This is an old revision of the document!
Table of Contents
Weight loss supplements
History
 The late 1800s patent medicine era was when the first availability of diet pills was seen (Whittemore, 2019). They were known as fat reducers, which increased the metabolic rate as they were centered on thyroid extract (Whittemore, 2019). Even though they were known to be an effective way of losing weight, the pills had many side effects that were not expected including weakness, an increase in heart rate, and even death (Whittemore, 2019). Even with all the risks becoming noticeable, the availability of these pills continued until the 1960s (Whittemore, 2019).
The late 1800s patent medicine era was when the first availability of diet pills was seen (Whittemore, 2019). They were known as fat reducers, which increased the metabolic rate as they were centered on thyroid extract (Whittemore, 2019). Even though they were known to be an effective way of losing weight, the pills had many side effects that were not expected including weakness, an increase in heart rate, and even death (Whittemore, 2019). Even with all the risks becoming noticeable, the availability of these pills continued until the 1960s (Whittemore, 2019).
Then in the 1930s, dinitrophenol turned out to be a common medication for weight reduction (Whittemore, 2019). This drug led to several accidents that contributed to the Food and Drug Administration gaining more control and making new laws.
In the 1950s, the drug, amphetamine, was the popular choice (Whittemore, 2019). Soldiers were given this drug to keep them attentive during World War II. Since one of the effects was suppression of appetite, it led to people using these pills for weight reduction (Whittemore, 2019).
Ephedrine was used with caffeine in the 1970s to help patients that had asthma (Whittemore, 2019). People eventually started using this for weight loss (Whittemore, 2019). The Dietary Supplement Health and Education Act passed in 1994 in the United States (Whittemore, 2019). This act classified the herb, ephedra, which does not need FDA approval (Whittemore, 2019). This caused the spread in usage of ephedrine for obesity and also as an appetite suppressant(Whittemore, 2019).
In 1973, fenfluramine was a drug used for the treatment of weight loss (Whittemore, 2019). This drug became really popular in the 1990s where this drug became prescribed over 180,000,000 times in one year (Whittemore, 2019).
Many herbal-based weight loss supplements boomed in the 21st century (Whittemore, 2019). Throughout the years, researchers continuously developed new supplements and learned through their mistakes through testing (Whittemore, 2019).
Myths
1) Weight loss being a linear process A common misconception is that losing weight is a linear process. Everyone has days or even weeks where his or her body weights fluctuate. An example for women is that during their menstrual cycle, water weight fluctuates a lot. No matter how much the weight is fluctuating, as long as the weight is generally going down over time, the individual will achieve weight loss in the long term
2) Supplements help with weight loss A lot of supplements claim they have huge effects, but once they are studied, they are observed not to be very effective. There are a few that have modest effects and help weight loss through several months. One of the main reasons weight loss occurs for people that take supplements is the placebo effect of people wanting these supplements to work and end up being more conscious of their eating habits.
Regulations
Weight loss supplements are currently not approved by the Food and Drug Administration (FDA). Companies that manufacture supplements are responsible for the safety of the products and claims that they make. However, the FDA has the ability to investigate and take measures to remove products from the market, or issue warning letters, and criminally prosecute those who manufacture illegal diet products (“Beware of Products Promising Miracle Weight Loss”, 2018). The FDA also provides warning signs on their website and advice for consumers who wish to purchase weight loss supplements. The website outlines reported accounts of harm such as stroke, seizure, high blood pressure, and heart palpitations, among many other health risks. Moreover, the FDA has approved prescription medications for weight loss but they have guidelines for usage.
Why are Supplements a Popular Choice?
Despite the lack of regulation and potential risks associated with weight loss supplements, researchers have explored possible reasons for why patients are attracted to these products. Research by Saper, Eisenberg, and Phillips (2004) shows common reasons why patients that are overweight or obese choose supplements for weight loss. This includes:
- The desire for quick results
- Stigma in society associated with obesity
- They are available without prescription
- Does not require lifestyle changes associated with diet or exercise
- Previous negative experiences with diet or exercise
- Advertisement claims are inflated
- Advertised as “natural”
Different Types of Weight Loss Supplements
Different types of weight loss supplements can include various ingredients such as amino acids, minerals, herbs, vitamins, metabolites, and other substances such as enzymes (Boucher, Shafer, & Chaffin, 2001). In addition to this, they can come in different forms including tablets, powder, liquid or sprays. Pills are known as the most efficient form of weight loss supplements (Studley, 2019). Contrastingly, individuals who choose not to take pills usually consume shakes or drink mixes. These are made up of powders that are mixed with water. Tea blends are also common, which are usually caffeinated, and most blends consist of green tea. Sprays or ointments are the newest form, and are applied directly to areas that need to be targeted. Due to the novelty of this form of supplement, there is little research to demonstrate its effectiveness and reliability.
The effects of weight loss supplements on the body can vary depending on the type of supplement. One way supplements can enhance weight loss is by boosting metabolism (Studley, 2019). This outcome usually occurs from products containing stimulants such as caffeine, and they increase the number of calories that are burned. Another effect that weight loss supplements have on the body is suppressing appetite (“Dietary Supplements for Weight Loss”, 2017). These supplements mostly contain herbal extracts or fibre that make the individual feel full, so they consume less calories. Furthermore, there are enzyme supplements, which produce different effects in the body compared to the others. L-carnitine is a common enzyme found in these products (Pooyandjoo, Nouhi, Shab‐Bidar, Djafarian, & Olyaeemanesh, 2016). Its role in this case is to transport proteins and fats from storage, and convert them to energy.
Garcinia Cambogia
Description:
Garcinia Cambogia is a trendy weight loss supplement that is derived from the fruit of the same name (Chuah et al., 2013). Furthermore, Garcinia cambogia is a yellow or green fruit that is shaped like a pumpkin. Traditionally, Asian nations have utilized Garcinia to add flavor and as a substitute for tamarind or lemon (Sergio, 1988). It is commonly found in the Western Ghats mountain range in India and in the the Shola forests of Nilgiri. Garcinia has been associated with a wide variety of health effects including: antiobesity, antioxidative, antidiabetes, anti-inflammatory and anticancer effects (Chuah et al., 2013). The peel of the fruit contains hydroxycitric acid and this substance is responsible for the weight loss benefits associated with Garcinia. Garcinia is known to reduce body weight and food intake. Studies show that Garcinia can help with the obesity related complications of inflammation, oxidative stress, and insulin resistance. Several studies support the effects of hydroxycitric acid (HCA) on body weight loss, reduced fat intake, increased fat oxidation and greater energy expenditure. However, there is controversy regarding its efficacy and safety as an obesity dietary supplement.
Mechanism
There are two main methods by which Garcinia Cambogia can facilitate weight loss. It can either block fat production or reduce appetite.
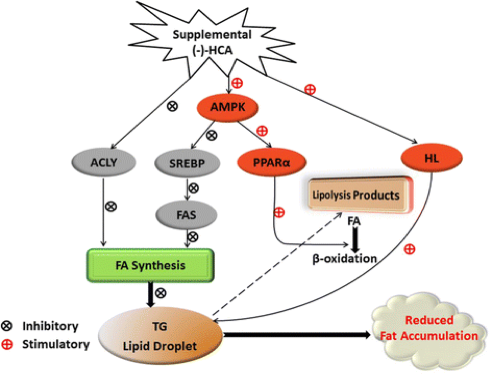
Hydroxcytric acid, the principal ingredient in Garcinia supplements, is a significant inhibitor of ATP. It specifically acts on ATP citrate lyase which is the primary enzyme that generates acetyl CoA from citrate in the cytosol of mammalian cells (Watson et al., 1969). Additionally, studies show that Hydroxcytric acid inhibits fatty acid synthesis in the liver through a series of biomechanical pathways. In summary, HCA inhibits lipogenesis by inhibiting ACLY, SREBP-1c and FAS expression, and accelerates lipolysis through amplifying HL activity and PPARα expression (Han, 2016). The following figure summarizes the methods by which hydroxycytric acid can reduce fat accumulation.
The exact mechanism by which hydroxcytric acid suppresses appetite is unclear, however, rat studies suggest that Garcinia Cambodia can raise levels of serotonin, an appetite suppressant (Ohia et al., 2001). Researchers have shown that hydroxcytric acid can increase the release and availability of serotonin in the synaptic cleft in cells of the rat cortex. Serotonin is known to regulate eating behavior and appetite control by which higher levels tend to reduce appetite (Ohia et al., 2001).
Clinical Studies
Various studies in animals support the use of hydroxcitrate as a supplement (Rao & Sakariah, 1988). In a particular study, Albino rats were fed a lipogenic diet with and without the addition of (−) hydroxycitrate for 15 days. After sacrificing the rats, the blood, liver and epididymal fat contents were measured and subsequently analyzed. Results indicated that rats who consumed hydroxycitrate had a significant reduction in food consumption, epididymal fat, serum triglyceride and an overall decrease in the feed efficiency ratio (Rao & Sakariah, 1988).
Some human trials also support the efficacy of hydroxcytric acid in reducing weight and fat (Preuss et al., 2004). A double-blind, placebo-controlled, human trial was conducted in thirty obese individuals for eight weeks in India. Results indicate that body weight and body mass index decline by 6.3% in the HCA group. Furthermore, total cholesterol and triglyceride levels were reduced by 6.3% and 8.6% while food intake was reduced by 4%. Urine analysis revealed a 125-258% increase in excretion of fat metabolites such as acetaldehyde, formaldehyde and acetone. Thus, it is evident that fat accumulation and weight loss can indeed occur by consuming HCA. Additionally, the study also noted that test participants had a 40% increase in serotonin, an appetite suppressant, which further provides support for the reduction in appetite (Preuss et al., 2004).
Side effects & recommended dosage:
Research suggest that garcinia cambogia is safe at dosages of 2,800 mg of HCA per day or lower (Chuah et al., 2012). Commonly, participants are encouraged to take 500 mg, three times per day and about 30–60 minutes before eating meals. The most common side effects of garcinia cambogia are gastrointestinal adverse events, headaches and skin rashes (Onakpoya et al., 2011). Some cases indicate that the supplement may cause hepatoxicity and liver failure in patients, however, more research is needed (Lunsford, 2016).
Conclusion
Overall there is controversy regarding the efficacy of HCA in inducing weight loss. In a particular study of 89 mildly overweight females, researchers found no support for the use of HCA in reducing weight, energy intake and other appetitive variables. Recently, studies on rats show that that garcinia cambogia may have anti-diabetes effects of reducing insulin levels, inflammation and raising insulin sensitivity (Sripradha & Magadi, 2015). Ultimately, further research is required on its efficacy as a clinical intervention.
Hydroxycut
Introduction
 Hydroxycut (C22-H18-11) has been labelled as a safe supplement, which aims to reduce the fat percentage in the body (Sharma et al., 2010). The developers initially reported no side effects, but in 2009 the FDA announced to the public, that the drug was reported to cause liver damage (Sharma et al., 2010). Hydroxycut products consisted of ephedra but were immediately told to remove it by the FDA in 2004, because of the side effects (LiverTox, 2018). Since Hydroxycut is used in many supplements, ingredients do vary but, they commonly have caffeine, green tea extract and various mixtures of plants with no known concentrations (LiverTox, 2018).
Hydroxycut (C22-H18-11) has been labelled as a safe supplement, which aims to reduce the fat percentage in the body (Sharma et al., 2010). The developers initially reported no side effects, but in 2009 the FDA announced to the public, that the drug was reported to cause liver damage (Sharma et al., 2010). Hydroxycut products consisted of ephedra but were immediately told to remove it by the FDA in 2004, because of the side effects (LiverTox, 2018). Since Hydroxycut is used in many supplements, ingredients do vary but, they commonly have caffeine, green tea extract and various mixtures of plants with no known concentrations (LiverTox, 2018).
Mechanism
The exact mechanism of action is unknown, but it has been said that green tea extract within the Hydroxycut supplements tend to impact the liver in a harmful manner. Green tea consists of catechins, these antioxidants, undergo oxidization via fermentation, that result in black tea (LiverTox, 2018). There are various types of catechins, but the most active type is called epigallocatechin 3-gallate (EGCG) (LiverTox, 2018). In intensive research studies, it has been determined that in higher dosages, this active ingredient tends to cause hepatocellular injury in animal models, such as mice (LiverTox, 2018). The dosage that was administered in the model animals was equivalent to 30-90 mcg/kg, which is very high compared to most weight loss supplements (LiverTox, 2018).
Clinical Studies
In a study conducted by Fong and colleagues (2010) hepatoxicity due to Hydroxycut was investigated. 24 cases from the FDA were obtained and out of the 24, 9 were chosen for further analysis. All patients previously were healthy but developed an acute hepatic injury due to Hydroxycut. It was assumed that participants in the study, all took the recommended dosage that the manufacturers of the supplement stated. All cases of the patients were then assessed to find the causality and damage of each patient via the Drug- Induced Liver Injury Network (DILIN) study (Fong et al., 2010). The results showed that 8 patients who had an acute liver injury, occurred after taking Hydroxycut, all patients were hospitalized. Three of the eight patients required a liver transplant (Fong et al., 2010). This study demonstrated the impact of Hydroxycut on the users.
In another study conducted by Dehoney and Wellein (2009), rhabdomyolysis was diagnosed in patients that had taken the supplement. The researchers looked into a specific case study of an 18-year-old Caucasian man who was sent to the emergency room since he was suffering from bilateral leg pain and weakness (Dehoney & Wellein., 2009). When he was asked by the practitioners, if he took any medications, he had stated that he had taken Hydroxycut, 4 tablets a day. It was determined from this information and further tests, that he had elevated levels of creatine kinase, serum creatinine and aspartate transaminase (Dehoney & Wellein., 2009). He was sent home the same day, and was told to not use Hydroxycut. Two weeks later, his creatine kinase and liver enzymes reached homeostasis (Dehoney & Wellein., 2009). Therefore, from this study, we can conclude that Hydroxycut does result in the symptoms of rhabdomyolysis and results in fluctuations in the body serum.
Side Effects & Recommended dosage
The main side effect from using Hydroxycut is liver failure, but other side effects include seizures, cardiovascular disorders, rhabdomyolysis, nausea, vomiting, light-coloured stools, excessive fatigue, and etc. (MedicineNet, 2009). Users are told to take no more than two capsules within the time span of four hours and no more than 4 capsules in a day (LiveStrong, n.d.). These instructions are labelled on every bottle. Since one of the main ingredients of Hydroxycut is caffeine (100 mg), it is highly recommended to drink about eight glasses of water after taking the supplement (LiveStrong, n.d.).
Synephrine
Introduction
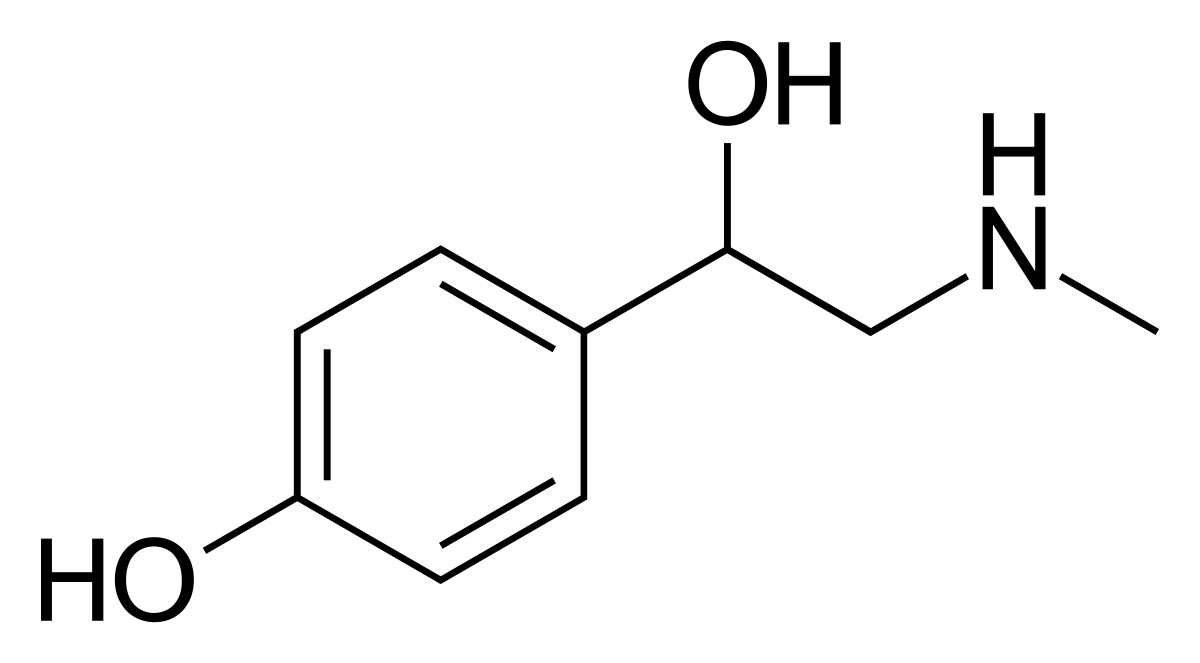
The phytochemical, synephrine, is an alkaloid molecule that is actively present in many vegetable species and citrus fruits, most commonly as an extract from the peel of bitter orange, known as Citrus aurantium (CA). Synephrine can exist in three different isomeric forms which include meta-synephrine (m-synephrine), ortho-synephrine (o-synephrine) and para-synephrine (p-synephrine), however, many studies show that only p-synephrine is found naturally in CA fruits (Rossato et al., 2011). With that said, p-synephrine is a potent stimulant that is widely included as an ingredient in many sports performance products, weight management and weight loss supplements. Historically, the use of bitter orange has been previously used in for clinical purposes in traditional Chinese medicine to treat digestive problems like diarrhea, as well as in South American folk medicine to treat anxiety and epilepsy (Stohs, Preuss, & Shara, 2011). In addition, p-synephrine is similar in chemical structure to ephedrine, which has been banned by the FDA, and is widely used in lieu of ephedrine as it’s much safer (Rossato et al., 2011).
Mechanism
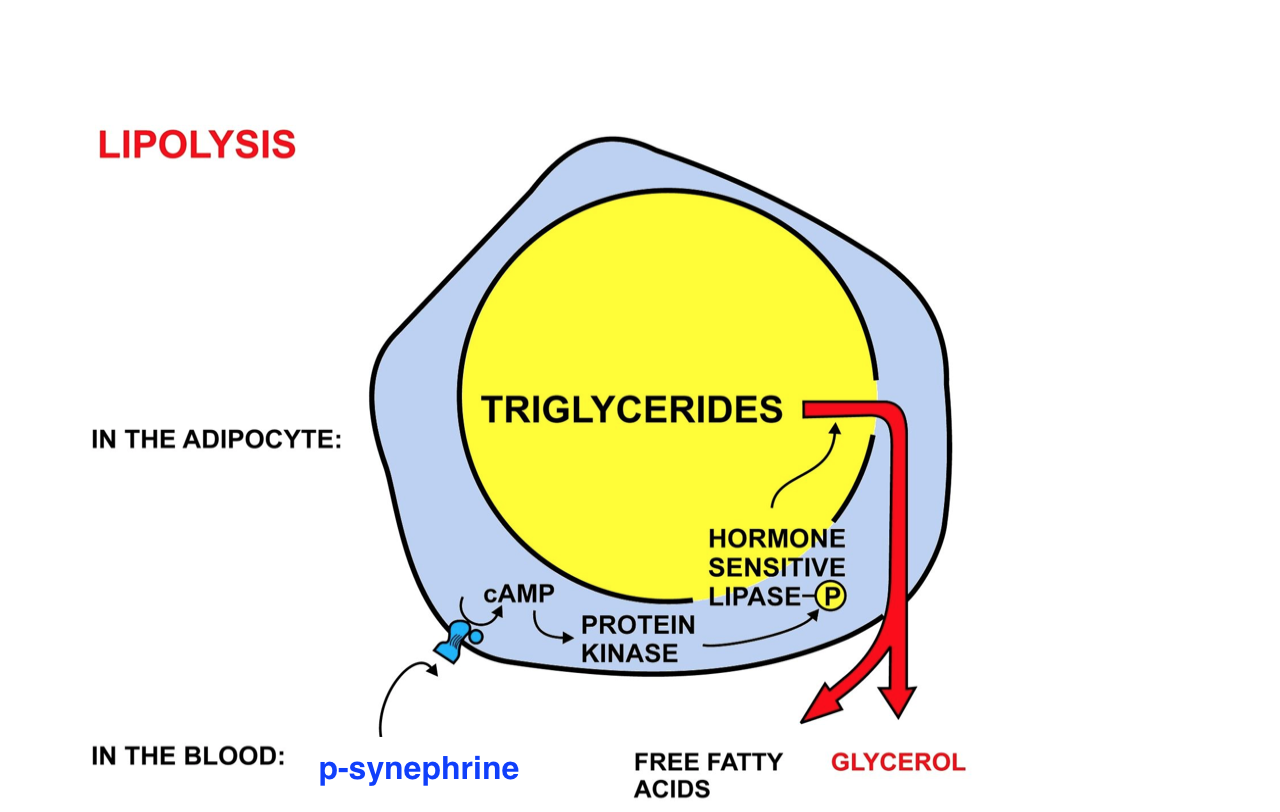
P-synephrine acts in the body through high affinity receptor binding to adrenergic receptors, a class of G protein-coupled receptors that stimulate the sympathetic nervous system (SNS). Predominantly, p-synephrine acts as a beta-3 adrenergic receptor agonist to increase thermogenesis and lipolysis, both of which aid in weight loss through appetite suppression, lipid metabolism and lipid breakdown (Stohs et al., 2011). That being said, these beta-3 adrenergic receptors are located within white and brown adipose tissue and the binding with p-synephrine leads to a cascade of events mediated by the adenylyl cyclase/cAMP/protein kinase A signaling cascade with downstream production of nitric oxide (NO). In a study by Alemzadeh et al. (2008), they looked at the effects of activated beta-3 adrenergic receptor function in adipose tissues on obese rats and observed reduction in food intake and significant weight loss. Thus, p-synephrine consumption leads to the following:
- Reduced food intake & weight gain
- Enhanced thermogenesis & lipolysis
- Improved insulin resistance, glycemic control & cholesterol metabolism
- Increased blood pressure & heart rate
Figure 1: Illustration of the lipolysis pathway where p-synephrine binds to beta-3 adrenergic receptors on adipose tissue and activates to simulate fat breakdown and thermogenesis.
Clinical Study
A clinical study by Gutiérrez‐Hellín & Coso (2016), examined the lipid oxidative effects of acute p-synephrine injection during exercise and rest in a double-blind, randomized trial. Here, they gathered a sample size of 18 healthy individuals and performed 2 experimental trials after consumption of 3 mg kg-1 of p-synephrine. As a result, the acute ingestion did not change energy consumption or lipid oxidation when at rest when compared to the placebo control. On the other hand, during exercise the rate of fat oxidation increased significantly and the individual’s energy consumption was not affected (Gutiérrez‐Hellín & Coso, 2016).
Side Effects and Recommended Dosage
There are some side effects reported from consuming products that contain p-synephrine, which include elevated blood pressure, alternation of heart rate and cardiotoxicity, hypertension, nausea and vomiting. The FDA regulates bitter orange and the amount of p-synephrine intake that is allowed in a substance. The daily dosage limit for bitter orange is 4-6 g in drugs, 2-3 g in tincture and 1-2g in extract. In addition weight loss formulas typically contain around 100 to 200 mg of bitter orange extract, which provides 10-40mg of p-synephrine (Integrated Laboratory Systems, 2004).
Conclusion
When selecting and determining the efficacy of fat loss supplements it is important to examine the various ingredients listed on the label. The supplement industry is a for-profit business and oftentimes, there may be unsubstantiated and exaggerated claims of weight loss and it is therefore more crucial to look at relevant scientific studies to validate such statements. Furthermore, it is also imperative to critically analyze the studies that were performed and determine whether it the results can be applicable to human dosages. For example, studies done on raspberry ketones may show some promise in rodents, but has no evidence of efficacy in humans. Moreover, when converting rodent to human dosage, the required supplementation becomes astronomically high. Other critical variables regarding dietary supplements also include safety and toxicity. It is strongly advised for individuals looking at fat loss supplements to do their own extensive research on individual compounds and risks involved.
References
[1] Alemzadeh, R., Karlstad, M. D., Tushaus, K., & Buchholz, M. (2008). Diazoxide enhances basal metabolic rate and fat oxidation in obese Zucker rats. Metabolism: Clini
[2] Dehoney, S., & Wellein, M. (2009). Rhabdomyolysis associated with the nutritional supplement Hydroxycut. American Journal of Health-System Pharmacy, 66(2), 142–148. https://doi.org/10.2146/ajhp070640
[3] LiverTox. (2018). Hydroxycut. Retrieved January 27, 2019, from https://livertox.nih.gov/Hydroxycut.htm#structure
[4] Jill Corleone. (n.d.). How to Use Hydroxycut Effectively | Livestrong.com. Retrieved January 27, 2019, from https://www.livestrong.com/article/375287-how-to-use-hydroxycut-effectively/
[5] MedicineNet. (2009). FDA Warns to Stop Using Hydroxycut Products Information on MedicineNet.com. Retrieved January 27, 2019, from https://www.medicinenet.com/script/main/art.asp?articlekey=99871
[6] Fong, T.-L., Klontz, K. C., Canas-Coto, A., Casper, S. J., Durazo, F. A., Davern, T. J., … Seeff, L. B. (2010). Hepatotoxicity Due to Hydroxycut: A Case Series. The American Journal of Gastroenterology, 105(7), 1561–1566. https://doi.org/10.1038/ajg.2010.5
[7] Sharma, T., Wong, L., Tsai, N., & Wong, R. D. (2010). Hydroxycut(®) (herbal weight loss supplement) induced hepatotoxicity: a case report and review of literature. Hawaii Medical Journal, 69(8), 188–90. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20845283
[8] Dara, L., Hewett, J., & Lim, J. K. (2008). Hydroxycut hepatotoxicity: a case series and review of liver toxicity from herbal weight loss supplements. World Journal of Gastroenterology, 14(45), 6999–7004. https://doi.org/10.3748/WJG.14.6999
[9] Chuah, L. O., Ho, W. Y., Beh, B. K., & Yeap, S. K. (2013). Updates on Antiobesity Effect of Garcinia Origin (-)-HCA. Evidence-Based Complementary and Alternative Medicine : ECAM, 2013, 751658. https://doi.org/10.1155/2013/751658
[10] Chuah, L. O., Yeap, S. K., Ho, W. Y., Beh, B. K., & Alitheen, N. B. (2012). In Vitro and In Vivo Toxicity of Garcinia or Hydroxycitric Acid: A Review. Evidence-Based Complementary and Alternative Medicine, 2012, 1–12. https://doi.org/10.1155/2012/197920
[11] Gutiérrez‐Hellín, J., & Coso, J. D. (2016). Acute p-synephrine ingestion increases fat oxidation rate during exercise. British Journal of Clinical Pharmacology, 82(2), 362–368. https://doi.org/10.1111/bcp.12952
[12] Han, J., Li, L., Wang, D., & Ma, H. (2016). (−)-Hydroxycitric acid reduced fat deposition via regulating lipid metabolism-related gene expression in broiler chickens. Lipids in Health and Disease, 15(1), 37. https://doi.org/10.1186/s12944-016-0208-5
[13] Lunsford, K. E., Bodzin, A. S., Reino, D. C., Wang, H. L., & Busuttil, R. W. (2016). Dangerous dietary supplements: Garcinia cambogia -associated hepatic failure requiring transplantation. World Journal of Gastroenterology, 22(45), 10071. https://doi.org/10.3748/wjg.v22.i45.10071
[14] Ohia, S. E., Awe, S. O., LeDay, A. M., Opere, C. A., & Bagchi, D. (n.d.). Effect of hydroxycitric acid on serotonin release from isolated rat brain cortex. Research Communications in Molecular Pathology and Pharmacology, 109(3–4), 210–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11758650
[15] Onakpoya, I., Hung, S. K., Perry, R., Wider, B., & Ernst, E. (2011). The Use of Garcinia Extract (Hydroxycitric Acid) as a Weight loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Journal of Obesity, 2011, 1–9. https://doi.org/10.1155/2011/509038
[16] Preuss, H. G., Bagchi, D., Bagchi, M., Rao, C. V. S., Satyanarayana, S., & Dey, D. K. (2004). Efficacy of a novel, natural extract of (–)-hydroxycitric acid (HCA-SX) and a combination of HCA-SX, niacin-bound chromium and Gymnema sylvestre extract in weight management in human volunteers: a pilot study. Nutrition Research, 24(1), 45–58. https://doi.org/10.1016/J.NUTRES.2003.09.007
[17] Rao, R. N., & Sakariah, K. K. (1988). Lipid-lowering and antiobesity effect of (−)hydroxycitric acid. Nutrition Research, 8(2), 209–212. https://doi.org/10.1016/S0271-5317(88)80024-1
[18] Rossato, L. G., Costa, V. M., Limberger, R. P., Bastos, M. de L., & Remião, F. (2011). Synephrine: From trace concentrations to massive consumption in weight-loss. Food and Chemical Toxicology, 49(1), 8–16. https://doi.org/10.1016/j.fct.2010.11.007
[19] Sergio, W. (1988). A natural food, the Malabar Tamarind, may be effective in the treatment of obesity. Medical Hypotheses, 27(1), 39–40. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3205204
[20] Sripradha, R., & Magadi, S. G. (2015). Efficacy of Garcinia Cambogia on Body Weight, Inflammation and Glucose Tolerance in High Fat Fed Male Wistar Rats. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH, 9(2), BF01-4. https://doi.org/10.7860/JCDR/2015/12045.5577
[21] Stohs, S. J., Preuss, H. G., & Shara, M. (2011). The Safety of Citrus aurantium (Bitter Orange) and its Primary Protoalkaloid p-Synephrine. Phytotherapy Research, 25(10), 1421–1428. https://doi.org/10.1002/ptr.3490
[22] Watson, J. A., Fang, M., & Lowenstein, J. M. (1969). Tricarballylate and hydroxycitrate: Substrate and inhibitor of ATP: Citrate oxaloacetate lyase. Archives of Biochemistry and Biophysics, 135, 209–217. https://doi.org/10.1016/0003-9861(69)90532-3
[23] White, C. P., Hitchcock, C. L., Vigna, Y. M., & Prior, J. C. (2011). Fluid retention over the menstrual cycle: 1-year data from the prospective ovulation cohort. Obstetrics and gynecology international, 2011.
[24] WHITTEMORE, F. (2019). The History of Diet Pills | Livestrong.com. Retrieved from https://www.livestrong.com/article/74336-history-diet-pills/
[25] Gunnars, K. (2019). Top 12 Biggest Myths About Weight Loss. Retrieved from https://www.healthline.com/nutrition/top-12-biggest-myths-about-weight-loss#section11