Table of Contents
Chronic Traumatic Encephalopathy (CTE)
Introduction
Chronic Traumatic Encephalopathy (CTE) is a type of neurodegenerative disease found most commonly in people who have had multiple injuries to the head, mainly athletes that competed in contact sports (Asken et al., 2017). Symptoms include cognitive issues, changes in behavior, and lapses in memory. Most symptoms first start appearing years after the injuries, and will often get worse with time. In many cases, CTE can deteriorate into dementia (Asken et al., 2017). The only definitive means of diagnosis is through autopsy (Asken et al., 2017). It has been found to occur in approximately 30% of people with multiple head injuries (Asken et al., 2017). However, it is unclear how many head injuries increase the risk for developing the disease (Asken et al., 2017). Currently, there are no known treatments for CTE. Research is being conducted in an effort to find early diagnostic markers. Prevention of head injuries are the best methods of minimizing the risk of developing the disease.
Epidemiology
CTE is linked to exposure to repetitive mild traumatic brain injury (MTBI) (Gardner & Yaffe, 2015). The largest modern autopsy series that looked at 85 patients (consisting of athletes and veterans) with exposure to repetitive MTBI, found CTE in 80% of patients, proving this correlation (Gardner & Yaffe, 2015).
Furthermore, in a review, McKee et al. discovered 90% of 51 cases of CTE occurred in athletes primarily playing sports such as football, boxing, soccer and hockey (Saulle & Greenwald, 2012). The exact incidence of CTE is unknown, but believed to vary on factors such as sport, career duration, number of head injuries, age of first head injury and genetics (Saulle & Greenwald, 2012).
The first diagnosis of CTE was made in 2002 based on a professional American football player (Gardner & Yaffe, 2015). American football continues to be the sport with the highest association with autopsy-proven CTE (Gardner & Yaffe, 2015). For instance, there was a recent autopsy series reporting 50-97% prevalence rates of CTE among professional American football players; however, these reports are limited by referral bias (Gardner & Yaffe, 2015).
Despite these findings, the epidemiology of CTE in the general population is largely unknown because it is a neuropathological diagnosis (Gardner & Yaffe, 2015). Researchers have been unable to conduct large population-based studies of CTE because of the absence of consensus clinical criteria (Gardner & Yaffe, 2015). This means incidence rates can only be inferred, either from autopsies or by looking at the prevalence of suggestive clinical syndromes in populations exposed to repetitive MTBI (Gardner & Yaffe, 2015). These populations include athletes or veterans for example. However the challenge with this approach is that the lack of consensus clinical criteria creates referral bias (Gardner & Yaffe, 2015). Referral bias is when results cannot be applied to the general population. Furthermore, by looking at the association between neurodegenerative syndromes and TBI in cases that do not have autopsy confirmation, the clinical diagnosis of the neurodegenerative syndrome may not be accurate (Gardner & Yaffe, 2015). CTE consensus clinical criteria is also required because of the overlap between CTE symptoms and other common neurodegenerative syndromes (Gardner & Yaffe, 2015). Therefore, it is crucial for researchers to discover unique clinical features of CTE and refine CTE biomarkers (Gardner & Yaffe, 2015). It is also important to conduct longitudinal studies of high risk groups to have better quantification of MTBI exposure with risk of CTE and risk factors for CTE (Gardner & Yaffe, 2015). This is because it is difficult to know the amount of MTBI exposure required to produce CTE pathology. There are ongoing efforts to already examine this in boxers and veterans.
Other challenges in determining the epidemiology of CTE include how CTE symptoms may not appear until years or decades after MTBI exposure and this can be influenced by issues such as recall bias (Gardner & Yaffe, 2015). Recall bias is when patients with affected memory may be more likely or less likely to report MTBI exposure.
Etiology
Cause of CTE:
Chronic traumatic encephalopathy or CTE is a disease that is caused by repetitive head trauma or repetitive hits to the head (DeKosky, Blennow, Ikonomovic & Gandy, 2013). This progressive degenerative disease affects individuals who play contact sports such as American football, members of the military who experience blast injuries, individuals who repeatedly bang their heads, and from physical abuse (“What is CTE?”, 2018). Individuals who play contact sports such as American football, wrestling, and boxing, are most prone to such brain injuries (DeKosky, Blennow, Ikonomovic & Gandy, 2013). Many experience concussions and knockouts that occur when when a hit to the head or a sudden jolt shakes the head and causes the brain to move around inside the skull (Lakhan & Kirchgessner, 2012). Studies show that American football players have the highest risk to developing CTE, with over 200 cases per year, followed by boxers at 65+ annual cases and hockey players at 20 + cases (“What is CTE?”, 2018).
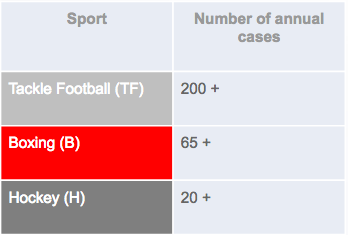 Figure 1: A table showing the risk of contact sports in developing CTE (“What is CTE?”, 2018).
Figure 1: A table showing the risk of contact sports in developing CTE (“What is CTE?”, 2018).
Risk Factors
The major risk for developing CTE is trauma to the central nervous system, therefore individuals who experience more head trauma are at a higher risk (DeKosky, Blennow, Ikonomovic & Gandy, 2013). Other factors such as the age at first head trauma and the length of the exposure to the head trauma also need to be taken into account and can increase risk to developing the disorder. Studies show that individuals who experience their first head trauma before the age of 12 are at a higher risk for developing CTE and have worse outcomes (Stamm et al., 2015). Furthermore, individuals who have a career with longer exposures to head trauma have a higher risk for CTE than those with a shorter career (Lakhan & Kirchgessner, 2012). Further research needs to be done on the genetic risk factors of CTE, as some have been proposed but not yet confirmed (DeKosky, Blennow, Ikonomovic & Gandy, 2013).
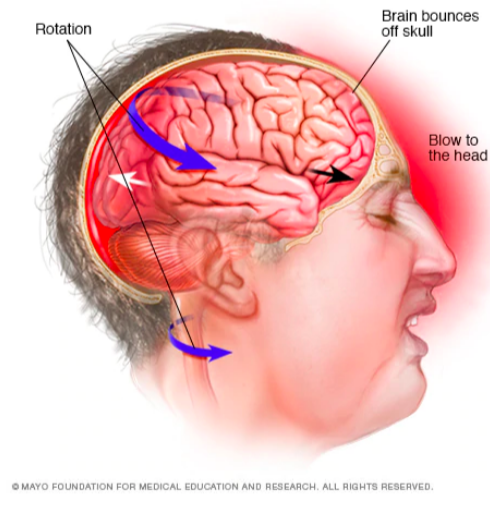 Figure 2: Repetitive head trauma is the number one risk factor for CTE ((“Chronic traumatic encephalopathy - Symptoms and causes”, 2018).
Figure 2: Repetitive head trauma is the number one risk factor for CTE ((“Chronic traumatic encephalopathy - Symptoms and causes”, 2018).
Symptoms
Symptoms of Chronic traumatic encephalopathy (CTE) have not been well-defined yet due to lack of research and limitations in studies. However, based on present knowledge, the symptoms develop in 3 stages. First, there are deteriorations in memory, attention and concentration. Symptoms observed in the first stage include disorientation, headaches, confusion, short-term memory loss and dizziness. Next, as deteriorations progress, patients have poor critical skills, lack of insight and can develop dementia. Some of the symptoms seen in stage 2 are erratic behavior, social instability and preliminary symptoms of Parkinson disease. With progressive cognitive dysfunction, overt dementia is seen. Advanced dementia in addition to gait and speech abnormalities appear during the third stage (McKee et al., 2009).
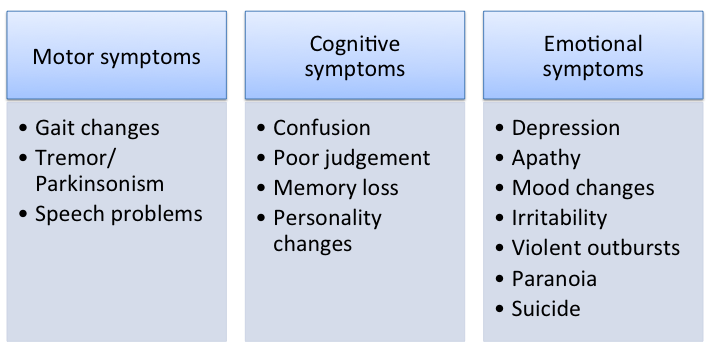 Figure 3: Overview of the various symptoms of CTE.
Figure 3: Overview of the various symptoms of CTE.
Other suspected symptoms include dysphagia (difficulty with swallowing), dysarthria (motor speech disorder), irritability, suicidal thoughts and vision problems (“Chronic Traumatic Encephalopathy”, 2016). Advanced cases usually include impeded speech, vertigo, deafness and slowing of muscular movements. The severity of CTE is correlated with the amount and rigorousness of traumatic brain injuries (McKee et al., 2009).
Diagnosis
There is currently no dependable way to diagnose CTE. Since the diagnosis requires evidence of brain tissue degeneration and of deposits of tau and other proteins in the brain, it can only be detected upon examination after death (autopsy). Researchers are working persistently to find a test that can detect CTE while the patient is still alive. Some of the tests researchers are working on include finding biomarkers and brain imaging to diagnose CTE (Reams et al., 2016).
Biomarkers: Biomarkers hold the promise of furthering our understanding of the clinical and pathologic spectrum of CTE, and is believed to have the ability to diagnose CTE (Reams et al., 2016). However, at the present time, there are no reliable biomarkers existing to detect CTE. While significant decreases in CSF Apolipoprotein E (ApoE) and Beta amyloid (Aβ) concentrations have been reported that correlated with severity of the injury after traumatic brain injury (TBI), there have been no similar studies in CTE (McKee et al., 2009).
Brain-imaging tests: Brain-imaging tests are currently being used to diagnose TBI. Although there are no tests that can diagnose CTE currently, researchers believe the following tests have the potential to detect CTE in the future.
Specialized Magnetic resonance imaging (MRI) tests:
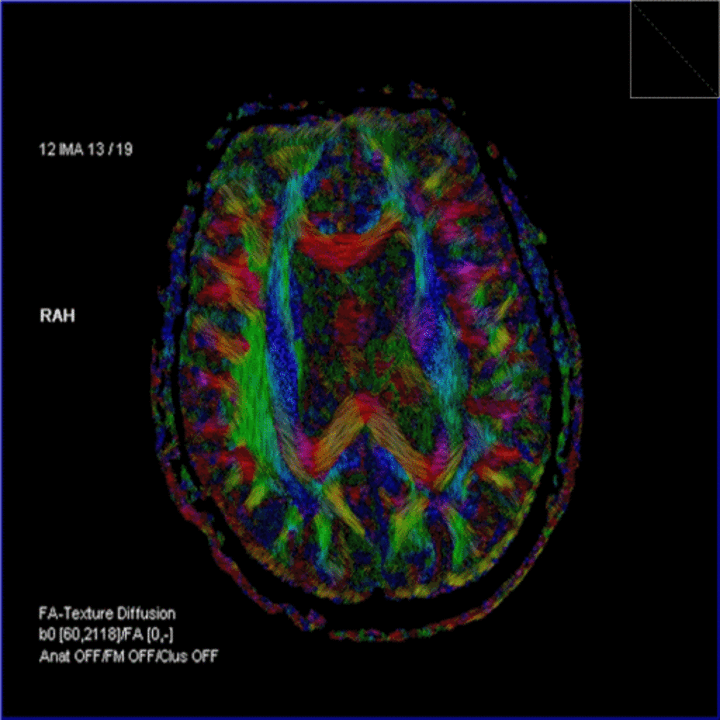
- Diffusion tensor imaging (DTI) is a type of MRI that reveals the movement of water and the path of white matter in the brain, which can show brain abnormalities. It shows promise for detecting CTE, but needs to become more accurate and precise.
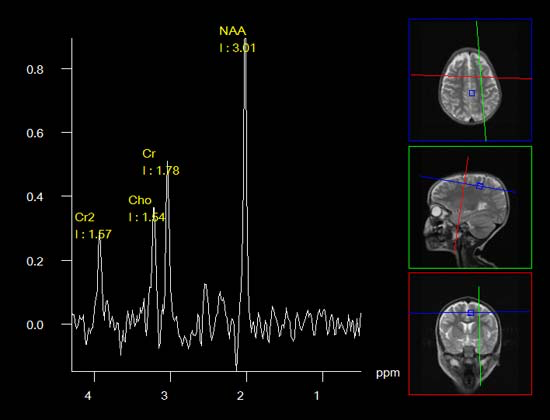
- Magnetic resonance spectroscopy (MRS) is similar to MRI but may be able to provide greater details about neurological damage. For instance, MRS allows researchers and physicians to derive biochemical information about the tissues of the human body in a non-invasive way, whereas MRI only gives them information about the structure of the body.
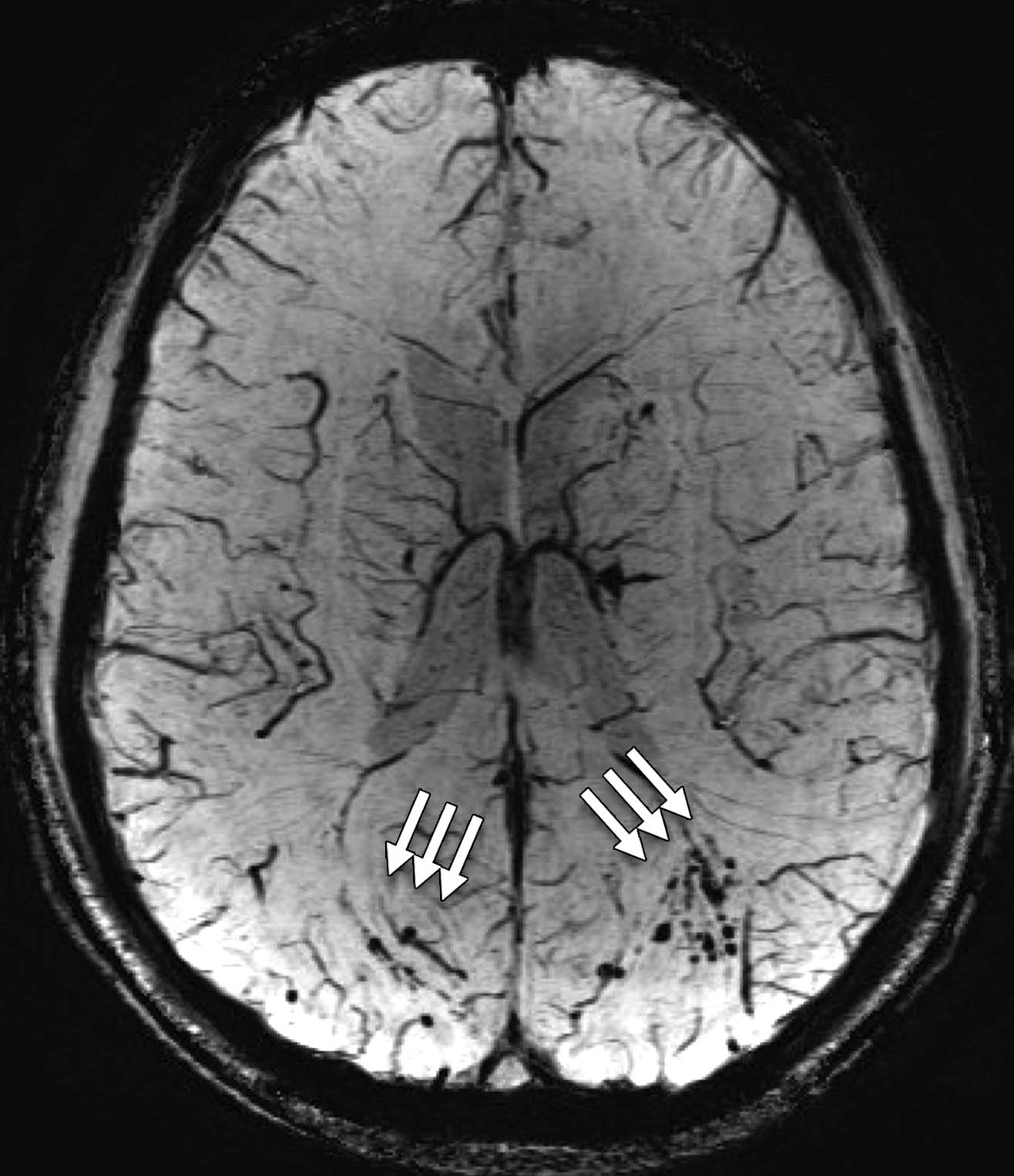
- Susceptibility-weighted imaging (SWI) is a type of MRI that shows tiny bleeds (hemorrhages) that result from injury to the central nervous system (Baugh et al., 2012).
Figure 4: (Left to right) Image of a DTI scan of the brain; image of a MRS scan of the brain; and image of a SWI scan of the brain
Event-related potentials (ERPs) and quantitative EEG: These noninvasive tests use electroencephalography (EEG), in which a mesh cap covered with electrodes is placed on a person's head. It allows doctors to detect, record and analyze brain waves, which may find brain changes that result from multiple traumatic brain injuries (Baugh et al., 2012).
Positron emission tomography (PET): A PET scan uses a low-level radioactive tracer that is injected in a vein. Then, a scanner tracks the tracer's flow through the brain. Researchers are actively working to develop PET markers to detect tau abnormalities associated with neurodegenerative diseases. The goal is to develop a marker to identify the tau pathology of CTE in people who are alive. Researchers are using various substances that bind to tau and other proteins on PET scans. (Reams et al., 2016).
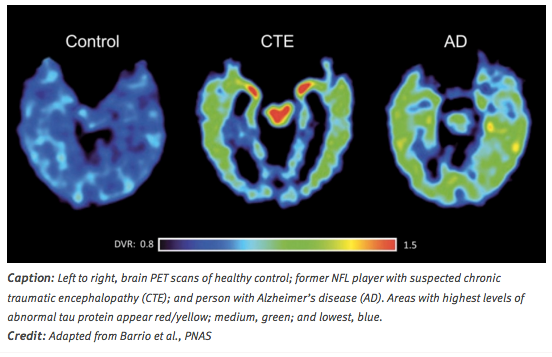 Figure 5: (Left to right) PET scans of a healthy brain, CTE patient and a person with Alzheimer's disease.
Figure 5: (Left to right) PET scans of a healthy brain, CTE patient and a person with Alzheimer's disease.
Pathophysiology and Mechanisms
Currently, the neurological effects of chronic traumatic encephalopathy (CTE) can only be studied post-mortem. The pathophysiology of the disease is unclear as there is a lack of clinical evidence and the symptoms can also be attributed to other factors such as aging, medications, and other diseases (Petraglia et al., 2014).
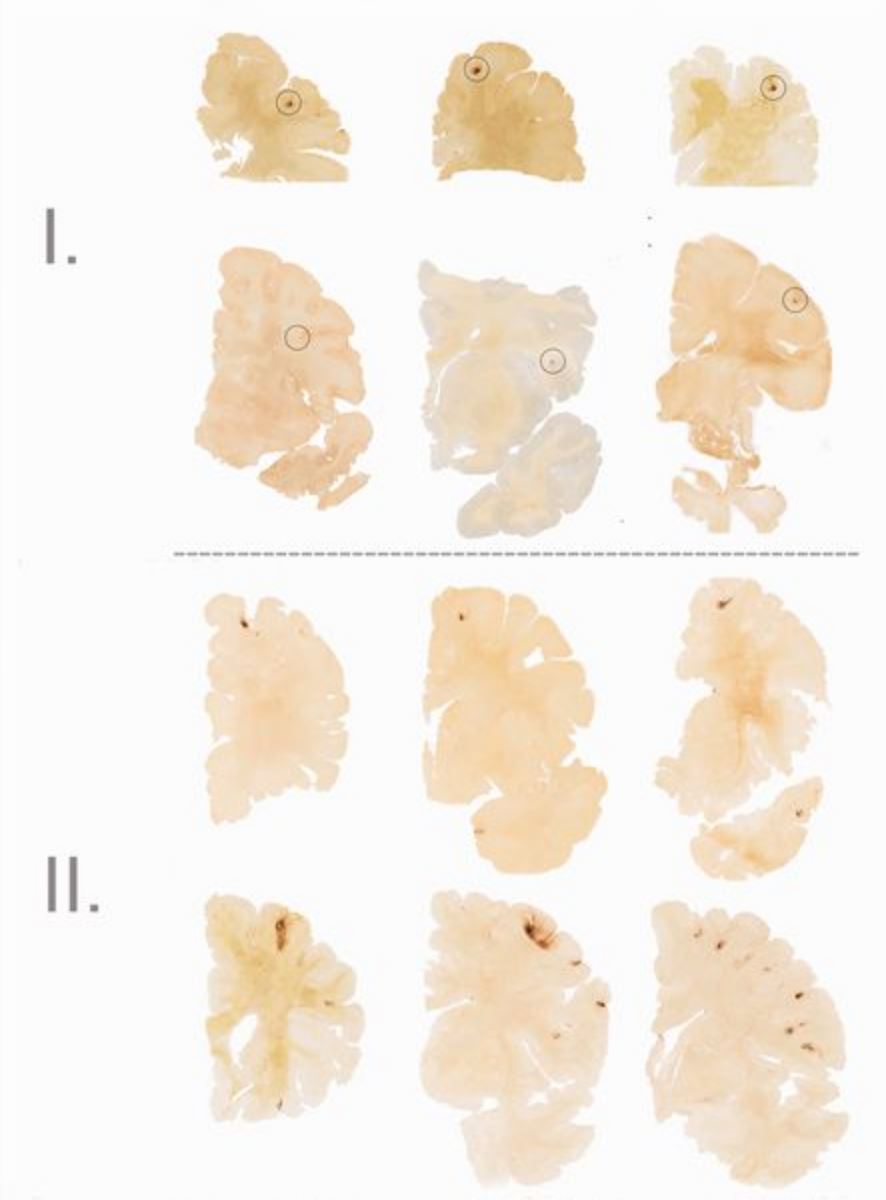
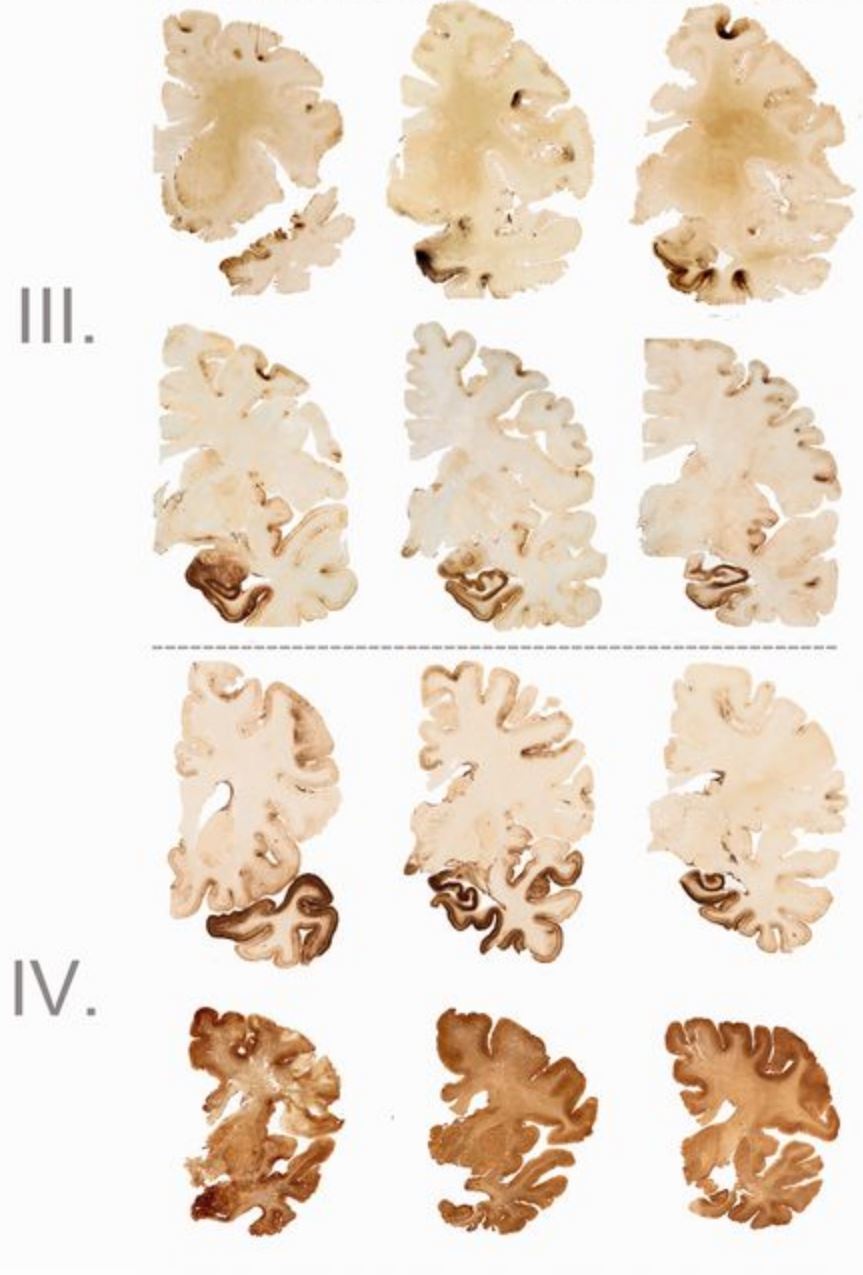
To be diagnosed with CTE as opposed to other neurological diseases such as Alzheimer’s disease, the following four criteria need to be present in the brain tissue: the presence of phospho-tau (p-tau) around the brain's blood vessels, irregular cortical distribution of p-tau immunoreactive neurofibrillary tangles and astrocytic tangles (with a preference for the cerebral sulci), groups of subpial and periventricular astrocytic tangles in the cerebral cortex, diencephalon, basal ganglia and brain stem, and finally the presence of neurofibrillary tangles located preferentially in the superficial layers of the cerebral cortex (McKee et al., 2013). Other pathological findings from individuals diagnosed with CTE are general atrophy (with greatest concentration in the frontal and temporal lobes, somewhat in parietal lobe, and nearly none in occipital lobe), scarring of cerebellar tonsils, a fenestrated cavum septum pellucidum, and enlargement of the ventral and third ventricles (Yi, Padalino, Chin, Montenegro, and Cantu, 2013).
Figure 5: (Left to right) Degeneration of brain tissue associated with each stage of CTE (McKee et al., 2013)
In one study, researchers evaluated the development of CTE by exposing unanesthetized mice to closed head impacts. There were three groups in this study: control, single-hit, and repetitive. The study found increased p-tau immunoreactivity in the single-hit mice group and the repetitive-hit group after one month. Both groups also had elevated inflammatory cytokine levels, mediated by microglial and astroglial cells. However, after six months, the single-hit group recovered substantially while the repetitive-hit group’s p-tau immunoreactivity increased significantly. The repetitive-hit group also had substantial and widespread microglial and astroglial activation, indicating a major neuroinflammatory response (Petraglia et al., 2014).
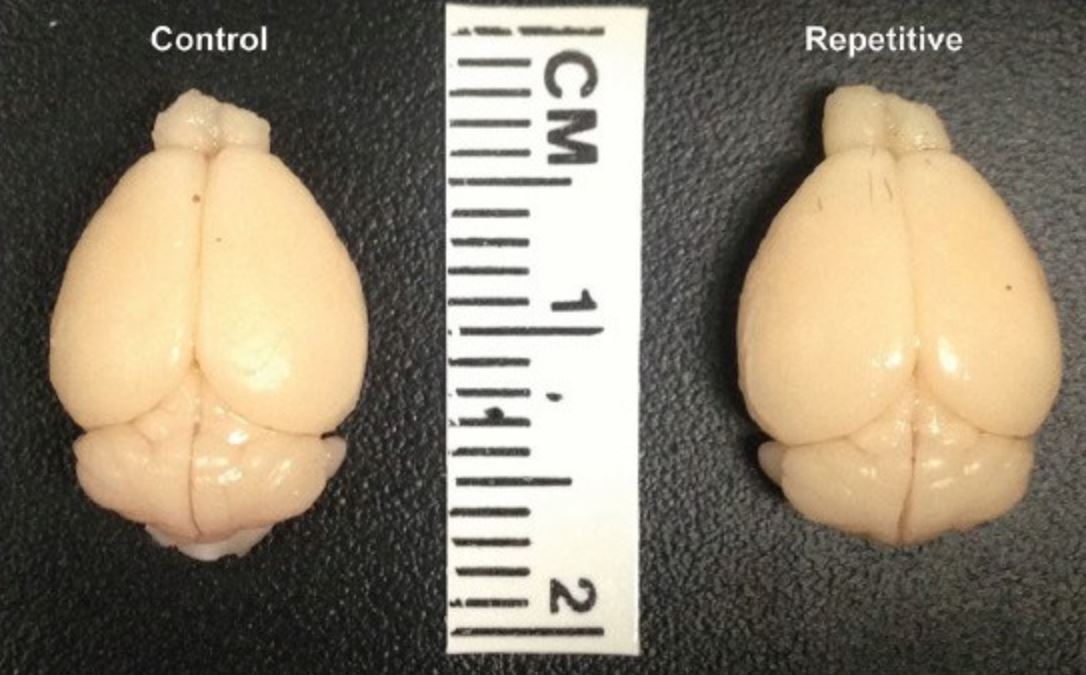 Figure 6: Comparison of brains taken from a control mouse and a repetitive-hit mouse (Petraglia et al., 2014)
Figure 6: Comparison of brains taken from a control mouse and a repetitive-hit mouse (Petraglia et al., 2014)
In another study, immunoexcitotoxicity was explored as a potential mechanism for CTE. Excitotoxicity refers to the apoptotic reaction that occurs when neurons are exposed to excessive amounts of glutamate. When any brain injury occurs, glutamate is released by the microglia and astrocytes, to facilitate the entry of proinflammatory cytokines for our immune response. These cytokines increase neuronal sensitivity to glutamate, which can result in damages due to the increased sensitivity. These damages include mitochondrial suppression, synaptic injury, damage to microtubules, dendritic retraction, and the accumulation of hyperphosphorylated tau (Blaylock and Maroon, 2011).
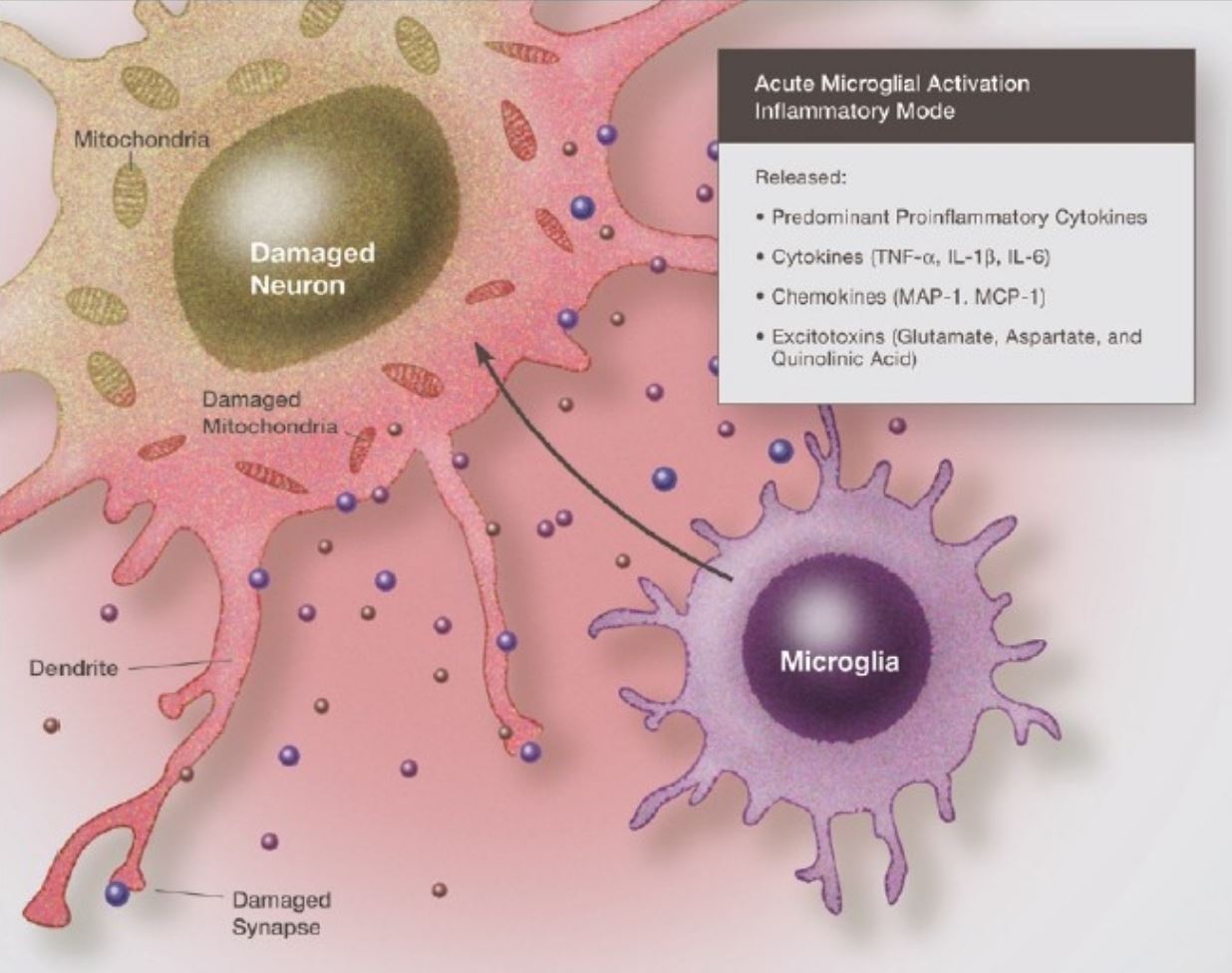 Figure 7: Illustration of the neurotoxic effects of proinflammatory cytokines and chemokines acting together with amino acids (mainly glutamate) (Blaylock and Maroon, 2011)
Figure 7: Illustration of the neurotoxic effects of proinflammatory cytokines and chemokines acting together with amino acids (mainly glutamate) (Blaylock and Maroon, 2011)
Treatment
Currently, the treatment methodologies for CTE are entirely preventative (Saulle & Greenwald, 2012). Prevention of CTE is highly problematic due to the nature of CTE-inducing sports such as American football, where head collisions are a part of the sports identity (Saulle & Greenwald, 2012). Therefore, prevention requires various approaches involving administrators, coaches, players, referees, team physicians, and equipment (Saulle & Greenwald, 2012).
Administrators create policies and penalize athletes for dangerous hits and sets equipment standards (Saulle & Greenwald, 2012). Coaches are responsible for educating their players on safe techniques on tackling and hitting, and to limit full contact practice drills (Saulle & Greenwald, 2012). It is the role of the players to understand the potential consequences of head trauma so that they can protect themselves during their career (Saulle & Greenwald, 2012). The role of referees is to create a safe playing environment and upholding rules to protect players (Saulle & Greenwald, 2012). The task of the team physicians is to remove injured players from play and manage their MTBI until they are safe to play again (Saulle & Greenwald, 2012).
Another crucial aspect of prevention is the improvement of protective gear worn by athletes (Saulle & Greenwald, 2012). It has been shown that properly fitting and padded helmets as well as mouthguards function well in protecting players from severe head injury (Saulle & Greenwald, 2012). A study conducted by Viano and Halstead has shown that newer American football helmets are heavier, more padded, and larger than older helmets, and were better at absorbing impacts (Saulle & Greenwald, 2012).
Despite being untreatable, the symptoms of CTE can be treated and managed. According to the Alzheimer's Association (n.d.), treatment of CTE symptoms is similar to treatment of the symptoms of Alzheimers. An example is the treatment of aggression using behavioral methods, or the treatment of depression using medication and therapy (Alzheimer's Association n.d.). Furthermore, as with Alzheimer’s and dementia patients, a calming environment helps the patient to maintain focus and function and modified tasks help section tasks into easier steps (Alzheimer's Association n.d.).
Research
The first known case of CTE in football was published in 2005 by forensic pathologist Bennet Omalu along with his team, based on the autopsy conducted on former NFL player Mike Webster (Ommalu et al. 2005). Another paper was published the following year citing the same condition found in the brain of Terry Long, another former NFL player. Currently, there has not been enough research conducted on CTE to determine in development and progress. As of 2018, the majority of research has been focused on building a database from brain donations of professional athletes. However, research will continue to be slow in progression since brains can only be analyzed after death.
In 2013, former president Barack Obama announced the creation of federally funded research projects aimed at investigating long term effects of brain injury, including CTE (Bryant, 2013). The projects are being lead by Virginia Commonwealth University along with research groups across the United States (Fact Sheet: Largest federal grant in VCU's history, 2013). As of recently, the focus has been on conducting longitudinal studies aimed at following participants over time and studying the disease’s progression.
Final Remarks
In conclusion, discovery of CTE has been relatively new and clouded with controversy. Due to its recent discovery, not much is known about the disease or its development. However, it has been directly linked to people that have suffered multiple brain injuries. Therefore, protecting the brain from concussions and trauma is the best way to prevent the development of CTE. Hopefully, future research will provide more information on the disease and its pathology, as well as early diagnostic methods.
References
Alzheimer's Association (n.d.). Chronic Traumatic Encephalopathy (CTE). Retrieved from https://www.alz.org/alzheimers-dementia/what-is-dementia/related_conditions/chronic-traumatic-encephalopathy-(cte)
Asken B., Sullan M., DeKosky S., Jaffee M., Bauer R. (2017). Research Gaps and Controversies in Chronic Traumatic Encephalopathy: A Review. JAMA Neurology. 74 (10): 1255–1262. doi:10.1001/jamaneurol.2017.2396. PMID 28975240
Baugh, C. M., Stamm, J. M., Riley, D. O., Gavett, B. E., Shenton, M. E., Lin, A., Stern, R. A. (2012). Chronic traumatic encephalopathy: Neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging and Behavior, 6(2), 244-254. doi:10.1007/s11682-012-9164-5
Blaylock, R. L., & Maroon, J. (2011). Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy—a unifying hypothesis. Surgical neurology international, 2.
Bryant, J. (2013). Obama Introduces New PTSD and Education Programs. military.com.
Chronic Traumatic Encephalopathy. (2016). Retrieved October 26, 2018, from https://www.mayoclinic.org/diseases-conditions/chronic-traumatic-encephalopathy/symptoms-causes/syc-20370921
DeKosky, S., Blennow, K., Ikonomovic, M., & Gandy, S. (2013). Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nature Reviews Neurology, 9(4), 192-200. doi: 10.1038/nrneurol.2013.
Fact Sheet: Largest federal grant in VCU's history. (2013). spectrum.vcu.edu.
Gardner, R. C., & Yaffe, K. (2015). Epidemiology of mild traumatic brain injury and neurodegenerative disease. Molecular and Cellular Neuroscience, 66, 75-80.
Goldstein, L. E., Fisher, A. M., Tagge, C. A., Zhang, X. L., Velisek, L., Sullivan, J. A., … & Goletiani, C. J. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Science translational medicine, 4(134), 134ra60-134ra60.
Lakhan, S., & Kirchgessner, A. (2012). Chronic traumatic encephalopathy: the dangers of getting “dinged”. Springerplus, 1(1), 2. doi: 10.1186/2193-1801-1-2
McKee, A. C., Cantu, R. C., Nowinski, C. J., Hedley-Whyte, E. T., Gavett, B. E., Budson, A. E., Santini, V. E., Lee, H. S., Kubilus, C. A., … Stern, R. A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. Journal of neuropathology and experimental neurology, 68(7), 709-35.
McKee, A. C., Stein, T. D., Nowinski, C. J., Stern, R. A., Daneshvar, D. H., Alvarez, V. E., … & Riley, D. O. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain, 136(1), 43-64.
Omalu B., DeKosky S., Minster R., Kamboh I., Hamilton R., Wecht C. (2005). Chronic Traumatic Encephalopathy in a National Football League Player, Neurosurgery, Volume 57, Issue 1, Pages 128–134, https://doi.org/10.1227/01.NEU.0000163407.92769.ED
Petraglia, A. L., Plog, B. A., Dayawansa, S., Dashnaw, M. L., Czerniecka, K., Walker, C. T., … & Huang, J. H. (2014). The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surgical neurology international, 5.
Reams, N., Eckner, J. T., Almeida, A. A., Aagesen, A. L., Giordani, B., Paulson, H., Lorincz, M. T., … Kutcher, J. S. (2016). A Clinical Approach to the Diagnosis of Traumatic Encephalopathy Syndrome: A Review. JAMA neurology,73(6), 743-9.
Saulle, M., & Greenwald, B. D. (2012). Chronic traumatic encephalopathy: a review. Rehabilitation research and practice, 2012.
Stamm, J., Bourlas, A., Baugh, C., Fritts, N., Daneshvar, D., & Martin, B. et al. (2015). Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology, 84(11), 1114-1120. doi: 10.1212/wnl.0000000000001358
What is CTE?. (2018). Retrieved from https://concussionfoundation.org/CTE-resources/what-is-CTE
Yi, J., Padalino, D. J., Chin, L. S., Montenegro, P., & Cantu, R. C. (2013). Chronic traumatic encephalopathy. Current sports medicine reports, 12(1), 28-32.