This is an old revision of the document!
Table of Contents
Chronic Obstructive Pulmonary Disease (COPD)
Introduction
A Genetically Modified Organism is any organism created by gene splicing techniques and often involves merging DNA from different species (31). Scientists directly manipulate an organism’s genome to isolate traits or characteristics deemed of value. Currently, the safety of GMOs is under research as they are relatively recent developments (22).
What is COPD
Prevalence and Epidemiology of the Disease
Economic Burden of COPD
Mechanism of Disease
Causes and Pathophysiology
Environmental Factors - Air Pollution
Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Matheson, M.C., et al., performed a cross sectional study of the risk factors of COPD relating to job history of 1200 participants. Those exposed to biological dust had increased risk of chronic bronchitis, emphysema and COPD with incidence being higher in women compared to men even though they reported less exposure to biological dust than men. Biological dust includes microbes such as plant and animal, bacteria, fungi, allergens, endotoxins, peptidoglycans, glucans and pollens. Occupations affected by biological dust are those in the cotton industry, farmers, grain handlers, bakers, saw mill workers, nurses, artists, and cleaners. The duration and type of exposure plays a role in increasing risk of COPD. Gas, fumes, and mineral dust do not pose a risk. The only way to reduce risk is to reduce exposure to these in the workplace (Matheson, M., 2005).
Outdoor Air Pollution Urban areas have the worst air pollution with some countries having more air pollution than others. Air pollutants have harmful effects on the airways, they increase oxidative stress, pulmonary and systemic inflammation, amplification of viral infections and reduction in air ciliary activity (Ko, F. & Hui, D., 2012). Higher traffic density is proven to be associated with higher pollution and lower forced expiratory volume (Ko, F. & Hui, D., 2012). Therefore it is biologically possible that air pollutants damage lungs, but it is not determined to be a causative factor due to lack of long-term studies.
Indoor Air Pollution Indoor air pollutants include tobacco smoke and biological allergens/ exposure are the major indoor pollutants. A cross sectional study in China and the USA proved that tobacco smoke from others is associated with development of COPD (Ko, F. & Hui, D., 2012). Biomass as a fuel source includes burning wood, crop residues, and animals during cooking or heating the home which releases toxins. Toxins are released because these fuels have low combustion efficiency. In rural and developing countries biomass fuel burning happens inside resulting in increased amounts of indoor air pollution (Ko, F. & Hui, D., 2012). Women are also more affected by indoor and outdoor air pollution (Ko, F. & Hui, D., 2012). COPD results from the combination of genetic and environmental factors; having more than one risk factor increases odds of getting the disease.
Environmental Factors- Smoking
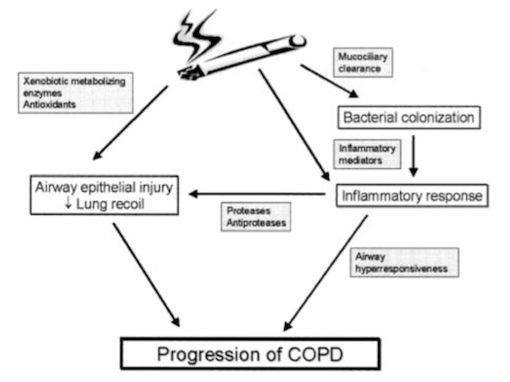
Noxious agents produced from cigarette smoke have a detrimental effect on the airways. The toxins damage airway epithelium and drive the processes that lead to certain airway inflammation and structural changes (Bourdin et al., 2009). Accordingly, cigarette smoking is the leading cause of COPD in North America (Bourdin et al., 2009). All smokers exhibit inflammation within their lungs, but those who have developed COPD demonstrate amplified or abnormal responses to inhaling noxious agents (MacNee, 2006). Airway remodelling may be a protective mechanism of airway epithelium to injury caused by cigarette smoke. Furthermore, the subsequent structural changes may be a reparative mechanism in response to damage caused by the toxic substance (Chung, 2005).
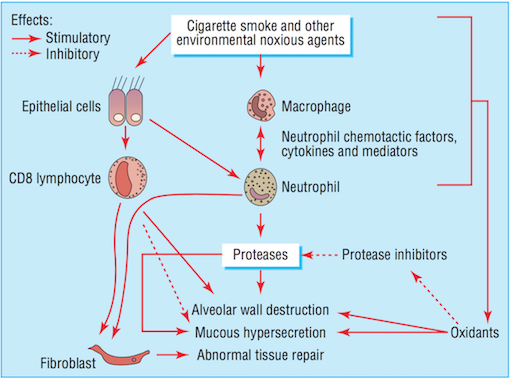
Bronchiolar epithelium is altered in smokers, notably in COPD patients (Chung, 2005). Bronchiolar epithelial cells are triggered by interleukin (IL)-1, interleukin (IL)-8, and granulocyte colony-stimulating factor causing them to release increased levels of neutrophil and monocytic chemotactic activities (Chung, 2005). Structural changes to the airway take place as a result of abnormal repair processes promoted by activated fibroblasts. Fibroblast activation is induced by the increased production of neutrophil, macrophages, and lymphocytes (MacNee, 2006). Moreover, an influx of neutrophils and macrophages result in an imbalance between proteases and anti-proteases. The imbalance of proteases and imbalances ultimately leads to destruction of the alveolar wall, while an overabundance of proteases alone results in the increased production and release of mucous (MacNee, 2006). Alveolar wall destruction and the presence of excessive mucous are both distinct characteristics of COPD (Bourdin et al., 2009).
Biological Perspectives- Structural Changes
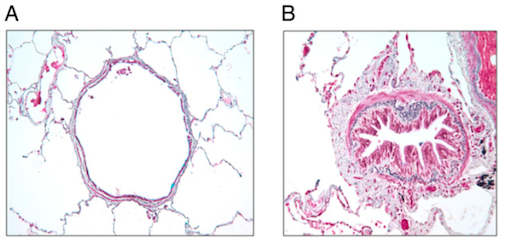
Chronic airflow obstruction is associated with airway remodelling and inflammation of peripheral airways. Airway remodelling, consistent with structural changes found in the airways of COPD patients, are more prominent in the small airways than in the large airways (Chung, 2005). Small airways are defined as peripheral airways that are less than 2mm in internal diameter (Berge et al., 2011). A strong association exists between disease progression and an increase in epithelium, lamina propria, adventitial compartments of the airway walls, and smooth muscle. The amount of smooth muscle is increased by nearly 50% in patients diagnosed with more severe COPD, stages 3 or 4, as categorized by the Global Initiative on Obstructive Lung Disease (GOLD) (Chung, 2005). Lung functionality is inversely correlated with the amount of smooth muscle found in the small airway (Chung, 2005). Structural changes, such as muscle thickening, may lead to muscle shortening against elastic loads. Muscle thickening also increases ability to generate force, which may play a part in bronchial hyper-responsiveness, loss of lung recoil, and fibrosis of the small airways (Chung, 2005).
Smooth muscles of the small airways not only demonstrate contractile properties, but may also contribute to the inflammatory and airway wall remodelling processes. This takes place as a result of the expression or release of cytokines, chemokines, proteases, and growth factors (Chung, 2005). Increased production of cytokines and chemokines increases the number and activation of neutrophil, a granulocyte associated with COPD (Bourdin et al., 2009). Notable cytokines and chemokines include interleukin (IL)-8 and CXC chemokine ligand 10 (CXCL10), respectively. IL-8 is a powerful chemoattractant and activator of neutrophil, and the receptor is present on blood and sputum neutrophils and epithelial cells (Bourdin et al., 2009). CXCL10 is also a chemoattractant for neutrophil as well as for human monocytes, natural killer cells, and T cells (Chung, 2005).
Neutrophil levels are elevated in COPD patients, resulting in the increased release of proteases and oxidants (Bourdin et al., 2009). The imbalance of proteases and anti-proteases, as well as the imbalance of oxidants and antioxidants (MacNee, 2006) perpetuate the progression of processes in favour of lung destruction (Bourdin et al., 2009).
Biological Perspectives- Genetics
Genes might play a significant role in COPD, and might even explain why some people who smoke develop the disease and others do not. There is a genetic component accounting for a small number of COPD cases associated with alpha1-antitrypsin protein deficiency (AATD). Treatment for this involves increasing amounts of AAT protein in the body through intravenous injection of the protein derived from human plasma, therefore halting further damage to lung (Castalsi., P.J., et al., 2010). AATD inhibits proteases and protects tissues from enzymes of inflammatory cells, when it is absent neutrophils can break eastin down resulting in pulmonary complications including COPD (Castalsi., P.J., et al., 2010). Many researchers have performed genome-wide association studies (GWAS) which provides an unbiased and complete search of the genome for susceptibility to COPD. Pillai and colleagues studies the association between COPD and the CHRNA3/CHRNA5/IREB2 region on chromosome 15 (Pillai, S., et al., 2009). MeMeo and colleagues performed gene expression studies comparing normal to COPD lung tissue resulting in the discovery of IREB2 region on chromosome 15 as a contributing genetic factor (DeMeo, D., et al., 2009). Large population genome studies have also found that SNPs near HHIP can also be associated with COPD (Hancock, D., et al., 2010). Overall, although many things were found the overall conclusions amongst the COPD community and researchers is that there is strong evidence concluding that IREB2, HHIP, and FAM13A loci are association with COPD susceptibility (Hancock, D., et al., 2010).
Comparison of monozygotic and dizygotic twins used to assess environmental versus genetic effects (MZ share 100% genes, DZ share 50%) showing heritability ranges from 0.5-0.8 (Hancock, D., et al., 2010). The study discovered genes coding for various proteins can exacerbate COPD. Proteases and antiproteases, such as MMP-12, play a role in tissue repair associated with inflammation on chromosomes 11,14,16,20,22 (Pendas, A., et al, 1996). Presence of MMP-12 polymorphisms increase the risk of developing COPD when smoking; in MMP-12 knockout mice they did not develop emphysema when exposed to cigarette smoke (Hautamaki, R., et al., 1997). It is the Asn357Ser polymorphism associated with decline in lung function (Hautamaki, R., et al., 1997). Xenobiotic Metabolizing Enzymes, such as EPHX, are involved in metabolizing high reactive epoxide intermediates formed from cigarette smoke that cause injury (Joos, L., Pare, P., Sandford, A., 2002). Two polymorphisms in EPHX gene with 2 substitutions at exon 3 and 4 result in slower metabolizing enzymes more commonly found in COPD patients and emphysema patients, (Smith, C & Harrison, D., 1997). Additionally, genes coding for inflammatory mediators, antioxidants, and mucociliary clearance factors can all have mutations causing these proteins not to work perfectly resulting in increased COPD symptoms (Joos, L., Pare, P., Sandford, A., 2002).
Diagnosis
There are several ways to diagnose COPD including spirometry, chest x-rays and blood tests.
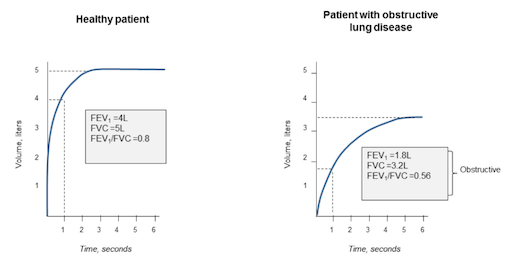
Spirometry is a way to test if the lungs are functioning properly. The patient will breath into the spirometer, and after inhalation, a bronchodilator will be released to widen the airways. The spirometer measures the volume of air the patient can exhale in one second and the total amount of air they inhale. The measurements can then be compared to standardized results for the patient’s age group, to find out if their airways are obstructed.
Chest x-rays are used to look at the lungs for problems that may cause symptoms of COPD.
Blood tests can detect other conditions that cause symptoms similar to COPD, including anemia and polycythaemia (NHS).
Management and Treatment
Pulmonary Rehabilitation
Across North America, pulmonary rehabilitation has become an extremely popular method for long-term management of COPD (Goldstein et al., 1994). It aims to manage and improve some of the disabilities that are associated with COPD, such as decreased motor function and weight loss (Goldstein et al., 1994). There are three facets of pulmonary rehabilitation are: the multidisciplinary nature of, individualized programs and attention to physical and social function (Ries & Squier, 1996). The collaboration between various kinds health care professionals makes pulmonary rehabilitation very successful because it encompasses a variety of health care fields. Individuals involved include: physicians, nurses, occupational therapists, psychologists, nutritionists and exercise specialists. (Ries & Squier, 1996). Additionally, the emphasis on an individualized rehabilitation plans leads to successful outcomes because patients are able to focus on the areas that they need to develop the most (Ries & Squier, 1996). Finally, by focusing on both the physical and social function of these individuals, pulmonary rehabilitation allows patients to work on emotional issues. This aspect has been correlated with better outcomes in physical symptoms, such as lung function and exercise tolerance (Ries & Squier, 1996).
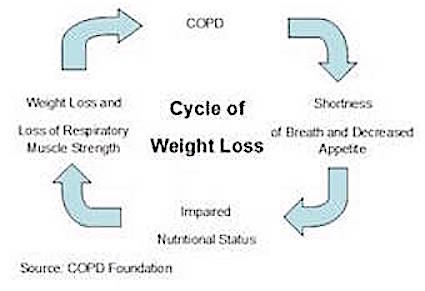
The main elements of pulmonary rehabilitation include: exercise training, education on the disease, nutritional intervention and psychosocial support (Ries et al., 2007). Due to the decreased appetite of COPD patients, individuals often experience weight loss (Schols et al., 1998). This weight loss is mainly stemming from a loss of muscle, specifically respiratory muscles and consequently, this makes breathing even more difficult for these individuals (Schols et al., 1998). By focusing on both nutritional support and strength training exercise, pulmonary rehabilitation helps rebuild the muscle loss as well as prevent future loss in COPD patients. Often times, this kind of rehabilitation can be offered as an outpatient treatment, thus making it accessible to patients who are not hospitalized (Ries et al., 2007). Overall, by making the disabilities of COPD individuals more manageable, pulmonary rehabilitation improves the overall quality of life of individuals by making them more independent and improving health status (Goldstein et al., 1995; Ries et al., 2007).
Outcomes of Pulmonary Rehabilitation
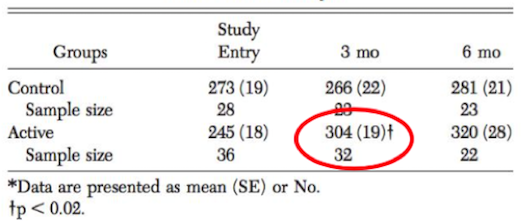
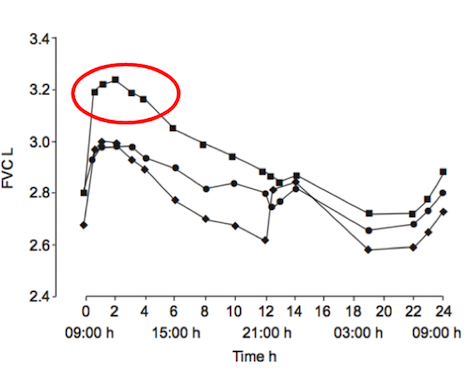
Although pulmonary rehabilitation does not reverse the pathophysiology of the disease it is able to reduce the symptoms and disabilities associated with COPD (Ries et al., 2007). This kind of therapy is able to help individuals become more physically active and teaches them how to cope with the disease, both with better understanding of the condition and emotional support (Lacasse et al., 2006). Physical improvements are often seen in COPD patients and are due to the exercise and nutritional supports that pulmonary rehabilitation provides, which can last up to 9 months after discontinuation of the program (Cambach et al., 1999). In a clinical study done by Berry et al. (1999), when compared to a control group that did not participate in pulmonary rehabilitation, an active group showed significant improvements in exercise tolerance. After participating in a 6-week pulmonary rehabilitation program, these COPD patients saw significant improvements in regards to the amount walked in six minutes. Improvements were maintained for at least 3 months after the conclusion of the experiment (Berry et al., 1999). These findings are illustrated in the diagram below.
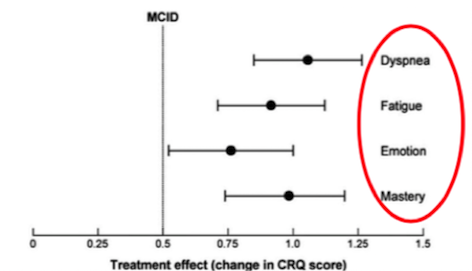
Furthermore, a study done by Lacasse et al. (2006), showed significant improvements with the participation in pulmonary rehabilitation in other symptoms common to COPD patients as well. It was proven that pulmonary rehabilitation relieved symptoms of dyspnea (labored breathing) and fatigue, which is common found in COPD patients due to the muscle loss and increased energy expenditure of movement (Lacasse et al. 2006). Additionally, improvements to the emotional state of COPD patients were shown to enhance an individual’s sense of mastery and control over their condition (Lacasee et al., 2006). Results of this study are shown below. After much research, the significant yield of results illustrate why pulmonary rehabilitation is a crucial component in the long-term management of COPD.
Popular Aspects of Pulmonary Rehabilitation
Diet and Nutrition
Being underweight and/or unintentional weight loss is associated with increased mortality and morbidity in COPD patients. So it is very important to help patients avoid losing weight or help them regain the weight that they lost. Many patients will experience a decline in respiratory muscles, physical performance and health status resulting from a loss of lean muscle mass. Detecting and reversing weight loss should be done as soon as possible to limit this functional decline. Increasing dietary intake or including energy dense foods may help to combat the onset of COPD. Some epidemiological studies have shown that diet and nutrition may contribute to COPD risk and could play a role in prevention (Burg, 2004).
There is not enough evidence to give dietary advice to reduce the risk of COPD, however studies show the benefits of fatty fish, fruits, antioxidants and high protein intake. Polyunsaturated fatty acids found in fish have been found to indirectly inhibit the production of inflammatory factors. Multiple cohort studies have indicated a protective effect from fruit intake and COPD incidence and mortality. Antioxidant nutrients, like vitamin C found in fruits and vegetables, may be responsible for preventing oxidative stress in the lungs from air pollution (Burg, 2004). COPD patients need higher supplies of energy and protein to fuel greater energy expenditure from the increased effort of breathing and the inflammatory processes of the disease. Based on a study done by Bauer (2013) it is recommended to COPD patients to take high protein oral nutritional supplementation with 20% kcal from protein.
Smoking Cessation
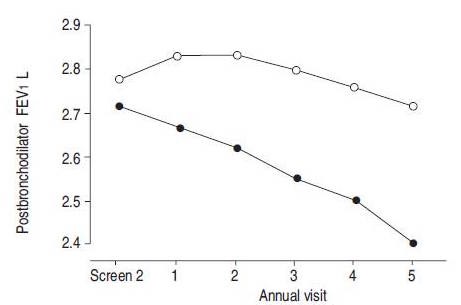
Stopping smoking is one of the main treatments for patients with COPD to effectively slow down disease progression (Willemse, 2005). The most evident clinical characteristics in COPD is respiratory symptoms and the accelerated decline in forced expiratory volume in one second (FEV1). By not smoking there will be an improvement to respiratory symptoms, which may be because of the decrease of goblet cells. It will also stabilize the accelerated decline in FEV1, strongly indicating that stopping smoking will positively influence inflammatory and remodelling processes in the lungs. It was seen that during the first year after stopping smoking, FEV1 improved by 57 mL in quitters, but fell by 32 mL in patients that continued to smoke. An improvement of inflammation is also seen, resulting from a combination of increased anti-inflammatory receptor levels and reduced neutrophil chemoattractants (Willemse, 2004).
Medical Treatment
Bronchodilators
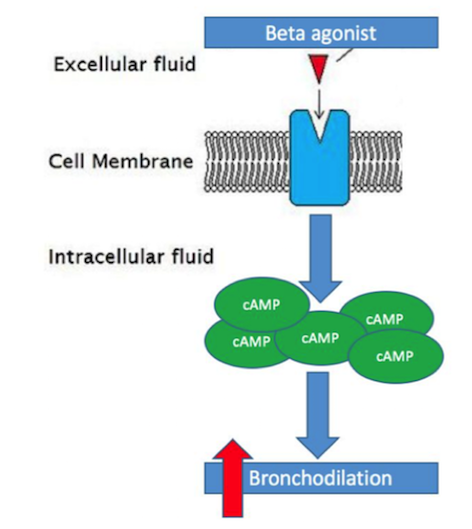
Bronchodilators are a class of medications that are widely used to treat COPD and aid in preventing airflow obstruction in COPD patients (Shim, 1989). These drugs provide relief of some symptoms commonly associated with COPD, such as dyspnea or decreased exercise tolerance, through the relaxation of smooth muscle that line airways (Barnes, 1995). Due to the increase build-up of smooth muscle in COPD patients and subsequent impairment of lung function, these bronchodilators are extremely important in the prevention/reduction of airway obstruction (Berge et al., 2011). The two main bronchodilators used in the treatment of COPD are beta-agonist’s and anti-cholinergic’s (Barnes, 1995). Beta-agonist’s are the most widely used bronchodilator typically used to treat COPD patients (Barnes, 1995). Beta-agonist’s act by binding to the adrenergic receptors on smooth muscle cells and cause an increase of cyclic-AMP (cAMP), a secondary messenger, within the smooth muscle cell. The increase in cAMP causes an intra-cellular cascade that ultimately leads to a relaxation of the smooth muscle surrounding the airway and thus causing bronchodilation (Tashkin & Fabbri, 2010). Long-acting beta-agonist’s in specific, such as formoterol or salmeterol, have been proven to more effectively than regular beta-agonist medications, primarily due to their rapid onset and long duration of action (Barnes, 1995).
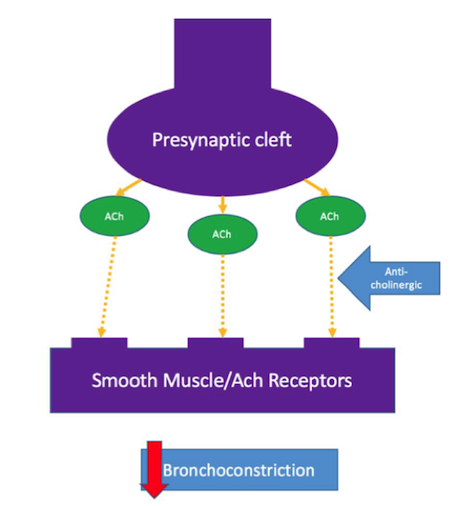
The other kind of bronchodilator used are anti-cholinergic’s, also known as muscarinic antagonists. These not used as often as beta-agonist’s but have been shown to aid in improvement of airway flow in COPD patients as well (Barnes, 1995). Anti-cholinergic’s act by blocking the binding of acetylcholine, which is released from the pre-synaptic cleft of a neuron, to the smooth muscle acetylcholine receptor. By blocking the binding, anti-cholinergic’s are able to prevent bronchoconstriction (Tashkin & Fabbri, 2010).
Efficacy of Bronchodilators
Studies have shown the long-acting beta-agonist’s (LABAs), such as formoterol, are the best course of medical treatments for individuals with COPD (Rossi, Khirani & Cazzola, 2008). These LABAs are more effective due to their rapid onset and prolonged duration (Barnes, 1995). When used, COPD patients saw improvement with common symptoms, such as dyspnea and decreased exercise tolerance, within minutes-hours after ingestion. Additionally, these drugs also helped improved lung function, reduce exacerbations and overall improve the health status of symptomatic patients with moderate-severe COPD (Rossi, Khirani & Cazzola, 2008). In a clinical study done by Van Noord et al. (2005), results showed that the efficacy of LABAs improved with the combined use of anti-cholinergic drugs. These results were only found in patients with severe COPD and only for the first 12-24 hours after ingestion (Van Noord et al., 2005).
Conclusion
Overall, COPD is becoming an increasingly prevalent cause of death worldwide. The disease is thought to be caused by a variety of genetic factors, which can be triggered or exacerbated by environmental factors, such as smoking or air pollution. Unless caused by an alpha-1-antitrypsin deficiency, there is no cure for COPD but only management options for helping individuals struggling with this disease. Pulmonary rehabilitation, which includes exercise, nutritional and psychosocial support, combined with bronchodilator medication have been proven to improve common symptoms of COPD and improve long-term outcomes of COPD patients.
References
Barnes, P. J. (1995). Bronchodilators: basic pharmacology. In Chronic obstructive pulmonary disease (pp. 391-417). Springer US.
Bauer, J., Biolo, G., Cederholm, T., Cesari, M., Cruz-Jentoft, A. J., Morley, J. E., … & Visvanathan, R. (2013). Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. Journal of the American Medical Directors Association, 14(8), 542-559.
Berge, M. V., Hacken, N. H., Cohen, J., Douma, W. R., & Postma, D. S. (2011). Small Airway Disease in Asthma and COPD. Chest, 139(2), 412-423. doi:10.1378/chest.10-1210
Bourdin, A., Burgel, P., Chanez, P., Garcia, G., Perez, T., & Roche, N. (2009, September 5). Recent advances in COPD: Pathophysiology, respiratory physiology and clinical aspects, including comorbidities. European Respiratory Review, 18(114), 198-212. Doi: 10.1183/09059180.00005509.
Brug, J., Schols, A., & Mesters, I. (2004). Dietary change, nutrition education and chronic obstructive pulmonary disease. Patient education and counseling, 52(3), 249-257.
Cambach, W., Wagenaar, R. C., Koelman, T. W., van Keimpema, T., & Kemper, H. C. (1999). The long-term effects of pulmonary rehabilitation in patients with asthma and chronic obstructive pulmonary disease: a research synthesis. Archives of physical medicine and rehabilitation, 80(1), 103-111.
Castaldi, P., et al. (2010). The COPD genetic association compendium: a comprehensive online database of COPD genetic associations. Human Molecular Genetis. 19 (3), 526-534. Doi:10.1093/hmg/ddp519
Chung, K. F. (2005, June 22). The Role of Airway Smooth Muscle in the Pathogenesis of Airway Wall Remodeling in Chronic Obstructive Pulmonary Disease. Proceedings of the American Thoracic Society, 2(4), 347-354. doi:10.1513/pats.200504-028sr.
DeMeo, D., et al. (2009). Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. American journal of human genetics. 85 (4), 493-502. Doi:10.1016/j.ajhg.2009.09.004
Goldstein, R. S., Gort, E. H., Avendano, M. A., Stubbing, D., & Guyatt, G. H. (1994). Randomised controlled trial of respiratory rehabilitation. The Lancet,344(8934), 1394-1397.
Hancock, D., et al. (2010). Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nature genetics. 42 (1), 45-52. Doi:10.1038/ng.500
Hautamaki, R., et al. (1997). Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science (New York, N.Y.). 277 (5334), 2002-4. Doi:10.1126/science.277.5334.2002
Joos, J., Pare, P., Sandford, A. (2002). Genetic risk factors for chronic obstructive pulmonary disease. Wolters Kluwer Health. 8 (2), 87-97. Doi: 2002/03/smw-09752
Ko, F. & Hui, D. (2012). Air pollution and chronic obstructive pulmonary disease. Respirology. 17 (3), 395-401. Doi: 10.1111/j.1440-1843.2011.02112.x
MacNee, W. (2006, May 20). ABC of chronic obstructive pulmonary disease: Pathology, pathogenesis, and pathophysiology. Bmj, 332(7551), 1202-1204. doi:10.1136/bmj.332.7551.1202.
Matheson, M., et al. (2005). Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax. 60 (8), 645-51. Doi: 10.1136/thx.2004.035170
Pendas, A., et al. (1996). Fine physical mapping of the human matrix metalloproteinase genes clustered on chromosome 11q22.3. Genomics. 37 (2), 266-8. Doi: 10.1006/geno.1996.0557
Pillai, S., et al. (2009). A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS genetics. 5 (3). Doi: 10.1371/journal.pgen.1000421
Ries, A. L., & Squier, H. C. (1996). The team concept in pulmonary rehabilitation. Lung Biology in Health and Disease, 91, 55-66.
Ries, A. L., Bauldoff, G. S., Carlin, B. W., Casaburi, R., Emery, C. F., Mahler, D. A., … & Herrerias, C. (2007). Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. CHEST Journal,131(5_suppl), 4S-42S.
Rossi, A., Khirani, S., & Cazzola, M. (2008). Long-acting β2-agonists (LABA) in chronic obstructive pulmonary disease: efficacy and safety. International Journal of Chronic Obstructive Pulmonary Disease, 3(4), 521–529.
Schols, A. M., Slangen, J. O. S., Volovics, L., & Wouters, E. F. (1998). Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine, 157(6), 1791-1797.
Shim, C. (1989). Response to bronchodilators. Clinics in chest medicine, 10(2), 155-164.
Smith, C. & Harrison, D. (1997). Association between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysema. The Lancet. 350 (9078), 630-633. Doi: 10.1016/S0140-6736(96)08061-0
Tashkin, D. P., & Fabbri, L. M. (2010). Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respiratory research, 11(1), 1.
Tests for COPD. (n.d.). NHS. Retrieved from http://www.nhs.uk/Conditions/Chronic-obstructive-pulmonary-disease/Pages/Diagnosis.aspx
Van Noord, J. A., Aumann, J. L., Janssens, E., Smeets, J. J., Verhaert, J., Disse, B., … & Cornelissen, P. J. G. (2005). Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. European Respiratory Journal, 26(2), 214-222.
Willemse, B. W. M., Ten Hacken, N. H. T., Rutgers, B., Lesman-Leegte, I. G. A. T., Postma, D. S., & Timens, W. (2005). Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. European Respiratory Journal, 26(5), 835-845. Willemse, B. W. M., Ten Hacken, N. H. T., Rutgers, B., Lesman-Leegte, I. G. A. T., Timens, W., & Postma, D. S. (2004). Smoking cessation improves both direct and indirect airway hyperresponsiveness in COPD. European respiratory journal, 24(3), 391-396.