Genomic Structure & Sequence
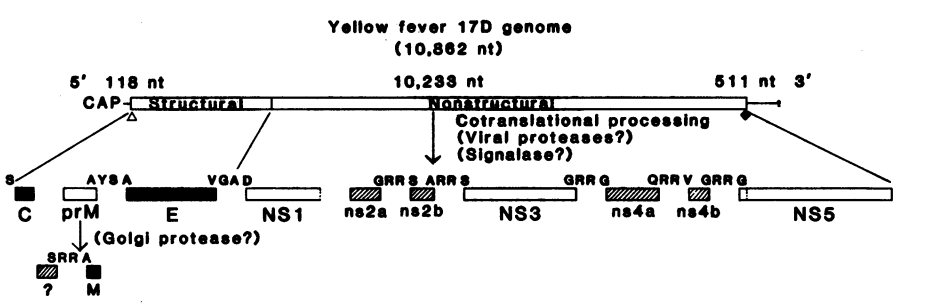
The Yellow Fever Virus (YFV) is a single-stranded, positive RNA Virus. It's RNA genome is 11kb long with the 5' end having a methylated nucleated cap which allows for translation to commence on the 5' end of the genome. The 3' end is not polyadenylated and forms a loop structure (i.e. this cannot be translated into mRNA). This non-polyadenylated tail is important for nuclear export, translation and stability of the mRNA of nucleotides. (Volk et al., 2009). Interestingly, the entire YFV genome consists of only one Open Reading Frame (ORF). An ORF is a continuous stretch of codons that do not contain a stop codon and that can potentially code for proteins or peptides (Volk et al., 2009). This one ORF is depicted in Figure #06 below and codes for 10 proteins in total: 3 structural proteins (C, M & E) and 7 non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5) which are responsible for functions ranging for translation, cytoplasmic transport and viral genome release (Patkar et al., 2007).
Figure 06:Genomic sequence showing the genome and the structural and non-structural proteins coded for in the one ORF of the YFV (Patkar et al., 2007).
By being positive-sense, the viral RNA has the ability to be directly translated into the viral protein since the RNA can be considered the mRNA as well (Rice et al., 1985). Furthermore, YFV, unlike other mosquito-borne diseases is viscerotropic, as it preferentially affects the main organs in the internal cavity of the body such as the kidneys (Rice et al., 1985).
Yellow Fever Virus: Interactions in the Human Body
Recall that the YFV can only be spread to humans via a vector and not through any direct human-human or human-animal interaction. The virus enters the human body during a mosquito bite when the blood from an infected individual on the mouth of the mosquito enters into the blood stream of a non-affected individual.
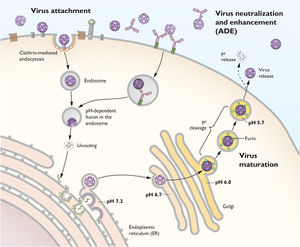
Once the YFV enters the bloodstream it is able to infect those cells that its E proteins are able to preferentially bind to. These susceptible cells are monocytes (Macrophages and / or Dendritic Cells) (Pulendran, 2009). Once the E-proteins on the YFV binds to the receptors on these monocytes the virus is able to enter the cell via Receptor Mediated Phagocytosis. Once it enters the cell an endosome forms around the entered virus where H+ enters the endosome raising the pH of the endosome-virus vesicle (Pulendran, 2009). This increase in pH allows the viral RNA to be released. This viral RNA attaches onto the Rough Endoplasmic Reticulum of the cell where it begins to replicate in “sacs”. In order to replicate it utilizes the host's own RNA-dependent-RNA-polymerase to initiate the replication by switching the positive strand to negative strand and allowing replication to proceed (Pulendran, 2009).
Following replication, immature viral particles enter the Golgi network through vesicles in which the viral particles mature. Maturation simply implies that the viral RNA takes its icosahedron shape and the E proteins on the outer layer of the virion form a dimer. Once they have matured, the vesicles with progeny virion are released into outer environment as the vesicle fuses with the cellular membrane (Pulendran, 2009). The above process is outlined in the diagram below:
Figure #07: Diagram depicting Viral replication of the Yellow Fever Virus.
Immune System Response
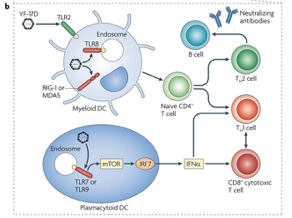
Research has shown that the immune system responses to combat yellow fever can be observed after administering the yellow fever vaccine. Once an individual is vaccinated, researchers are able to analyze the types of immune cells that interact and limit the spread of yellow fever infection (Pulendran, 2009). This is possible either through using a genetic screen that identifies genes which are expressed by immune cells or using flow cytometry to determine specific proteins that are expressed by immune cells. Using these methods, the immune cells that have been identified are dendritic cells (DC), a variety of T cells and B cells (Querec et al., 2009; Gaucher et al., 2008). DCs are important for the immune response against yellow fever as they are the main activators of other adaptive immune cells that directly kill yellow fever infected cells. Research has shown that specific receptors present on the DC called toll-like receptors are able to bind to yellow fever antigens. Upon binding, the DC can then present the yellow fever antigen to naïve CD4+ T cells resulting in the activation of the apoptotic CD8+ T cells as well as neutralizing antibodies that can directly interfere with yellow fever activity in the blood. Please see Figure below for details. Ultimately, immune system recognition of yellow fever results in a well rounded and robust response that involves effective communication between multiple cell types (Pulendran, 2009).
Figure #08: The image represents a diagram showing the different parts involved in the immune response to the Yellow Fever virus (Pulendran, 2009).
Yellow Fever Treatment
Currently, the only way to diagnose someone with Yellow Fever is via a blood test but unfortunately, no specific anti-viral treatment is present to treat the affected individual (Bukowski, 2015). Instead, it is treated by providing the patient with intensive and supportive care in order to allow their adaptive immune system to kick in so that it can combat the YFV (Bukowski, 2015). These care procedures include: a vasoactive medication which works as a vasoconstrictor, thus increasing the patients' blood pressure (Bukowski, 2015). Symptoms such as excessive bleeding and vomiting will cause a huge fluid imbalance in the body. However, administering intravascular fluid will keep the body hydrated and also keep the blood pressure high (Bukowski, 2015). Also, ventilator management is used to support patients' breathing, as these machines give oxygen to the lungs (Bukowski, 2015). Furthermore, you would have to administer the treatment of disseminated intravascular coagulation (DIC), hemorrhage, secondary infections, and renal and hepatic dysfunction. For actively bleeding patients, the administration of fresh frozen plasma is recommended to maintain a prothrombin time of 25-30 seconds (Bukowski, 2015).
Yellow Fever Vaccination
Yellow Fever was the first pathogenic virus for which a live-attenuated vaccine was created back in 1936 (Roukens & Visser, 2008). A live-attenuated vaccine carries a live virus however this virus has been weakened or altered so that it cannot actually infect the vaccinated individual allowing instead, the vaccinated individual to build an 'immunity' for that vaccinated virus. The first two strains created of the virus were: FNV & YF-17D, however, following their dispersion it was seen that the FNV virus caused vaccine related encephalitis and thus it was discontinued (Roukens & Visser, 2008). The YF-17D virus was further developed and today it exists in two forms: YF-17DD & YF-17D-204 (Roukens & Visser, 2008).
These vaccinations can prevent yellow fever and the current vaccine for yellow fever confers 95% lifelong immunity in patients (Roukens & Visser, 2008). Based on the severity of Yellow fever in certain areas, it is recommended to have routine immunization. Currently, world health organization strives for an 80% coverage for the vaccine to achieve immunity for many people to prevent an outbreak (Roukens & Visser, 2008). For travelers from non-endemic areas to endemic areas it is crucial to take this vaccine.
Safety and Side-effects of Current Vaccination Procedures
This vaccine has been administered to over 500 million people worldwide, and has been regarded as a safe drug (Roukens & Visser, 2008). The adverse effects usually occur within 2-6 days after vaccination and include low grade fever, headache, myalgia headache, and redness at the site of the injection (Roukens & Visser, 2008). Only 10-20 % of these symptoms are reported. This vaccination is advised for adults and children over the age of 9 months and under 65 years (Roukens & Visser, 2008). Furthermore, anaphylaxis can occur after vaccination with a risk of 0.8 in 100000 doses. Obtaining history on patients for hypersensitivity to eggs or egg products can reduce the risk of anaphylaxis (Roukens & Visser, 2008). The reason for this is that because this vaccine is grown in an embryonate chicken egg. To test hypersensitivity of patients with egg allergies, they give 0.1ml to patients under strict supervision (Roukens & Visser, 2008). Then a controlled saline dose is given to the other arm and both sites are checked after 30 minutes. If the diameter of the cutaneous wheal of the yellow fever virus is less than twice the control diameter, hypersensitivity to the vaccine is highly unlikely. If it exceeds the diameter two folds, the yellow fever vaccine will not be administered. Moreover, yellow fever vaccination in early pregnancy stages is safe (Roukens & Visser, 2008).
Future procedures?
Over the past 60 years the YF-17D vaccine has not changed. There are limitations on the amount of vaccinations that can be produced on a short notice. This a concern to the Global Alliance of Vaccines, which is supporting the stock pile of over 6 million doses of yellow fever vaccines (Roukens & Visser, 2008). According to the WHO, shortages in vaccine supply is soon to become a problem. In order to make it more economically friendly, they will reduce standard dosages fivefold from 0.5ml to 0.1ml (Roukens & Visser, 2008). Furthermore, injecting inactive yellow fever vaccines in people traveling to endemic areas may provide protection, as there has been trial with the inactive vaccine in mice, finding that all the mice were protected from the virus (Roukens & Visser, 2008).
Summary
To summarize, the yellow fever virus is a small single-stranded RNA virus that causes debilitating effects on the human host upon infection. Studying the yellow fever virus in greater depth provides valuable information for understanding other viruses that are found within the same family. As a flavivirus, YFV is similar to many other prevalent viruses in the world such as the Dengue virus and the Zika virus with respect to their genomes and proteins that are expressed. Unfortunately, variability with these types of viruses is high, as they are highly dependent on environmental changes. Stagnant water and higher climates all contribute to higher incidence of yellow fever virus because of the fact that they are mosquito-borne. A lot of speculation has been made regarding ways to manage the illness since there is no direct cure. Safely administered vaccinations have been constructed from a significant strain of the YFV but also have adverse side effects and age limitations. Ultimately, advancements in medical treatments will one day be able to cure the yellow fever virus; however, as of now, the virus continues to prevail, especially in equatorial countries or developing countries with a lack of a strong healthcare system.
References
1. Barnett, E. D. (2007). Yellow Fever: Epidemiology and Prevention. Clinical Infectious Diseases, 44(6), 850-856.
2. Busowski, M. T. (2015, June 26). Yellow Fever. Retrieved,from http://emedicine.medscape.com/article/232244-overview
3. CDC (2015, August 13). Yellow Fever: Transmission. Retrieved from http://www.cdc.gov/yellowfever/transmission
4. CDC (2015, August 13). Yellow Fever: Symptoms and Treatment. Retrieved from http://www.cdc.gov/yellowfever/symptoms/index.html
5. Fontenille, D., Diallo, M., Mondo, M., Ndiaye, M., & Thonnon, J. (1997). First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Transactions of the Royal Society of Tropical Medicine and Hygiene, 91(5), 533-535.
6. Gaucher, D., Therrien, R., Kettaf, N., Angermann, B. R., Boucher, G., Filali-Mouhim, A., … & Akondy, R. (2008). Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. The Journal of experimental medicine, 205(13), 3119-3131.
7. Monath, T. P. (2001). Yellow fever: An update. The Lancet Infectious Diseases, 1(1), 11-20.
8. Patkar, C.G., Jones, C.T., Chang, Y., Warrier, R., & Kuhn, R.J. (2007). Functional Requirements of the Yellow Fever Virus Capsid Protein. Journal of Virology, 81(12), 6471-6481.
9. Pulendran, B. (2009). Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nature Reviews Immunology, 9(10), 741-747.
10. Querec, T. D., Akondy, R. S., Lee, E. K., Cao, W., Nakaya, H. I., Teuwen, D., … & Kennedy, K. (2009). Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nature immunology, 10(1), 116-125.
11. Rice, C.M., Lenches, E.M., Eddy, S.R., Shin, S.J., Sheets, R.L., & Strauss J.H. (1985) Nucleotide Sequence of Yellow Fever Virus: Implications for Flavivirus Gene Expression and Evolution. Science, 229(4715), 726-733
12. Roukens, A. H., & Visser, L. G. (2008). Yellow fever vaccine: past, present and future. Expert opinion on biological therapy, 8(11), 1787-1795.
13. Stock, N.K., Laraway, H., Faye, O., Diallo, M., Niedrig, M., & Sall, A.A. (2013). Biological and Phylogenetic Characteristics of Yellow Fever Virus Lineages from West Africa. Journal of Virology, 87(5), 2895-2907.
14. Volk, D.E., May, F.J., Gandham, S.H., Anderson, A., Von Lindern, J.J., Beasley, D.W., Barrett, A.D., & Goreinstein, D.G. (2009). Structure of Yellow Fever Virus Envelope Protein Domain III. US National Library of Medicine, 394(1), 12-18.
15. WHO (2014, March 1). Yellow Fever. Retrieved from http://www.who.int/mediacentre/factsheets/fs100/en/