Table of Contents
What are Synthetic Antibody Mimics (SyAMs)?
Synthetic antibody mimics are synthetic organic molecules which are approximately one-twentieth the size of antibodies. These molecules have various advantages as they are composed of only 5 % of the molecular weight of natural antibodies, they are thermally stable (thus they are able to be stored at room structure) and can be orally administered. Other benefits include their inexpensive production and their limited adverse effects on the immune system. In essence, synthetic antibody mimics function by creating a link between target cells and immune cells to initiate a specific and targeted immune response (McEnaney et al., 2014).
Fighting Against Disease
The Body's Natural Defenses
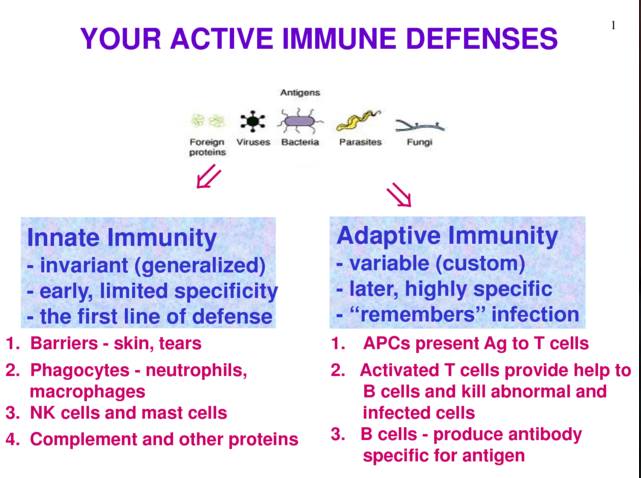
The human body is generally quite effective in targeting and eliminating disease-causing agents (e.g. viruses & bacteria). It accomplishes this via its immune system which has two branches: Innate immune system and Adaptive immune system. The innate immune system is quite general and fights anything foreign in the body immediately. Examples of the innate immune system include physical barriers such as epithelial layers as well as chemical barriers such as mucosal layers and innate infection fighting cells such as macrophages (Parham, 2014). The adaptive immune system is more specific and takes some time to activate; however, it is responsible for clearing those pathogens that are able to evade the innate immune system and infect various cells within the human body. The adaptive immune system is heavily reliant on antibodies. These antibodies are produced by Plasma cells which are B-cells primed by dendritic cells upon contact of infectious pathogen in lymphatic system of the human body (Parham, 2014).
Figure 1: Highlights the main differences and specific functions of innate and adaptive immune systems in response to pathogens (Chu, 2014).
Antibodies are composed of 4 polypeptide chains which include two identical heavy and light chains. The heavy chains are derived from 5 different types of crystallisable fragments (Fc) (Burke, 2015). These Fc regions in turn give rise to the 5 antibody isotypes: IgA, IgD, IgE, IgG & IgM. Each Fc region of a given antibody isotype is able to bind to specific Fc Receptors (FcR) on a target cell such as a virally infected cell. This leads to the recruitment of other immune cells (e.g. NK cells, cytotoxic T-cells etc.) through the release of signalling molecules. Ultimately, the interaction between the target cell and the antibody results in the degradation or destruction of the target cell (Burke, 2015).
Immunotherapy
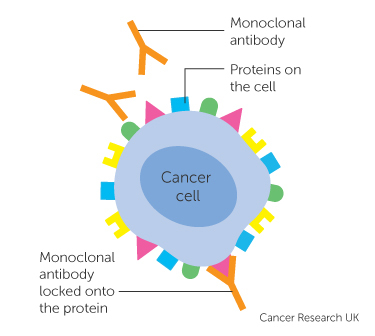
Sometimes the human body fails in fighting particularly evasive viruses or controlling tumour growth resulting in conditions such as cancer. In this case the body’s natural defenses can be manipulated via immunotherapy or biologic therapy. This type of therapy commonly uses monoclonal antibodies (mAbs), which are antibodies produced in-vitro via a single clone of cells (Weiner, Surana, & Wang, 2010). These mAbs are designed to attach to specific proteins on a pathogen so as to illicit an immune response by recruiting other immune cells to degrade or destroy the pathogenic cell. This is done in the case that the body produces its antibodies at too slow a rate or otherwise fails to recognize a pathogen as foreign or disease causing.
Figure 2: This figure illustrates the interaction between a monoclonal antibody and an antigen on a cancer cell. Once the mAb attaches to the cancer cell it can recruit other immune cells (e.g. NK cells, macrophages etc.) to destroy the associated cancer cell (Burke, 2015).
As promising as the potential of mAbs sound, they have several disadvantages including life-threatening allergic responses, greater difficulty in the penetration of tissues due to their large composition and high mass as well as high production costs (Chames, Van Regenmortel, Weiss, & Baty, 2009). Consequently, David Spiegel and his colleagues generated a new class of organic molecules: Synthetic Antibody Mimics (SyAMs). This new type of molecule was discovered when trying to bring the target prostate cancer cell closer to immune cell to elicit an immune response (McEnaney et al., 2014).
Synthetic Antibody Mimics to target Prostate Cancer Cells (SyAM-Ps)
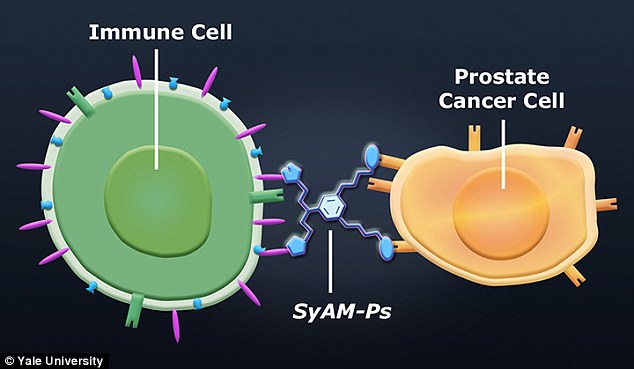
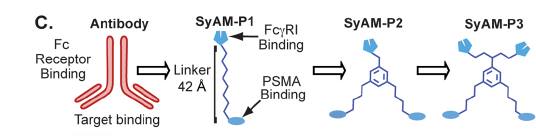
The science of synthetic antibody mimics to target disease was started in 2011 with the creation of synthetic antibody mimics to target prostate cancer cells (SyAM-Ps). Theoretically, the SyAM-P works by attaching itself to a specific molecule on the membrane of the prostate cancer cell (i.e. PSMA) while simultaneously attaching itself to receptors on immune cells (e.g. FcγRI) to elicit an immune response (Brigandi, 2016). This is depicted in Figure 3 below.
Figure 3:This figure depicts the ligand-receptor binding nature of the SyAM-P molecule (Gray, 2015).
Synthesis & Effector Function of SyAM-Ps
Prostate Specific Membrane Antigens
Prostate Specific Membrane Antigens (PSMA) are a type of membrane glycoprotein located on the surface of prostate cells. They are structurally composed of an intracellular segment, a transmembrane domain and an extracellular domain. In theory, PSMAs would serve as the most potent targets in treating prostate cancer as they are almost exclusively expressed in the prostate and is one of the most highly expressed proteins in prostate cancers. Thus, targeting PSMAs would result in a highly specific immune response that would minimize damage to healthy cells. While the exact function of PSMAs is poorly understood, researchers have hypothesized its role in immune-invasive procedures (McEnaney et al., 2014).
 Figure 4: Shows the crystallized asymmetric structure of PSMA (McEnaney et al., 2014).
Figure 4: Shows the crystallized asymmetric structure of PSMA (McEnaney et al., 2014).
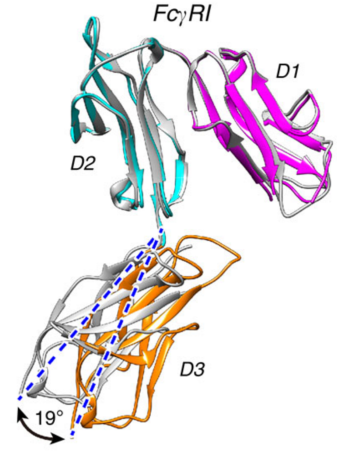
FcγRI
The second component of the SyAM-P is to attach itself onto an antibody. Typically, Immunoglobulin G (IgG) antibodies are chosen as they are the most abundant isotype in the body, making it preferable to be linked with the PSMA and the SyAM-P (McEnaney et al., 2014). The attachment process includes the SyAM-P molecule binding to the FcγRI (Fc gamma receptor 1) binding domain found on the IgG antibody. Once this interaction occurs, a set of intracellular reactions results in the release of cytokines that leads to pro-inflammatory responses against the cancer cell or leads to the activation of macrophages. In turn, these macrophages can engulf the cancer cells through a process called phagocytosis (Chu, 2014).
Figure 5: Shows the crystallized structure of FcγRI (McEnaney et al., 2014).
Synthesis
SyAM-Ps were created to bring together PSMA on prostate cancer cells, and FcγRI on antibodies to initiate an immune response as depicted in Figure 3 above. In order to synthesize SyAM-Ps, researchers analyzed different molecules which would selectively and favourably bind to PSMA and FcγRI.
Spiegel and colleagues identified a Glutamate Urea binding domain that was capable of binding PSMA with high affinity and specificity. Using this principle, they synthesized an antibody-recruiting molecule (ARM) that targeted prostate cancer cells in a specific manner. This ARM named as ARM-P8 was utilized for incorporation into the SyAM-P molecule (McEnaney et al., 2014). The other aspect of the SyAM-P includes a type of peptide called CP33 cyclic peptide that binds to the FcγRI in a highly selective manner. An ideal length of 40-42 Å was used as the linker length between the two ligands. Using these two ligands (ARM-P8 & CP33 cyclic peptide) three consecutive molecules of SyAM-P: SyAM-P1, SyAM-P2 and SyAM-P3 were constructed (McEnaney et al., 2014).
Figure 6: Figure showing different models of SyAMs: SyAM-P1, SyAM-P2 & SyAM-P3 (McEnaney et al., 2014).
As can be seen from the above figure, the differing trait between the 3 analogs is the number of receptors for PSMA and FcγRI (i.e. a greater # of ARM-P8s and CP33 cyclic peptides).
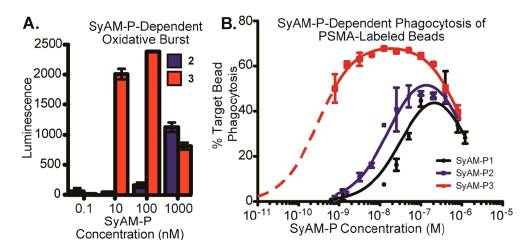
The results of the SyAM-P study demonstrated the greatest effectiveness of the SyAM-P3 analog in eliciting a maximal immune response. The molecular structure of SyAM-P3 is shown in Figure 8. Figure 7B illustrates this as the labeling of PSMA with SyAM-P3 led to the phagocytosis of 70% of beads in comparison to the phagocytosis of 40 % and 50 % of the beads from SyAM-P1 & SyAM-P2, respectively (McEnaney et al., 2014). Furthermore, the presence of oxidative burst (a process that causes target cell rupturing due to the release of reactive oxygen radicals (Chu, 2014)) was much more evident the SyAM-P3 assays compared to that of SyAM-P2. This is demonstrated in Figure 7A. This can be explained by the greater number of FcγRI-ligating motifs present on the SyAM-P3 molecule which lead to a more effective immune response in targeting the cancer cells. Thus, increasing the number of FcγRI-ligating motifs enhanced the potency and efficacy of SyAM-P molecules in eliciting a targeted immune response. The figure below also shows a significant concentration of PSMA is needed to initiate phagocytosis or oxidative burst. Therefore cells not part of the tumour are less likely to be targeted by SyAM-Ps to initiate an immune response.
Figure 7A & 7B: Depicts the efficacy of SyAM-P-Dependent Oxidative Burst and SyAM-P-Dependent Phagocytosis of PSMA labeled assays between SyAM-P1, SyAM-P2 & SyAM-P3 (McEnaney et al., 2014).
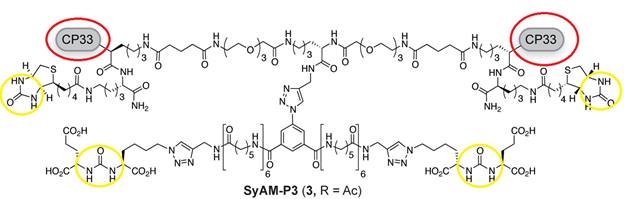
Figure 8: Molecular structure of SyAM-P3. Circled in red are the 2 FcγRI-ligating motifs of SyAM-P3 . Circled in yellow are the 4 glutamate binding motifs (which are the main components of the ARM-P8s) which bind to the PSMAs (McEnaney et al., 2014).
Effector Function
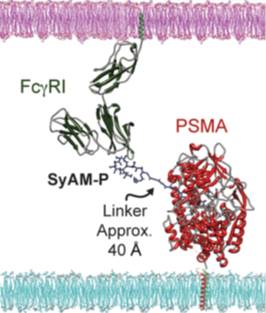
When complexed with the PSMA and FcγRI, SyAM-Ps are effective in initiating the body’s immune responses which include phagocytosis and oxidative burst. This is demonstrated above in Figure 7A & 7B. In addition, the smaller composition of SyAM-Ps leads to a greater affinity as they are able to minimize the distance between the FcγRI and its target ligand to 40 Å in comparison to the conventional 140 Å that occurs in the normal immune response (McEnaney et al., 2014). This phenomenon is illustrated Figure 8. The minimized length between the FcγRI receptor and the PSMA antigen is quite effective as it limits the interference of other molecules present on other cells that could attach to the FcγRI receptor. As a result, the desired immune response is achieved with a higher degree of efficacy when using SyAM-P molecules (McEnaney et al., 2014).
Figure 8: Shows the molecular interaction between PSMA on prostate cancer cell and FcγRI on antibodies (McEnaney et al., 2014).
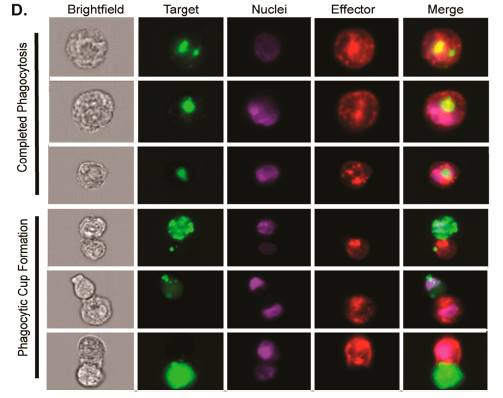
SyAM-P dependent phagocytosis was further studied using AMNIS (instrument used to observe and picture a standard flow cytometry (Sharpe & Freeman, 2002)). The process of phagocytosis results from the recruitment of macrophages or other phagocytic immune cells possessing the FcγRI. The FcγRI immune cells are then linked with the target prostate cancer cell that expresses PSMA which induces phagocytosis. This is demonstrated in Figure 8 where the target cell (green) is gradually engulfed by the macrophage (red). AMNIS showed that SyAM-P induced phagocytosis worked similar to natural immune responsive phagocytosis where the same steps take place: cell-cell attachment, phagocytic cup formation and complete target cell engulfment to allow for target cell degradation.
Figure 7 [5]: AMNIS showing the typical phagocytic processes that exemplify early, intermediate and late stages of phagocytosis. A bright-field view is also provided on the right of the diagram (McEnaney et al., 2014).
Critiques and Potential Drawbacks of SyAMs
Though the prospect of synthetic antibody mimics has the potential to be studied further and developed into treatments for various conditions, there is much to be researched and tested before a conclusion is reached regarding their efficiency.
Lack of Extensive Testing
SyAM research has been limited to the laboratory (in-vitro). No testing has been conducted in humans or animals; in fact there are no current studies in place that could potentially explore the effectiveness of SyAMs in-vivo. Although the necessary compounds for further testing have been isolated, the research process may take a further 1-2 years for in-vivo trials to begin and then a further six to ten years in order develop and produce a new drug containing SyAMs (Government of Canada, 2001). Within this time frame fundamental information must emerge on SyAMs of which there is currently no existing documentation including systemic and long-term effects, side effects, dosage information and success rate of SyAMs (Chames, Van Regenmortel, Weiss & Baty, 2009). Therefore, it is difficult to determine long-term effects and any disadvantages associated with SyAMs, as the research has not kept up with the science.
Limited Usage in Broad Spectrum of Disease
Given the high specificity of SyAM's, i.e. that each ligand is specifically created to target a specific receptor on a specific disease-causing cell, new SyAMs have to be created for each new disease or cancer type. This must be done in a relatively quick and efficient manner before the patient is forced to undergo chemotherapy since chemotherapeutic agents induce the expression of new death receptors: TNF and TNF-related apoptosis-inducing ligand receptors which render antibodies inefficient (Bracci, Schiavoni, Sistigu & Belardelli, 2014). Without effective antibodies the SyAMs would not be able to bind to the receptors on the antibody and therefore no immune response will occur, rendering SyAMs practically useless. Furthermore, patients that undergo chemotherapy will experience rapid mutations in the structure of their tumour cells and thus specific receptors on tumour cells may rapidly change in structural conformation, thereby preventing the SyAM to bind to the receptor and therefore no immune response will occur.
Over-reaching Claims: Curing HIV
Although SyAMs truly hold a lot of potential, a general myth has begun to emerge in that they may be used to target and eliminate HIV. Although current evidence shows that synthetic antibody mimics have the potential to be ingested similar to over-the-counter medications for prostate cancer, there is an overreaching claim made that the same concepts can be used to treat HIV (Shelton, 2014) (Grey, 2015).
HIV particles replicate and multiply within T-helper cells when HIV RNA is released into the cell and the overall effects cause a weakened immune system (Klein, Gnanapragasam, Galimidi, Foglesong, West & Bjorkman, 2008). HIV particles have very unique proteins on their outer membrane to which normal antibodies cannot bind (Klein et al., 2008). Furthermore, specific proteins that would preferentially bind to the HIV receptors are yet to be elucidated and without those proteins construction of SyAMs cannot begin.
Future Applications
Beyond its potential for treating prostate cancer, SyAms may have applications for treating other forms of cancer, and various bacterial and viral diseases. The ability to do so is possible by controlling the framework and properties of the antibody and by creating the optimal characteristics for the binding of some protein to receptor on the cancer or bacteria / virus. In-vitro construction of the SyAMs would allow the creation of accurately engineered binding specific proteins, which would ultimately cause the body to trigger a natural immune response via the created SyAM (Brigandi, 2016).
Moreover, SyAms are becoming more popular due to the fact that they are less likely to instigate an adverse immune response because of their small molecular mass, high specificity and thermal stability (McEnaney et al., 2014). The fact that antibodies are proteins makes them generally more challenging to produce (Sharpe & Freeman, 2002), and even when one is able to manufacture them, proteins denature over time - another disadvantage (Davis, 2005). On the other hand, SyAms are made of peptides instead of proteins, making them more resistant to denaturing. Additionally, SyAms are cheaper and easier to produce than antibodies (McEnaney et al., 2014). Patients would be able to take the SyAms orally like conventional drugs (McEnaney et al., 2014), which results in a more efficient immune response, viable because of their compact size. This can be vastly advantageous to patients with cancers and autoimmune diseases, like multiple sclerosis in which patients undergo maddening, monoclonal antibody therapies. Additionally, the small size of the antibody mimics can be used as an asset to eliminate tumors. Although SyAms are currently in the beginning stages, the efforts made by the immunologists and bioengineers show SyAms will have the potential for treating a broad range of bacterial diseases and other complications.
References
[1] Bracci, L., Schiavoni, G., Sistigu, A., & Belardelli, F. (2014). Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death & Differentiation, 21(1), 15-25.
[2] Brigandi, R. (n.d.). The development of synthetic antibody mimics to improve immunotherapy methods. Retrieved January 26, 2016, from http://www.pitt.edu/~rpb38/wassignment2.html
[3] Burke, J. (2015, September 14). Multiple Melanoma: The Antibodies are Coming!. Retrieved from http://www.rockymountaincancercenters.com/wp-content/uploads/2015/08/Monoclonal-antibody1.jpg
[4] Chames, P., Van Regenmortel, M., Weiss, E., & Baty, D. (2009). Therapeutic antibodies: successes, limitations and hopes for the future. British journal of pharmacology, 157(2), 220-233.
[5] Chu, A. (2014). Immune System Defenses. 5-9.
[6] Davis, M.I. (2005). Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. RCSB PDB. 102, 5981-5986.
[7] Government of Canada. (2001). How Drugs are reviewed in Canada [fact sheet]. Retrieved from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/fs-fi/reviewfs_examenfd- eng.php
[8] Gray, R. (2015). An immune system in a pill? First synthetic antibodies created that could one day treat cancer and even HIV. Science and Tech.
[9] Klein, J.S., Gnanapragasam, P. N. P., Galimidi, R. P., Foglesong, C. P., West, A. P., & Bjorkman, P.J. (2008). Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proceedings of the National Academy of Sciences, 106, 7385-7390.
[10] McEnaney, P. J., Fitzgerald, K. J., Zhang, A. X., Douglass Jr, E. F., Shan, W., Balog, A., … & Spiegel, D. A. (2014). Chemically Synthesized Molecules with the Targeting and Effector Functions of Antibodies. Journal of the American Chemical Society, 136(52), 18034-18043.
[11] Parham, P. (2014).The immune system. Garland Science.
[12] Sharpe, A.H. and Freeman, G.J. (2002). The B7-CD28 Superfamily. Nature Reviews Immunology, 2, 116-126.
[13] Shelton, J. (2014). New class of synthetic molecules mimics antibodies. Yale News. Retrieved January 29, 2016, from http://news.yale.edu/2014/12/17/new-class-synthetic-molecules-mimics-antibodies
[14] Weiner, L. M., Surana, R., & Wang, S. (2010). Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology,10(5), 317-327.
[15] Wine, Y. (2013). Molecular deconvolution of the monoclonal antibodies that comprise the polyclonal serum response. PNAS, 110(8), 2993-2998.