Table of Contents
Cellulases and Biofuels
Introduction
Biofuels are a source of energy that derives from renewable plants and animals. The most abundant fermentable source of cellulosic carbohydrates is obtained from plant biomass due to its high abundance in plant cell walls (Himmel, Ruth, & Wyman, 1999). One of the most studied and currently employed uses of this vast biomass is the conversion to ethanol.
Biofuels have increased in popularity because of rising oil prices and the need for energy security. Ethanol based biofuel is the predominant biofuel produced in the United States and Brazil. It is commonly made from corn and sugarcane, however some critics view this as a waste of valuable resources. Thus, enzymes known as cellulases are currently being employed as a means of fermenting dead and excess plant biomass into ethanol (Himmel et al., 1999).
Ethanol is produced by the action of microorganisms and enzymes through the fermentation of sugars, starches or cellulose. It can be used in petrol engines as a replacement for gasoline. Most existing car petrol engines can run on blends of up to 15% bioethanol with petroleum/gasoline. Ethanol has a smaller energy density than that of gasoline; meaning more ethanol is required to produce an equivalent amount of work done by gasoline. However, ethanol has higher burning efficiency compared to standardized car fuels (Himmel et al., 1999).
In the United States, bioethanol derived from corn contributes ~2% to the total transportation fuels mix. Therefore, shifting the global dependence to renewable biomass resources is crucial to creating a sustainable environment (Ragauskas et al., 2006).
Biomass and Cellulose Composition
The leaves and stalks of plants are the largest source of cellulose and hemicellulose. Fruits and seeds of plants primarily are made up of sucrose and starch. However, digestion and fermentation of cellulose and hemicellulose produce higher yields of ethanol than sucrose and starch (Lynd et al., 2008).
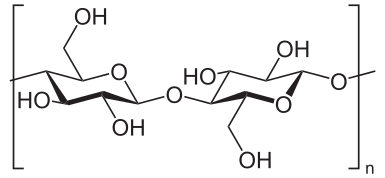
Cellulose is highly water insoluble, rigid, and densely packed into long chains held by numerous intermolecular forces. This inherent property makes degradation of cellulose a challenging task and require the use of multiple enzymes to catabolize it into glucose monomers (Bayer, Chanzy, Lamed, & Shoham, 1998).
There are two major areas of interest of cellulose polymers: Crystalline Regions are highly ordered and structured areas of the polymer. They appear as linear fragments within the cellulose polymer. Amorphous Regions are less structured and appear jagged (Bayer et al., 1998).
Cellulase Structure and Functionality
Cellulases are enzymes capable of hydrolyzing the β 1-4 glyosidic bonds which hold the cellulose polymers together. The function of cellulases have been well studied in specific species of fungi and bacteria. Degradation of cellulases requires the use of multiple enzymes which are collectively known as a cellulosome (Walker and Wilson, 1991).
The best characterized cellulosome systems have been found in aerobic fungi. The cellulosome contains three general enzymes: endo-1,4-beta-D-glucanase, exo-1,4-beta-D-glucanase( also known as cellobiohydrolase) , and beta-glucosidase (Rabinovich, Melnick and Bolobova, 2002).
Endo-1,4-beta-D-glucanases attacks the cellulose β 1-4 glyosidic bonds mostly at amorphous regions within the interior of the chain. It produces various oligomers in size including cellobiose; a disaccharide composed of two glucose monomers. Exo-1,4-beta-D-glucanases are similar, but attack towards the exterior of the fibre at reducing and non-reducing ends of the polymer chain. This enzyme also yields cellobiose. The cellobiose is then further catabolized by beta-glucosidase into two individual glucose monomers. After this, bacteria and fungi are now able to ferment this into ethanol (Rabinovich, Melnick and Bolobova, 2002).
Each enzyme has 3 major areas of interest including a: cellulose binding domain (CBD), catalytic domain (CD), and glycosylated linker chain. The CBD allows for the enzyme to bind to the cellulose polymer and treadmills down the chain. The CD is responsible for hydrolyzing the glyosidic bonds within the polymer chain. The CBD and CD are linked by a glycosylated chain. This provides physical separation which allows them to independently function (Linder and Teeri, 1997).
Cellulases are generally characterized by homology and mechanism of action in different species of fungi and bacteria. Some enzymes are particularly effective at hydrolyzing amorphous regions, while others are more specific to highly structured, crystalline regions (Rabinovich, Melnick and Bolobova, 2002).
Lignocellulosic Biomass
In the past few decades, the technologies employed used edible biomass material such as corn to produce ethanol. However, current methods are being utilized to harness energy from non-edible biomass to conserve our edible resources.
Lignocellulosic biomass is the non-edible portion of the plant material. It is the dry plant matter consisting of cellulose, hemicellulose, and encased in lignin. This type of biomass is the most the most abundant raw material on earth for the production of biofuels (Sukumaran, Singhania, Mathew, and Pandey, 2009).
Cellulose is coated with hemicellulose. The most abundant type of hemicellulose is xylan; a polymer of β-1,4-linked xylose which may have branches containing other sugars such as arabinose. The hydrolysis of both cellulose and hemicellulose releases glucose which can be then be fermented into ethanol (Sukumaran, Singhania, Mathew, and Pandey, 2009).
Lignin is a complex polymer consisting of hydroxylated and methoxy-lated phenylpropanoids. These phenylpropanoids cross-link plant secondary cell walls and provide mechanical strength and a physical barrier to invasive pathogens. The percentage of lignin content in cell wall varies between plants and is a crucial parameter affecting the decomposition efficiency of the polysaccharides (Sukumaran, Singhania, Mathew, and Pandey, 2009).
Ethanol Production
The production of ethanol from lignocellulosic biomass begins with several pretreatment steps, hydrolysis of cellulose polymers into glucose, and followed by ethanol recovery. (Van Zessen et al., 2003).
The purpose of the pretreatment is to remove lignin and hemicellulose, reduce cellulose crystallinity, and increase the porosity of the materials. The pretreatment should achieve the following requirements:
- improve the formation of sugars or the ability to subsequently form sugars by enzymatic hydrolysis
- avoid the degradation or loss of carbohydrate
- avoid the formation of byproducts inhibitory to the subsequent hydrolysis and fermentation processes
- cost effective
There are numerous methods to pre-treat the lignocellulosic biomass. Physical treatments such as grinding and drying the lignin can be employed. Drying is the removal of volatile compounds such as water from a material to a gaseous phase by thermal evaporation. Grinding reduced the size of the solid material by impact and compression. Chemical and enzymatic treatment can be also used to degrade and digest the lignin (Kumar, Parveen et al., 2009).
The combination of biological pretreatment by a white rot fungus Echin- odontium taxodii or a brown rot fungus Antrodia sp. 5,898 with mild acid pre- treatment (0.25 % sulfuric acid at varied temperature) were evaluated by Ma et al. (2010), under different pretreatment conditions for enzymatic hydrolysis and ethanol production from water hyacinth (E. crassipes). The reducing sugar yield from enzymatic hydrolysis of co-treated water hyacinth increased 1.13-2.11-fold than that of acid-treated water hyacinth at the same conditions. The following study on separate hydrolysis and fermentation with Saccharomyces cerevisiae indicated that the ethanol yield from co-treated water hyacinth achieved 0.192 g/g of dry matter, which increased 1.34-fold than that from acid-treated water hyacinth (0.146 g/g of dry matter). The discussion and conclusion of this research was that one pretreatment is often not the most efficient pathway as combining different types of pre-treatment and the order in which they occur are directly affect its efficiency.
Genetically modify the lignin component of plant cell wall by induced mutation or genetic manipulation exercising transcriptional control over the biosynthetic pathway genes. Genetic manipulation of the composition and amount of lignin in the cell walls to modify the material properties of biomass making it susceptible to thermochemical deconstruction followed by enzymatic degradation. A novel modification strategy that enables the synthesis of new phenolic molecules that can, when incorporated into the lignin polymer, can greatly improve its degradation. Research shows an increase in the number of total cellulose products when lignin is genetically modified (Fu et al., 2011)
Genetic Engineering for Biofuels
Genetic manipulation of plant cell wall polymer lignin is an effective route to ensure increased biomass production that can be translated to higher quality raw materials for industrial production of biofuels (Ciesielski et al., 2014). Lignin is a phenylpropanoid polymer component of the plant cell wall structure which has a natural tendency of biomass recalcitrance to resist microbial, chemical or enzymatic degradation (Himmel, 2007). Genetic engineering techniques are aimed at overcoming this barrier as the hydrophobic network of cross links that lignin forms with the polysaccharides inhibits their enzymatic degradation (Zeng, Zhao, Yang, & Ding, 2014).
There are various genetic engineering routes to overcoming biomass recalcitrance including:
- Obtaining localized lignification to promote accessibility to enzymes (Chen & Dixon, 2007).
- Genetic modification of lignin via mutation or transcriptional manipulation of biosynthetic pathway genes to bypass costly pretreatments and result in facilitated bioprocess consolidation (Chen & Dixon, 2007; Verma & Dwivedi, 2014).
- Increasing the thermochemical deconstruction susceptibility of biomass through lignin modification (Ciesielski et al., 2014).
- Incorporation of phenolic molecules into lignin polymer to improve its degradation (Verma & Dwivedi, 2014).
Waste Management
The remaining solids, which are not fermentable, can be burned to produce fuel for the operation of the conversion plants, as well as generate electricity (Morris, 2006). This way, the energy used in the production of cellulosic ethanol is self-generated with the excess portions of the plants. However, conversion plants that make ethanol from corn generate energy using natural gases and coal, depleting our source of fossil fuels and increasing greenhouse emissions.
Excess parts of the crops, which are considered unusable, can be returned to the soil. The addition of these excess organic materials back into carbon-depleted soils in turn increase the amount of carbon diffused in the soil. This would drastically affect climate change, since the carbon in the soil can absorb large quantities of carbon dioxide (CO2) present in the atmosphere (Nelson, 2007).
Sustainability
Cellulosic ethanol does not compete with production in the food industry. An alternative fuel, such as corn ethanol, interferes with the food industry greatly. This is because it requires croplands, which may normally be used for food growth. However, humans are unable to digest the polysaccharide cellulose because the necessary enzymes required to break beta acetal links are not present (Oyetunji, 2009). Thus, cellulose production does not interfere with food production.
The entire plant can also be harvested. Because plants are primarily made up of cellulose, a large portion of the plant can actually be harvested to produce ethanol for fuel. Since more of the plant content is being used, it produces much greater yields. Approximately 10 tons of ethanol is produced per acre using the cellulose portion of plants, compared to 4 tons of ethanol produced per acre using grain crops.
Feedstock to make cellulosic ethanol is abundant. Ethanol production using cellulosic biomass is extremely sustainable because it can be produced from a large variety of resources. For example, urban wastes such as wood, and agricultural crops such as switchgrass and corn stovers are large feedstocks of cellulosic ethanol (Nelson, 2007). In the United States alone, approximately 323-million tons of material, which is made up of cellulose, is thrown away as waste. Converting these wastes into ethanol itself will produce 30% of the present fuel usage (Nelson, 2007).
The expense to produce of cellulosic biomass is lower than the expense to produce corn. Expense of course plays a large factor in determining whether something is sustainable. The expense is lower because less inputs, such as fertilizers, herbicides and energy, are required to produce large yields. Switchgrass, a perennial plant, is a major livestock for cellulosic ethanol. It produces double the amount of ethanol/acre compared to corn, which means it requires less land for production (Montenegro, 2006). This translates to reduced habitat destruction and not endangering existing wildlife. Switchgrass also has long widespread roots, which aid in improving the quality of soil, either by reducing soil erosion and/or augmenting nutrient capture (Nelson, 2007). A 5-year study conducted by Schmer and colleagues determined that using switchgrass as the primary crop to produce ethanol produces 500% more energy than what is consumed for production itself (Schmer et al., 2008).
The usage of cellulosic ethanol as fuel accounts for reduced greenhouse gas emissions. It has been found that the burning of ethanol produced from cellulose discharges less sulphur and carbon monoxide into the atmosphere, compared to ethanol produced by corn and other fossil fuels (Huanga et al., 2009).
Enzyme Cost as a Limitation
Cellulase and hemicellulase are enzymes that break down the cellulose content in plants, and play an important role in the production of cellulosic ethanol. However, these enzymes are more expensive than the enzymes used in ethanol production with grain. The greater cost is attributed to needing a larger quantity of cellulase enzymes. A greater amount of cellulase enzymes is required because they are a few orders of magnitude lower in efficiency (Yang & Wyman, 2007). Thus, 40-100x more cellulase enzyme is required relative to the amount of enzyme required to make other forms of ethanol (Yang & Wyman, 2007).
Conclusion
Based on the available research findings, it could be prospected that biofuels will form a large share of the fuel industry in the near future. With scientific and technological innovations, more selective methods of extraction, production and refinement could become a reality which will greatly improve its cost efficiency without compromising quality. In addition, strategies to genetically engineer raw materials could also contribute to a large scale production and use of biofuels. Currently, biofuels are sought of as a viable substitute for non-renewable resources such as coal, petroleum and natural gas, and thus serving as part of the solution for the world’s energy crisis (Bhattarai, Stalick, Mckay, Geme, & Bhattarai 2011).
References
Bayer, E. A., Chanzy, H., Lamed, R., & Shoham, Y. (1998). Cellulose, cellulases and cellulosomes. Current opinion in structural biology, 8(5), 548-557.
Bhat, M. K., & Bhat, S. (1997). Cellulose degrading enzymes and their potential industrial applications. Biotechnology advances, 15(3), 583-620.
Bhattarai, K., Stalick, W. M., Mckay, S., Geme, G., & Bhattarai, N. (2011). Biofuel: An alternative to fossil fuel for alleviating world energy and economic crises. Journal of Environmental Science and Health Part A-Toxic/hazardous Substances & Environmental Engineering, 46(12), 1424-1442. doi:10.1080/10934529.2011.607042
Chen, F., & Dixon, R. A. (2007). Lignin modification improves fermentable sugar yields for biofuel production. Nature Biotechnology, 25(7), 759-761. doi:10.1038/nbt1316
Ciesielski, P. N., Resch, M. G., Hewetson, B., Killgore, J. P., Curtin, A., Anderson, N., Donohoe, B. S. (2014). Engineering plant cell walls: Tuning lignin monomer composition for deconstructable biofuel feedstocks or resilient biomaterials. Green Chemistry, 16(5), 2627-2635. doi:10.1039/c3gc42422g
Fu, C., Mielenz, J. R., Xiao, X., Ge, Y., Hamilton, C. Y., Rodriguez, M., … & Wang, Z. Y. (2011). Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proceedings of the National Academy of Sciences, 108(9), 3803-3808.
Himmel, M. E. (2007). Biomass recalcitrance: Engineering plants and enzymes for biofuels production (vol 315, pg 804, 2007). Science, 316(5827), 982-982.
Himmel, M. E., Ruth, M. F., & Wyman, C. E. (1999). Cellulase for commodity products from cellulosic biomass. Current Opinion in Biotechnology, 10(4), 358-364.
Huanga, H., Ramaswamya, S., Al-Dajania, W., Tschirnera, U., & Cairncrossb, R. A. (2009). Effect of biomass species and plant size on cellulosic ethanol: A comparative process and economic analysis. Biomass Bioenergy, 33, 234-246.
Linder, M., & Teeri, T. T. (1997). The roles and function of cellulose-binding domains. Journal of Biotechnology, 57(1), 15-28.
Ma, F., Yang, X, Yu, H., Zhang, X., & Chen, S. (2011). Effects of biopretreatment of corn stover with white-rot fungus on low-temperature pyrolysis products. Bioresource technology, 102(3), 3498-3503.
Montenegro, M. (2006). The numbers behind ethanol, cellulosic ethanol, and biodiesel in the U.S. Grist.
Morris, D. (2006). Putting the pieces together: Commercializing ethanol from cellulose. US Department of Energy. Retrieved 2014-09-29.
Nelson, R. (2007). Cellulosic ethanol: bioethanol in Kansas. Kansas State University Engineering Extension. Retrieved 2014-09- 29.
Oyetunji, R. (2009). Enzymatic hydrolysis Of cellulose in a NMMO/H2O solution. Electronic Theses, Treatises and Dissertations, Paper 2435.
Rabinovich, M. L., Melnick, M. S., & Bolobova, A. V. (2002). The structure and mechanism of action of cellulolytic enzymes. Biochemistry (Moscow), 67(8), 850-871.
Ragauskas, A. J., Williams, C. K., Davison, B. H., Britovsek, G., Cairney, J., Eckert, C. A., … & Tschaplinski, T. (2006). The path forward for biofuels and biomaterials. science, 311(5760), 484-489.
Schmer, M. R., Vogel, K. P., Mitchell, R. B. & Perrin, R. K. (2008). Net energy of cellulosic ethanol from switchgrass. Proc. Nat. Acad. Sci., 105(2), 464-469. Yang, B., & Wyman, C. E. (2007). Pretreatment: the key to unlocking low‐cost cellulosic ethanol. Biofuels, Bioproducts and Biorefining, 2(1), 26-40.
Sukumaran, R. K., Singhania, R. R., Mathew, G. M., & Pandey, A. (2009). Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renewable Energy, 34(2), 421-424.
Van Zessen, E., Reith, J. H., Den Uil, H., Weismann, M., Bakker, R. R., & Elbersen, H. W. (2003). Ligno Cellulosic-Ethanol. A second opinion. Netherlands Agency for Energy and the Environment Novem, Utrecht (Netherlands).
Verma, S. R., & Dwivedi, U. N. (2014). Lignin genetic engineering for improvement of wood quality: Applications in paper and textile industries, fodder and bioenergy production. South African Journal of Botany, 91(0), 107-125. doi:http://dx.doi.org.libaccess.lib.mcmaster.ca/10.1016/j.sajb.2014.01.002
Walker, L. P., & Wilson, D. B. (1991). Enzymatic hydrolysis of cellulose: an overview. Bioresource Technology, 36(1), 3-14.
Yang, B., & Wyman, C. E. (2007). Pretreatment: the key to unlocking low‐cost cellulosic ethanol. Biofuels, Bioproducts and Biorefining, 2(1), 26-40.
Zeng, Y., Zhao, S., Yang, S., & Ding, S. (2014). Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Current Opinion in Biotechnology, 27, 38-45. doi:10.1016/j.copbio.2013.09.008