Table of Contents
Cholera
 Figure 1: This image depicts a group of the bacteria species Vibrio cholerae
Figure 1: This image depicts a group of the bacteria species Vibrio cholerae
Cholera is an acute diarrheal infection that arises from consuming food or water that has been contaminated with the bacterium Vibrio cholerea. The bacterium causes severe dehydration, taking between 12 hours to 5 days to show symptoms after a person has come in contact with the bacterium (World Health Organization, n.d.). Cholera can affect both children and adults and can result in death within hours if left untreated. Cholera is an easily preventable disease with access to clean water and proper sanitation facilities, and good hygiene practices (World Health Organization, n.d.).
Cholera Discovery by John Snow
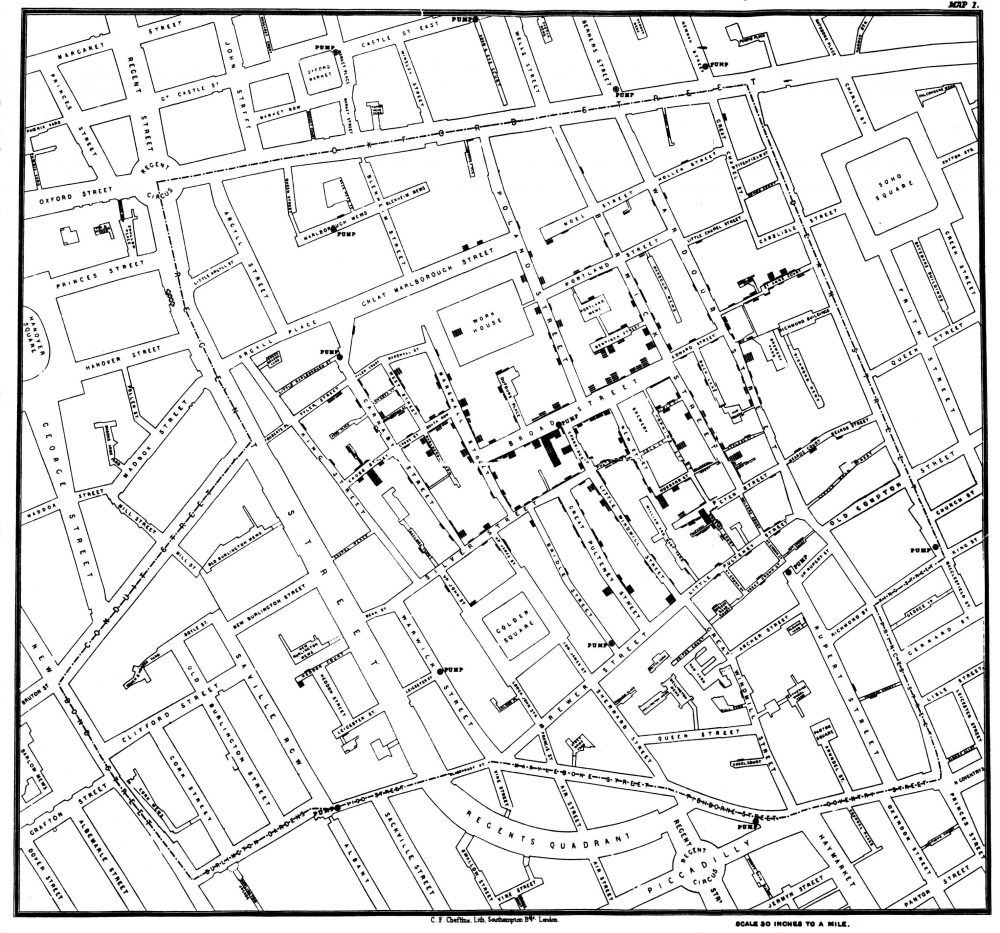
The first case of cholera was discovered in England in 1831. At the time, the outbreak was recognized as “Miasma Theory” which supposed that the cause of the disease was due to bad air coming from rotting organic matter. A doctor working during the outbreak, John Snow, was reluctant to believe this theory. He started to investigate the matter in 1854, and believed that the water from the river was contaminated due to sewage dump (Barton, 2018). Using information he gathered from hospitals and speaking to local residents, he created a dot map to illustrate the number of cases around the city. He identified that the source of the outbreak was due to a pump on Broadwick Street in Soho, London. The pump had been drawing water from the contaminated river. He took the information he gathered and presented it to health officials. Shortly after, the pump handle was removed and the cholera outbreak came to an end in London. It was later discovered that cholera was introduced into the water source from a baby’s diaper who had contracted cholera from another source. This baby’s diaper had been washed into the river, spreading the cholera bacterium (Barton, 2018). In 1883, a German scientist named Robert Koch, took Snow’s research a step further and isolated the bacterium Vibrio cholera for the first time. His research revealed that the bacteria is spread through the use of unsanitary water, proving Snow’s theory. Today, scientists and researchers consider Snow to be the pioneer of epidemiology because of his research and contributions to the public health field. His methods of tracking and identifying cases are still used for many disease outbreaks today (Tuthill, 2003).
Signs & Symptoms
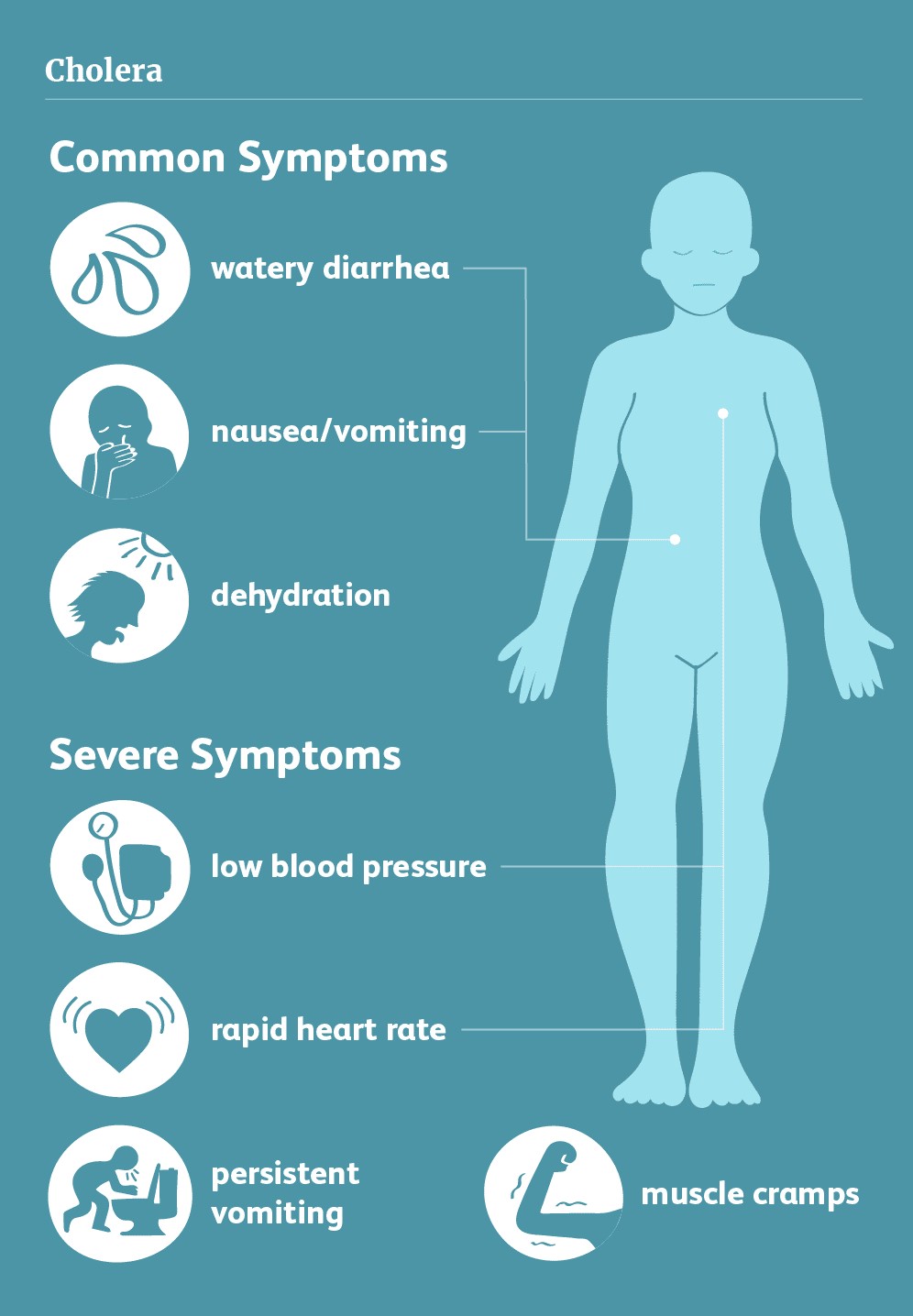
It can take between 12 hours to 5 days for a person to show symptoms after ingesting contaminated food or water, and can kill within hours if left untreated (WHO, 2019). The main sign and symptom of cholera disease is watery diarrhea that generally contains flecks of pale and white material that are mucus and a number of gastrointestinal lining epithelial cells (Davis, 2018). These whitish substances are approximately the size of a piece of rice. Therefore, cholera related diarrhea is also known as rice-water stool. Many bacterial infections can lead to diarrhea. However, the volume of diarrhea caused by cholera disease is normally in vast amounts. For instance, a 154-pound adult suffering from cholera can discharge about 250 cc per kilogram or about 10 to 18 litres of diarrheal fluid over 24 hours (Davis, 2018). The amount of fluid discharging during diarrhea highly depends on the severity of the disease as such individuals with severe disease may excrete high levels of diarrheal fluid (Harris, 2012). Additionally, patients may also develop one or more of the common symptoms simultaneously. These symptoms include watery diarrhea, rice-water stools, fishy smell to stools, vomiting, and thirst due to dehydration (Harris, 2012). Most of the specified symptoms result from extreme dehydration and loss of body water. Individuals who suffer from more severe cases of the disease may also experience additional symptoms such as abdominal and rectal pain, fever, weight loss, little or no urine output, rapid heart rate, low blood pressure, loss of skin elasticity, muscle cramps, dry mucus membrane, restless and irritability appearing more in children, and severe vomiting, and shock (Davis, 2018).
Cause
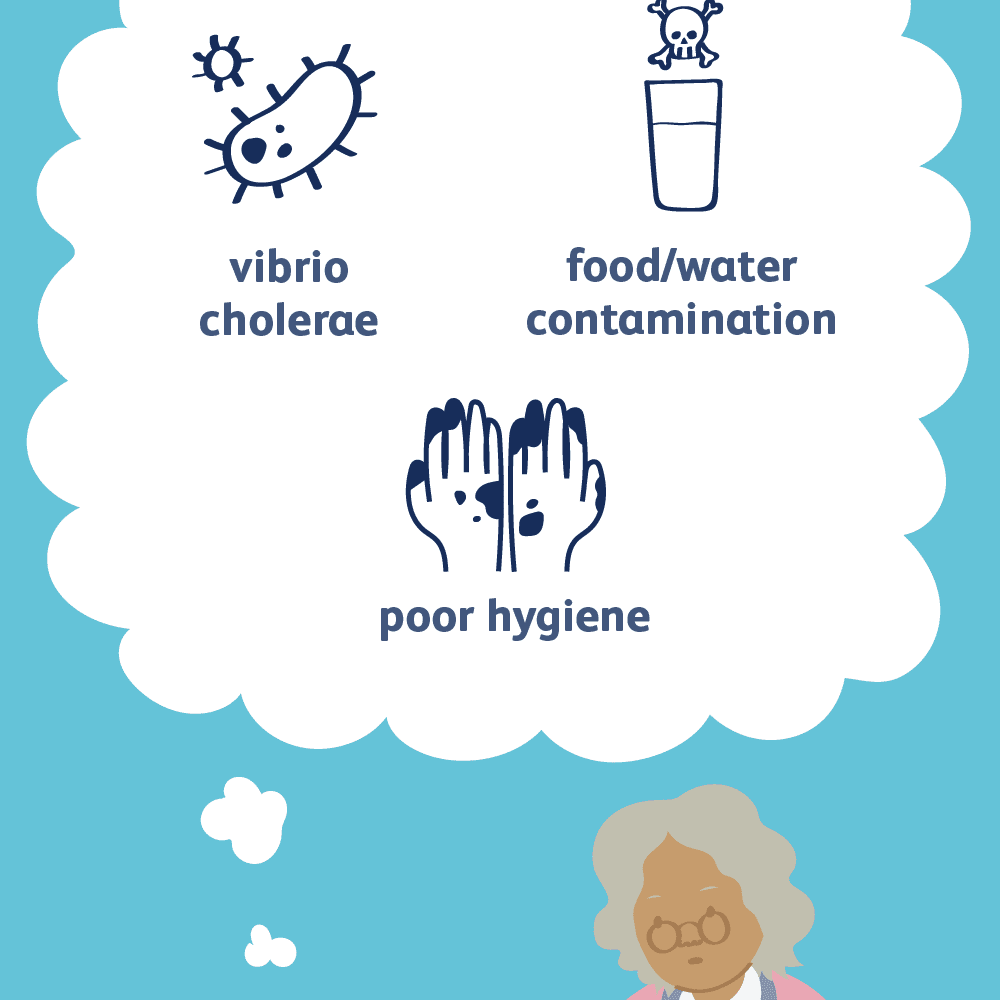
Cholera is caused by a bacterium known as Vibrio cholerae. There are different serogroups of Vibrio cholerae, but only two strains cause outbreaks - O1 and O139 (WHO, 2019). The deadly disease cholera is spread by drinking water or eating food contaminated with the Vibrio cholerae bacterium (CDC, 2018). The bacterium thrives in an environment of brackish water, i.e. water that is warm and salty. Vibrio cholerae produces a toxin in the small intestine, causing the deadly effects of cholera.
In a cholera epidemic, the source of contamination usually traces back to the feces of an infected person or through food washed in contaminated water (CDC, 2018). Cholera spreads rapidly in regions with inadequate sewage systems, poor water treatment, and poor sanitation/ hygiene. However, cholera is unlikely to be spread through direct contact from person-to-person. Although not every individual exposed to Vibrio cholerae will be affected, the bacterium can still pass with their feces to contaminate water and food supplies (WHO, 2019).
Mechanism
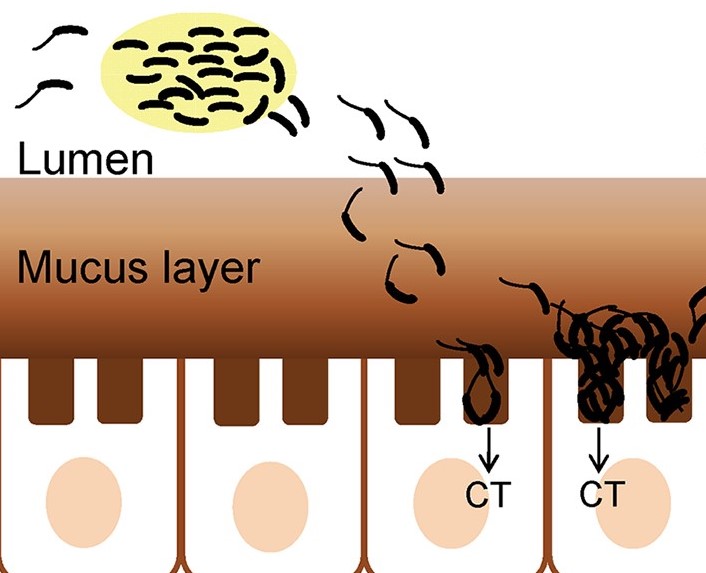
Vibrio cholerae enters via the fecal-oral route, from here the bacteria travels through the GI tract towards the small intestine. Before reaching their destination, the bacteria must survive the acidic conditions within the stomach and antimicrobial peptides in the small intestine (Almagro-Moreno et al., 2015). Those successful now focus on penetrating intestinal mucus using mucolytic enzymes followed by propulsion down a chemotactic gradient towards the intestinal mucosa.
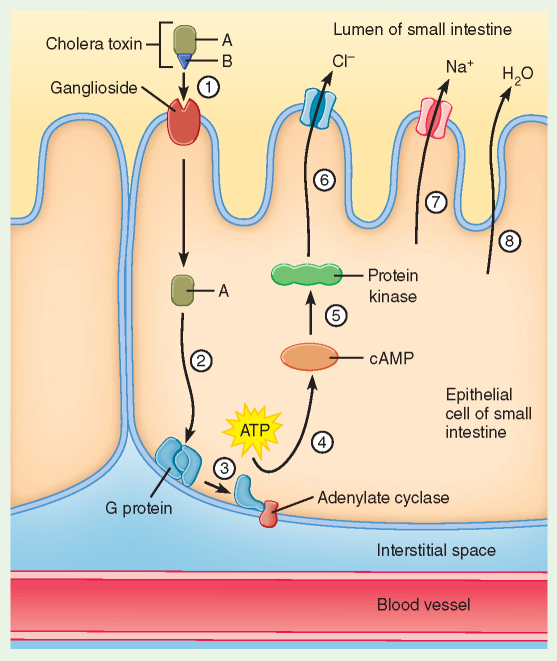
Fimbriae on the bacterial surface are then used to attach to the intestinal mucosa. Next, an appendage on the surface of Vibrio cholerae cells called toxin coregulated pilus helps with colonization by binding the bacteria together to create a microcolony, thus increasing the local concentration (Krebs & Taylor, 2011). The local chemical environment at the intestinal wall causes Vibrio cholerae to activate the expression of ToxT regulatory proteins. ToxT proteins then activate the expression of genes that produce cholera toxin, the protein responsible for the symptoms of cholera (Almagro-Moreno et al., 2015).
Cholera toxin is made up of six protein subunits: a single A subunit and five B subunits, connected by a disulfide bond. The five B subunits form a five-membered ring that binds to GM1 gangliosides on the surface of intestinal epithelium cells (O’Neal et al., 2005). Next, the toxin enters via receptor-mediated endocytosis and once inside the disulfide bond is reduced. The now free A subunit binds to ADP-ribosylation factor 6, binding changes the shape of A subunit exposing its active site, allowing it to catalyse ADP-ribosylation of alpha subunits within G proteins (O’Neal et al., 2005). ADP-ribosylation causes alpha subunits to remain in its activated state. Increased alpha subunit activation leads to increased adenylate cyclase activity, which increases the concentration of cAMP to more than 100-fold over normal which leads to over-activation of protein kinase A. These active protein kinase A then phosphorylate the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel proteins, which stimulates intestinal mucosal cells to actively pump large amounts of Cl- into the intestinal lumen (O’Neal et al., 2005). H2O, Na+, K+, and HCO3− passively enter the intestinal lumen after due to the osmotic and electrical gradients created by the loss of Cl-. The combined effects result in rapid fluid loss leading to severe dehydration and diarrhea.
Diagnosis
Cholera can be diagnosed through the findings of Vibrio cholerae serogroup O1 or O139 in stool samplings. Cary Blair media is ideal for the transport whereas the selective thiosulfate- citrate-bile salts agar (TCBS) is useful in the separation and recognition. In terms of epidemic settings commercially available rapid test kits are useful. In many countries where cholera is not uncommon, access to diagnostic laboratory testing is difficult. The Crystal VC dipstick rapid test allows for early warning to officials about an outbreak of cholera, although the sensitivity and specificity are not ideal (CDC, 2018).
In countries that are not common to Cholera cases, WHO recommends using the following definition. In areas where cholera has not been declared, patients who are 2 years of age or older with acute watery diarrhea and severe dehydration. In areas where there is a declared outbreak, any person presenting with or dying from acute watery diarrhea. The use of crystal VC dipstick rapid tests can provide early warning of an outbreak to areas with limited or no lab testing. The sensitivity is not optimal therefore it is said to be confirmed using the traditional culture-based methods suitable for Vibrio cholerae (CDC, 2018).
Treatment
Those infected with cholera have mild diarrhea or little to no symptoms, the about 5-10% which are infected with Vibrio cholerea O1 may require treatment at a healthcare facility. This includes rehydration therapy which includes oral rehydration salts and intravenous fluids and electrolytes. In doing so, the fatalities reduce to well under 1% to all patients. Those with acute malnutrition also receive a low-osmolarity ORS solution to help regain the lost sodium which is not available in the generic rehydration therapy (ReSoMal). ReSoMal does not contain the sufficient sodium content needed to replace the amount lost from cholera.
Another viable option is the use of antibiotic treatment which is generally the use of antibiotics for children. Although hydration is the mainstay of cholera treatment, it can be used with the conjunction of antibiotics to those who are severely ill. It is particularly recommended for patients who are severely /moderately dehydrated and pass a large volume of stool. Generally, Doxycycline is the first line of treatment for adults while azithromycin is for children and pregnant women. Antibiotic use allows for reduces resource requirements, by decreasing the duration of diarrhea and stool volume it allows for rapid recovery and shorter inpatient stays which allow for optimal allocation of resources in an outbreak setting. There is so far insufficient data on examining the effect of antibiotics on the secondary transmission of cholera (CDC, 2018).
Zinc treatment is another viable option as it reduces the duration and severity of diarrhea in children. A study in Bangladesh showed that Zinc supplementation significantly reduces the duration and severity of diarrhea in children. This study was conducted on 179 children aged 3-14 who were admitted to the hospital within 24 hours of cholera symptoms. All children were given antibiotics and rehydration but those in the intervention group also received zinc. Those who received zinc had 8 fewer hours of diarrheal illness and 10% less diarrheal stool volume on average. Zinc is shown to have a similar effect in children with diarrhea caused by other infections as well as a recommended treatment for pediatric diarrhea (CDC, 2018).
Cholera Epidemiology and Disease Burden
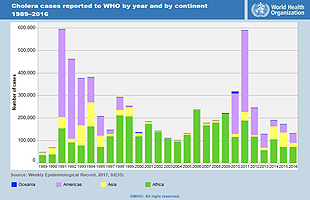
Investigators have estimated that there are approximately 1.3 to 4 million cases and 21000 to 143000 deaths due to cholera per year worldwide (WHO, 2019). According to WHO reports, the number of cholera cases have been rising over the last few years which is mainly due to overpopulation and scare access to sanitation facilities and usable clean water (WHO, 2019). Studies indicate that the universal burden of cholera is rising specifically in developing countries. Cholera can be either endemic or non-endemic (epidemic) (Mohammad Ali, 2012). A cholera-endemic area is a region where cholera cases were detected during the last three years with verification of domestic and local transmission while non-endemic regions are areas and countries where cholera does not occur on a regular basis. With regards to statistics, approximately 2.8 million and about 91000 people suffer and die from cholera disease, respectively in endemic countries every year. Additionally, another 87000 cases and 2500 deaths developed in non-endemic countries (Mohammad Ali, 2012). It is worth mentioning that, many cholera cases are missed to be reported worldwide mainly due to limitations in surveillance systems and fear of negative effects on both trade and tourism (Mohammad Ali, 2012).
Current Cholera Epidemic: The Civil War in Yemen
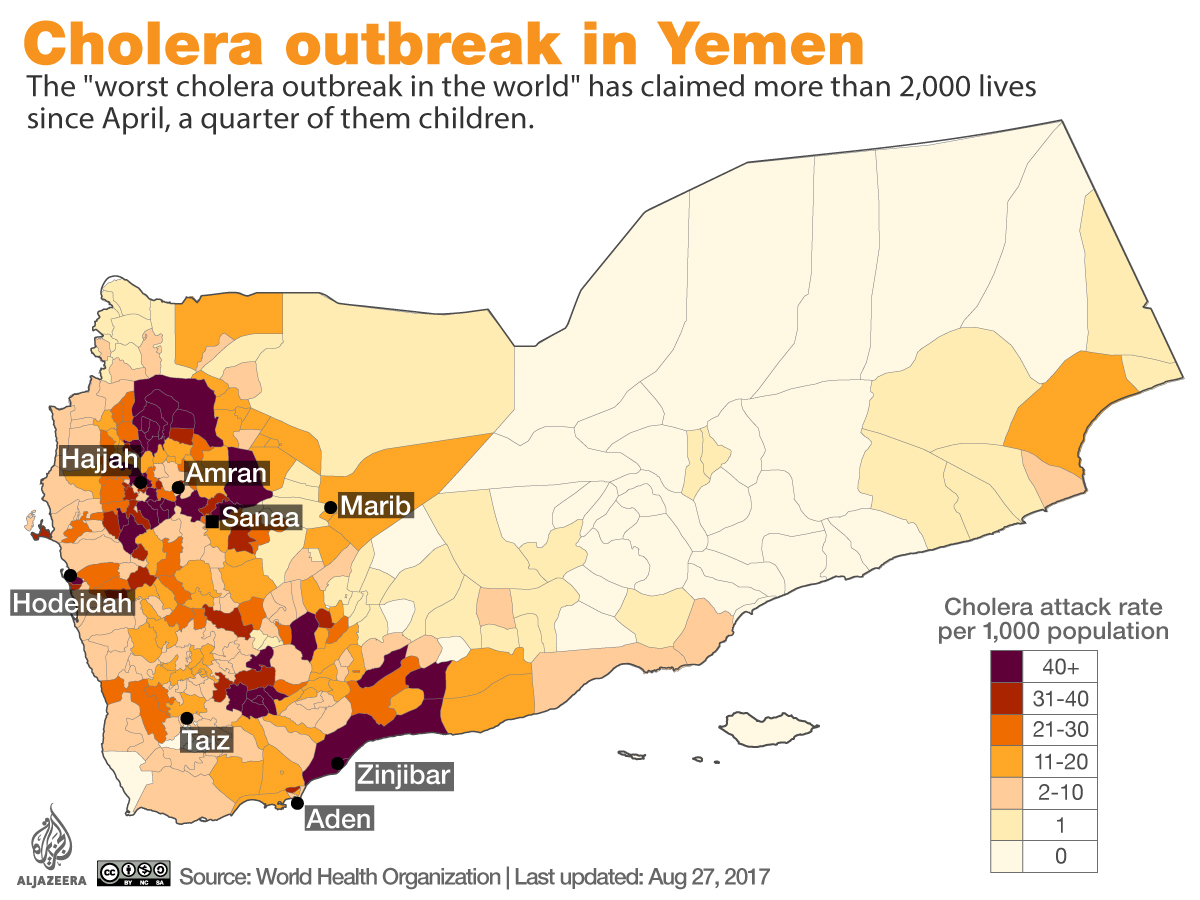
The civil war in Yemen has resulted in the world’s worst humanitarian crisis, causing a shattered health system in Yemen. The bombings have led to chronic shortages of supplies and staff, forcing half of the medical facilities to close since the civil war escalated (Doctors Without Borders, 2018). A lack of access to healthcare facilities and professionals has increased the spread of preventable infectious diseases, like cholera. The Yemeni population has become more vulnerable to deadly diseases. The recent cholera outbreak in Yemen is the world’s worst cholera epidemic, with roughly 10,000 suspected cases per week (WHO, 2018). Children under the age of five represent 25% of the suspected cholera cases in Yemen since January 2019 (WHO, 2019). According to the United Nations, the number of Yemenis with a suspected case of cholera passed one million in 2017 (United Nations, 2017). Yemenis suffering from malnutrition also suffer from a weakened immune system. Treating every patient that is starving is nearly impossible with the lack of healthcare infrastructure, staff, and supplies, further allowing Yemen to reach the state of a country-wide famine.
Researchers found that the cholera strain that first started the Yemen outbreak was related back to one seen in 2012 in South Asia. The strain spread globally before arriving in Yemen. Because of the fighting and blockade, around 16 million Yemenis do not have access to safe drinking water, sanitation, and basic healthcare, leading to the massive number of cholera cases (BBC, 2019).
Presentation
https://docs.google.com/presentation/d/1tqHuINjaMupkRVI-qkQpbWNar65LxENlei6vMskAl7I/edit?usp=sharing
References
Almagro-Moreno, S., Pruss, K., & Taylor, R. K. (2015). Intestinal Colonization Dynamics of Vibrio cholerae. PLOS Pathogens, 11(5). doi: 10.1371/journal.ppat.1004787
Barton, M. (2018). John Snow and the 1854 Cholera Outbreak. Retrieved from https://www.pastmedicalhistory.co.uk/john-snow-and-the-1854-cholera-outbreak/
Beaumont, P. (2019, April 17). Mounting concern over cholera health crisis in Yemen. Retrieved from https://www.theguardian.com/global-development/2019/apr/17/mounting-concern- over-cholera-health-crisis-in-yemen
Centers for Disease Control and Prevention. (2018, July 20). Diagnosis and Detection. Retrieved from https://www.cdc.gov/cholera/diagnosis.html
Centers for Disease Control and Prevention. (2018, July 20). Treatment. Retrieved from https://www.cdc.gov/cholera/diagnosis.html
Cholera. (n.d.). Retrieved from https://www.who.int/news-room/fact-sheets/detail/cholera
Davis, C. P. (2018, August 13). Cholera History, Causes, Spread, Treatment & Symptoms. Retrieved from https://www.medicinenet.com/cholera/article.htm#what_is_the_history_of_cholera
Eastern Mediterranean Region. (n.d.). Retrieved from http://www.emro.who.int/pandemic-epidemic-diseases/cholera/outbreak-update-cholera-in-yemen-1-september-2019.html
General Information. (2018, May 11). Retrieved from https://www.cdc.gov/cholera/general/index.html
Harris, J. B., LaRocque, R. C., Qadri, F., Ryan, E. T., & Calderwood, S. B. (2012). Cholera. Lancet (London, England), 379(9835), 2466–2476. https://doi.org/10.1016/S0140-6736(12)60436-X
Krebs, S. J., & Taylor, R. K. (2011). Protection and Attachment of Vibrio cholerae Mediated by the Toxin-Coregulated Pilus in the Infant Mouse Model. Journal of Bacteriology, 193(19), 5260–5270. doi: 10.1128/jb.00378-11
Nebehay, S. (2018, October 2). Yemen Cholera Outbreak Accelerates to 10,000+ Cases Per Week: WHO. Discover Thomson Reuters.
O’Neal, C., Jobling, M., Holmes, R., & Hol, W. (2005). Structural basis for the activation of cholera toxin by human ARF6-GTP. Science. doi: 10.2210/pdb2a5d/pdb
Suspected cholera cases in Yemen surpass one million, reports UN health agency | UN News. (2017). Retrieved from https://news.un.org/en/story/2017/12/640331-suspected-cholera-cases-yemen-surpass-one-million-reports-un-health-agency
The global burden of cholera. (2012, February 29). Retrieved from https://www.who.int/bulletin/volumes/90/3/11-093427/en/
Tuthill, K. (2003). John Snow and the Broad Street Pump on the Trail of an Epidemic. Cricket. 31(3), 23-31. Retrieved from https://www.ph.ucla.edu/epi/snow/snowcricketarticle.html World Health Organization. (n.d.). Cholera. Retrieved from https://www.who.int/health-topics/cholera#tab=tab_1
Yemen cholera epidemic strain 'came from eastern Africa'. (2019, January 3). Retrieved from https://www.bbc.com/news/world-middle-east-46746394
Yemen: Conflict and a shattered health system. (2018, October 26). Retrieved from https://www.doctorswithoutborders.ca/country/yemen-conflict-and-shattered-health-system