This is an old revision of the document!
Table of Contents
Chronic Obstructive Pulmonary Disease (COPD)
Introduction
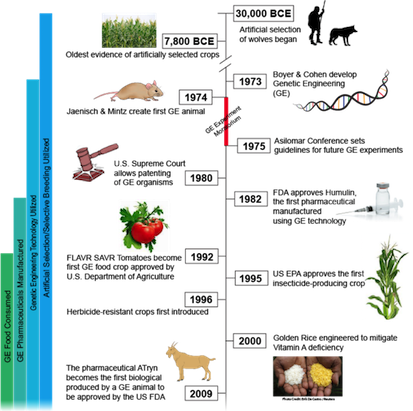
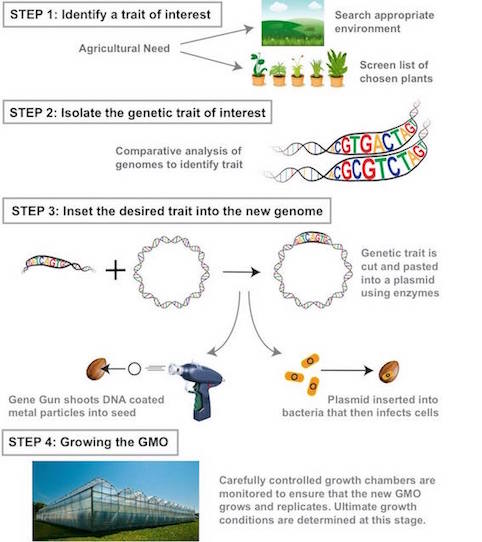
A Genetically Modified Organism is any organism created by gene splicing techniques and often involves merging DNA from different species (31). Scientists directly manipulate an organism’s genome to isolate traits or characteristics deemed of value. Currently, the safety of GMOs is under research as they are relatively recent developments (22).
What is COPD
Prevalence and Epidemiology of the Disease
Economic Burden of COPD
Mechanism of Disease
Causes and Pathophysiology
Environmental Factors - Air Pollution
The Relationship Between COPD Deaths and Air Pollution
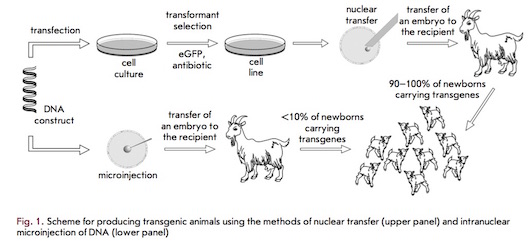
Advances in molecular and reproductive technology have driven the practice of commercial animal pharming over the past twenty years (14). Pharming is a branch of biotechnology which bridges together the contrasting fields of pharmaceuticals and farming. Transgenic plants or animals are utilized for producing pharmaceuticals synthesized for human or animal consumption (7). Animal pharming is promoted as a cost-effective method of biopharmaceutical production (14) and has the potential to become a highly lucrative industry (7).
The need for pharmaceutical intervention is evident amongst patients possessing hereditary antithrombin deficiencies. In 2006, an anticoagulant called Antithrombin became the first recombinant protein to be approved for commercialization by the United States’ Food and Drug Administration (FDA) (18). Antithrombin is extracted and purified from the milk of transgenic dairy goats (16). Transgenic animals, such as these goats, are produced via one of two contemporary methods: intra-pronuclear zygotic DNA microinjection (MI) or somatic cell nuclear transfer (NT). As linear DNA enters the nucleus, it is capable of integrating into the genome of cell lines or living organisms (18).
The most promising site for production of recombinant proteins is the mammary gland due to the large quantities of protein that can be produced (16). Since milk is often produced in abundance, deriving and producing pharmaceuticals in this manner is determined to be cost efficient (14).
Environmental Factors- Smoking
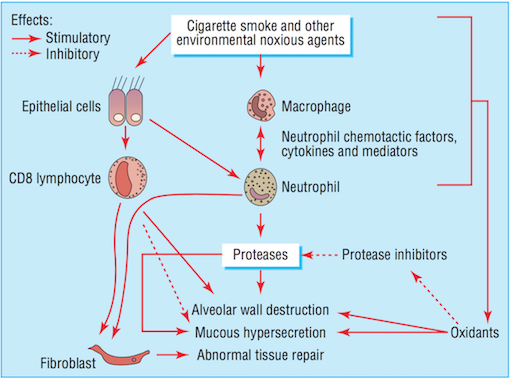
Noxious agents produced from cigarette smoke have a detrimental effect on the airways. The toxins damage airway epithelium and drive the processes that lead to certain airway inflammation and structural changes (Bourdin et al., 2009). Accordingly, cigarette smoking is the leading cause of COPD in North America (Bourdin et al., 2009). All smokers exhibit inflammation within their lungs, but those who have developed COPD demonstrate amplified or abnormal responses to inhaling noxious agents (MacNee, 2006). Airway remodelling may be a protective mechanism of airway epithelium to injury caused by cigarette smoke. Furthermore, the subsequent structural changes may be a reparative mechanism in response to damage caused by the toxic substance (Chung, 2005).
Bronchiolar epithelium is altered in smokers, notably in COPD patients (Chung, 2005). Bronchiolar epithelial cells are triggered by interleukin (IL)-1, interleukin (IL)-8, and granulocyte colony-stimulating factor causing them to release increased levels of neutrophil and monocytic chemotactic activities (Chung, 2005). Structural changes to the airway take place as a result of abnormal repair processes promoted by activated fibroblasts. Fibroblast activation is induced by the increased production of neutrophil, macrophages, and lymphocytes (MacNee, 2006). Moreover, an influx of neutrophils and macrophages result in an imbalance between proteases and anti-proteases. The imbalance of proteases and imbalances ultimately leads to destruction of the alveolar wall, while an overabundance of proteases alone results in the increased production and release of mucous (MacNee, 2006). Alveolar wall destruction and the presence of excessive mucous are both distinct characteristics of COPD (Bourdin et al., 2009).
Biological Perspectives- Structural Changes
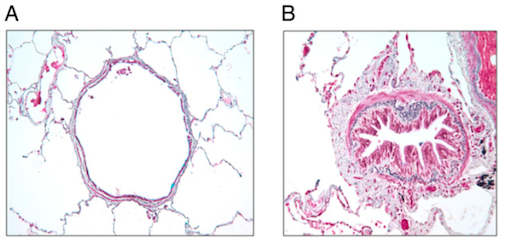
Chronic airflow obstruction is associated with airway remodelling and inflammation of peripheral airways. Airway remodelling, consistent with structural changes found in the airways of COPD patients, are more prominent in the small airways than in the large airways (Chung, 2005). Small airways are defined as peripheral airways that are less than 2mm in internal diameter (Berge et al., 2011). A strong association exists between disease progression and an increase in epithelium, lamina propria, adventitial compartments of the airway walls, and smooth muscle. The amount of smooth muscle is increased by nearly 50% in patients diagnosed with more severe COPD, stages 3 or 4, as categorized by the Global Initiative on Obstructive Lung Disease (GOLD) (Chung, 2005). Lung functionality is inversely correlated with the amount of smooth muscle found in the small airway (Chung, 2005). Structural changes, such as muscle thickening, may lead to muscle shortening against elastic loads. Muscle thickening also increases ability to generate force, which may play a part in bronchial hyper-responsiveness, loss of lung recoil, and fibrosis of the small airways (Chung, 2005).
Smooth muscles of the small airways not only demonstrate contractile properties, but may also contribute to the inflammatory and airway wall remodelling processes. This takes place as a result of the expression or release of cytokines, chemokines, proteases, and growth factors (Chung, 2005). Increased production of cytokines and chemokines increases the number and activation of neutrophil, a granulocyte associated with COPD (Bourdin et al., 2009). Notable cytokines and chemokines include interleukin (IL)-8 and CXC chemokine ligand 10 (CXCL10), respectively. IL-8 is a powerful chemoattractant and activator of neutrophil, and the receptor is present on blood and sputum neutrophils and epithelial cells (Bourdin et al., 2009). CXCL10 is also a chemoattractant for neutrophil as well as for human monocytes, natural killer cells, and T cells (Chung, 2005).
Neutrophil levels are elevated in COPD patients, resulting in the increased release of proteases and oxidants (Bourdin et al., 2009). The imbalance of proteases and anti-proteases, as well as the imbalance of oxidants and antioxidants (MacNee, 2006) perpetuate the progression of processes in favour of lung destruction (Bourdin et al., 2009).
Biological Perspectives- Genetics
Diagnosis
There are several ways to diagnose COPD including spirometry, chest x-rays and blood tests.
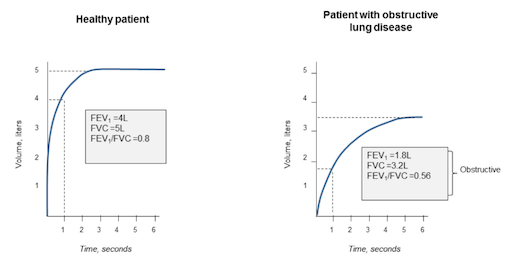
Spirometry is a way to test if the lungs are functioning properly. The patient will breath into the spirometer, and after inhalation, a bronchodilator will be released to widen the airways. The spirometer measures the volume of air the patient can exhale in one second and the total amount of air they inhale. The measurements can then be compared to standardized results for the patient’s age group, to find out if their airways are obstructed.
Chest x-rays are used to look at the lungs for problems that may cause symptoms of COPD.
Blood tests can detect other conditions that cause symptoms similar to COPD, including anemia and polycythaemia (NHS).
Management and Treatment
Pulmonary Rehabilitation
Across North America, pulmonary rehabilitation has become an extremely popular method for long-term management of COPD (Goldstein et al., 1994). It aims to manage and improve some of the disabilities that are associated with COPD, such as decreased motor function and weight loss (Goldstein et al., 1994). There are three facets of pulmonary rehabilitation are: the multidisciplinary nature of, individualized programs and attention to physical and social function (Ries & Squier, 1996). The collaboration between various kinds health care professionals makes pulmonary rehabilitation very successful because it encompasses a variety of health care fields. Individuals involved include: physicians, nurses, occupational therapists, psychologists, nutritionists and exercise specialists. (Ries & Squier, 1996). Additionally, the emphasis on an individualized rehabilitation plans leads to successful outcomes because patients are able to focus on the areas that they need to develop the most (Ries & Squier, 1996). Finally, by focusing on both the physical and social function of these individuals, pulmonary rehabilitation allows patients to work on emotional issues. This aspect has been correlated with better outcomes in physical symptoms, such as lung function and exercise tolerance (Ries & Squier, 1996).
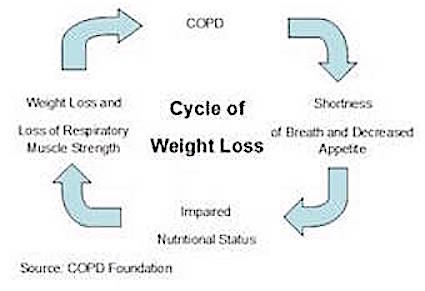
The main elements of pulmonary rehabilitation include: exercise training, education on the disease, nutritional intervention and psychosocial support (Ries et al., 2007). Due to the decreased appetite of COPD patients, individuals often experience weight loss (Schols et al., 1998). This weight loss is mainly stemming from a loss of muscle, specifically respiratory muscles and consequently, this makes breathing even more difficult for these individuals (Schols et al., 1998). By focusing on both nutritional support and strength training exercise, pulmonary rehabilitation helps rebuild the muscle loss as well as prevent future loss in COPD patients. Often times, this kind of rehabilitation can be offered as an outpatient treatment, thus making it accessible to patients who are not hospitalized (Ries et al., 2007). Overall, by making the disabilities of COPD individuals more manageable, pulmonary rehabilitation improves the overall quality of life of individuals by making them more independent and improving health status (Goldstein et al., 1995; Ries et al., 2007).
Outcomes of Pulmonary Rehabilitation
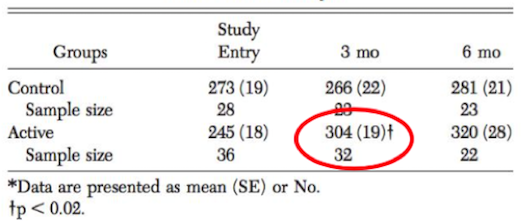
Although pulmonary rehabilitation does not reverse the pathophysiology of the disease it is able to reduce the symptoms and disabilities associated with COPD (Ries et al., 2007). This kind of therapy is able to help individuals become more physically active and teaches them how to cope with the disease, both with better understanding of the condition and emotional support (Lacasse et al., 2006). Physical improvements are often seen in COPD patients and are due to the exercise and nutritional supports that pulmonary rehabilitation provides, which can last up to 9 months after discontinuation of the program (Cambach et al., 1999). In a clinical study done by Berry et al. (1999), when compared to a control group that did not participate in pulmonary rehabilitation, an active group showed significant improvements in exercise tolerance. After participating in a 6-week pulmonary rehabilitation program, these COPD patients saw significant improvements in regards to the amount walked in six minutes. Improvements were maintained for at least 3 months after the conclusion of the experiment (Berry et al., 1999). These findings are illustrated in the diagram below.
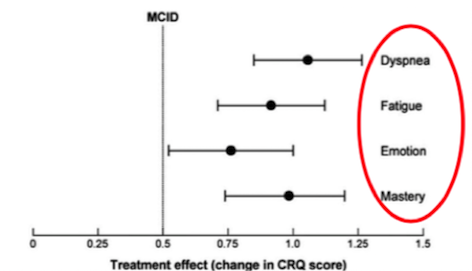
Furthermore, a study done by Lacasse et al. (2006), showed significant improvements with the participation in pulmonary rehabilitation in other symptoms common to COPD patients as well. It was proven that pulmonary rehabilitation relieved symptoms of dyspnea (labored breathing) and fatigue, which is common found in COPD patients due to the muscle loss and increased energy expenditure of movement (Lacasse et al. 2006). Additionally, improvements to the emotional state of COPD patients were shown to enhance an individual’s sense of mastery and control over their condition (Lacasee et al., 2006). Results of this study are shown below. After much research, the significant yield of results illustrate why pulmonary rehabilitation is a crucial component in the long-term management of COPD.
Popular Aspects of Pulmonary Rehabilitation
Diet and Nutrition
Being underweight and/or unintentional weight loss is associated with increased mortality and morbidity in COPD patients. So it is very important to help patients avoid losing weight or help them regain the weight that they lost. Many patients will experience a decline in respiratory muscles, physical performance and health status resulting from a loss of lean muscle mass. Detecting and reversing weight loss should be done as soon as possible to limit this functional decline. Increasing dietary intake or including energy dense foods may help to combat the onset of COPD. Some epidemiological studies have shown that diet and nutrition may contribute to COPD risk and could play a role in prevention (Burg, 2004).
There is not enough evidence to give dietary advice to reduce the risk of COPD, however studies show the benefits of fatty fish, fruits, antioxidants and high protein intake. Polyunsaturated fatty acids found in fish have been found to indirectly inhibit the production of inflammatory factors. Multiple cohort studies have indicated a protective effect from fruit intake and COPD incidence and mortality. Antioxidant nutrients, like vitamin C found in fruits and vegetables, may be responsible for preventing oxidative stress in the lungs from air pollution (Burg, 2004). COPD patients need higher supplies of energy and protein to fuel greater energy expenditure from the increased effort of breathing and the inflammatory processes of the disease. Based on a study done by Bauer (2013) it is recommended to COPD patients to take high protein oral nutritional supplementation with 20% kcal from protein.
Smoking Cessation
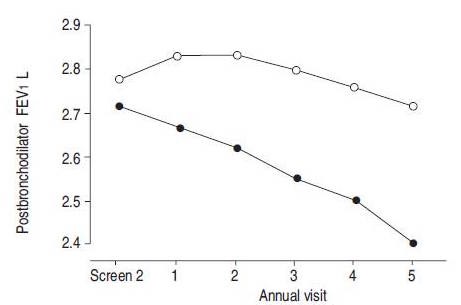
Stopping smoking is one of the main treatments for patients with COPD to effectively slow down disease progression (Willemse, 2005). The most evident clinical characteristics in COPD is respiratory symptoms and the accelerated decline in forced expiratory volume in one second (FEV1). By not smoking there will be an improvement to respiratory symptoms, which may be because of the decrease of goblet cells. It will also stabilize the accelerated decline in FEV1, strongly indicating that stopping smoking will positively influence inflammatory and remodelling processes in the lungs. It was seen that during the first year after stopping smoking, FEV1 improved by 57 mL in quitters, but fell by 32 mL in patients that continued to smoke. An improvement of inflammation is also seen, resulting from a combination of increased anti-inflammatory receptor levels and reduced neutrophil chemoattractants (Willemse, 2004).
Medical Treatment
Bronchodilators
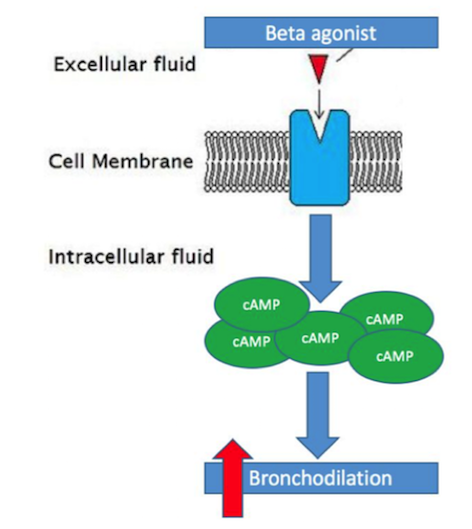
Bronchodilators are a class of medications that are widely used to treat COPD and aid in preventing airflow obstruction in COPD patients (Shim, 1989). These drugs provide relief of some symptoms commonly associated with COPD, such as dyspnea or decreased exercise tolerance, through the relaxation of smooth muscle that line airways (Barnes, 1995). Due to the increase build-up of smooth muscle in COPD patients and subsequent impairment of lung function, these bronchodilators are extremely important in the prevention/reduction of airway obstruction (Berge et al., 2011). The two main bronchodilators used in the treatment of COPD are beta-agonist’s and anti-cholinergic’s (Barnes, 1995). Beta-agonist’s are the most widely used bronchodilator typically used to treat COPD patients (Barnes, 1995). Beta-agonist’s act by binding to the adrenergic receptors on smooth muscle cells and cause an increase of cyclic-AMP (cAMP), a secondary messenger, within the smooth muscle cell. The increase in cAMP causes an intra-cellular cascade that ultimately leads to a relaxation of the smooth muscle surrounding the airway and thus causing bronchodilation (Tashkin & Fabbri, 2010). Long-acting beta-agonist’s in specific, such as formoterol or salmeterol, have been proven to more effectively than regular beta-agonist medications, primarily due to their rapid onset and long duration of action (Barnes, 1995).
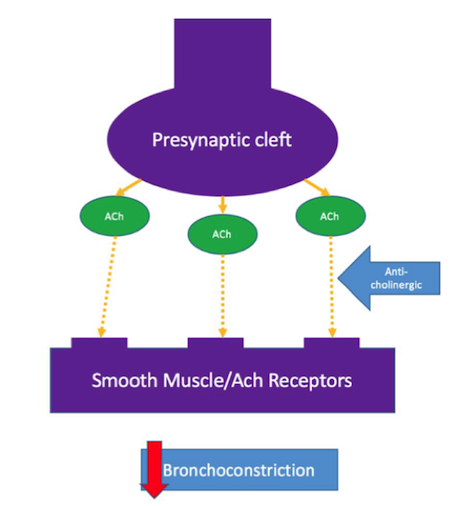
The other kind of bronchodilator used are anti-cholinergic’s, also known as muscarinic antagonists. These not used as often as beta-agonist’s but have been shown to aid in improvement of airway flow in COPD patients as well (Barnes, 1995). Anti-cholinergic’s act by blocking the binding of acetylcholine, which is released from the pre-synaptic cleft of a neuron, to the smooth muscle acetylcholine receptor. By blocking the binding, anti-cholinergic’s are able to prevent bronchoconstriction (Tashkin & Fabbri, 2010).
Efficacy of Bronchodilators
Studies have shown the long-acting beta-agonist’s (LABAs), such as formoterol, are the best course of medical treatments for individuals with COPD (Rossi, Khirani & Cazzola, 2008). These LABAs are more effective due to their rapid onset and prolonged duration (Barnes, 1995). When used, COPD patients saw improvement with common symptoms, such as dyspnea and decreased exercise tolerance, within minutes-hours after ingestion. Additionally, these drugs also helped improved lung function, reduce exacerbations and overall improve the health status of symptomatic patients with moderate-severe COPD (Rossi, Khirani & Cazzola, 2008). In a clinical study done by Van Noord et al. (2005), results showed that the efficacy of LABAs improved with the combined use of anti-cholinergic drugs. These results were only found in patients with severe COPD and only for the first 12-24 hours after ingestion (Van Noord et al., 2005).
Conclusion
Overall, COPD is becoming an increasingly prevalent cause of death worldwide. The disease is thought to be caused by a variety of genetic factors, which can be triggered or exacerbated by environmental factors, such as smoking or air pollution. Unless caused by an alpha-1-antitrypsin deficiency, there is no cure for COPD but only management options for helping individuals struggling with this disease. Pulmonary rehabilitation, which includes exercise, nutritional and psychosocial support, combined with bronchodilator medication have been proven to improve common symptoms of COPD and improve long-term outcomes of COPD patients.
References
1. Aktar, M. W., Sengupta, D., & Chowdhury, A. (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary Toxicology, 2(1), 1–12. http://doi.org/10.2478/v10102-009-0001-7
2. Aris A., Leblanc S. (2011, May). Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Reproductive Toxicology 31(4): 528-33. Doi:10.1016/j.reprotox
3. Bauman, D. (1999, February 5). Bovine somatotropin and lactation: From basic science to commercial application. Domestic Animal Endocrinology, 17(2-3), 101-116. doi:10.1016/s0739-7240(99)00028-4
4. Benfey, T. (2014). Opinion: Sizing up GM Salmon; On the Potential Benefits and Risks of Genetically Modified Fish Entering the Marketplace. The Scientist. Retrieved 23 September from http://www.the-scientist.com/?articles.view/articleNo/40086/title/Opinion--Sizing-Up-GM-Salmon/
5. Boyle, R. (2011). How to Genetically Modify a Seed, Step by Step. Popular Science. Retrieved from http://www.popsci.com/science/article/2011-01/life-cycle-genetically-modified-seed
6. Cannell, R.Q & Hawes, J.D. (1994). Soil Till Res. 30. 245.
7. Engelhard, M., Hagen, K., & Thiele, F. (2007, November). Pharming A New Branch of Biotechnology. Europäische Akademie, 43, 7-12.
8. Institute for Green Energy and Clean Environment. (2009). Phytoremediation and Phytosensing Technologies. Retrieved from: http://systemsbiology.usm.edu/BrachyWRKY/WRKY/Phytoremediation.html
9. Inui, H. & Ohkawa, H. (2005). Herbicide resistance in transgenic plants with mammalian P450 monooxygenases genes. Pest Management Science. 61:286-291.
10. Ismael, Y., Bennett, R., Morse, S. (2002). Agricultural Biology Forum. 5 (4).
11. Jaenisch, R. Mintz, B. (1974). Simian Virus 40 DNA Sequences in DNA of Healthy Adult Mice Derived from Preimplantation Blastocysts Injected with Viral DNA. NCBI, 71(4). Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC388203
12. James, C. (2002). Global review of commercialized transgenic crops: (Feature: Bt Cotton), International Service for the Acquisition of Agri-biotech Applications. 26. 12.
13. Johnson, B. (2003). Problems of plant conservation in agricultural landscapes: can biotechnology help or hinder? Methods for Risk Assessment of Transgenic Plants. (109-120). Peterborough, UK: English Nature
14. Kind, A., & Schnieke, A. (2008, July 29). Animal pharming, two decades on. Transgenic Res Transgenic Research, 17(6), 1025-1033. doi:10.1007/s11248-008-9206-3
15. Kole, R.K., Banerjee, H., Bhattacharyya, A. (2001). Monitoring of market fish samples for endosulfan and hexachlorocyclohexane residues in and around Calcutta. Bull Environ Contam Toxicol. 67(4). 554-9.
16. Kues, W. (2004). The contribution of farm animals to human health. Trends in Biotechnology, 22(6), 286-294. doi:10.1016/j.tibtech.2004.04.003
17. Langer, G. (2016). Skepticism of Genetically Modified Foods. ABC News. Retrieved from http://abcnews.go.com/Technology/story?id=97567&page=1
18. Maksimenko, O. G., Deykin, A. V., Khodarovich, Y. M., & Georgiev, P. G. (2013). Use of Transgenic Animals in Biotechnology: Prospect and Problems. Acta Naturae, 5(16), 1st ser., 33-46.
19. Northoff, E. (2016). 2050 A third more mouths to feed. Food and Agriculture Organization of the United Nations. Retrieved from http://www.fao.org/news/story/en/item/35571/icode/
20. Oerke, E.C., Dehen, H.W., Schonbeck F. & Weber, A. (1994). Crop Production and Crop Protection: Estimated Losses in Major Food and Cash Crops. Elsevier.
21. P. A., & J. R. (2001, September). Recombinant proteins for neurodegenerative diseases: The delivery issue. Trends in Neurosciences, 24(9).
22. Powell, C. (2015). How to Make a GMO. Science in the News, Harvard University. Retrieved from http://sitn.hms.harvard.edu/flash/2015/how-to-make-a-gmo/
23. Pretty, J. (2001). The rapid emergence of genetic modification in world agriculture: Contested risks and benefits. Environmental Conservation, 28(3), 248-262. doi:10.1017/S0376892901000261
24. Qaim, M., & Zilberman, D. (2003). Yield effects of genetically modified crops in developing countries. Science, 299(5608), 900-902.
25. Rangel, G. (2015). Form Corgis to Corn. Science in the News, Harvard University. Retrieved from http://sitn.hms.harvard.edu/flash/2015/from-corgis-to-corn-a-brief-look-at-the-long-history-of-gmo-technology/
26. RT Autonomous Nonprofit Organization “TV-Novosti” (2013, November 26) GMOs linked to gluten disorders plaguing 18 million Americans . https://www.rt.com/usa/gmo-gluten-sensitivity-trigger-343
27. Salt, D.E., Smith, R.D., Raskin, I. (1998). Phytoremediation. Annual Rev Plant Physiology Plant Mol Biol. 49: 643-668.
28. Snow, A. A., Andow, D. A., Gepts, P., Hallerman, E. M., Power, A., Tiedje, J. M., & Wolfenbarger, L. L. (2005). Genetically engineered organisms and the environment: current status and recommendations. Ecological Applications,15(2), 377-404.
29. Späth, A. (2013). Unleashing the Frankenfish. News24. Retrieved 23 September from http://www.news24.com/Columnists/AndreasSpath/Unleashing-the-Frankenfish-20130603
30. Spisak S., Solymosi N., Ittzes P., Bodor A., Kondor D., Vattay G., et al. (2013, July 30). Complete Genes May Pass from Food to Human Blood. PLoS ONE 8(7): e69805. Doi: 10.1371/journal.pone.0069805
31. Suzuki, D. (2014). Understanding GMO. The David Suzuki Foundation. Retrieved from http://www.davidsuzuki.org/what-you-can-do/queen-of-green/faqs/food/understanding-gmo/
32. Tuszynski, M. H. (2002, May). Growth-factor gene therapy for neurodegenerative disorders. The Lancet Neurology, 1(1), 51-57. doi:10.1016/s1474-4422(02)00006-6
33. United States Department of Agriculture (2000). Genetically engineered crops: has the adoption reduced pesticide use? Retrieved from: www.ers.usda.gov/epubs/pdf/agout/aug2000/ao273f.pdf
34. United States Department of Agriculture (2014). Adoption of Genetically Engineered Crops by US Farmers has Increased Steadily for Over 15 years. Retrieved from: http://www.ers.usda.gov/amber-waves/2014-march/adoption-of-genetically-engineered-crops-by-us-farmers-has-increased-steadily-for-over-15-years.aspx#.V-wfE5MrKt9.
35. U.S. Geological Survey. (1999). The quality of our nation's waters – nutrients and pesticides. Retreieved from:http://water.usgs.gov/pubs/circ/circ1225/
36. World Health Organization (WHO). (2013, November). Evaluation of certain veterinary drug residues in food. Joint FAO/WHO Expert Committee on Food Additives, (78), 988th ser. doi:10.1002/food.19880320917
37. Wolfenbarger, L. L., & Phifer, P. R. (2000). The ecological risks and benefits of genetically engineered plants. Science, 290(5499), 2088-2093.