This is an old revision of the document!
Table of Contents
Introduction
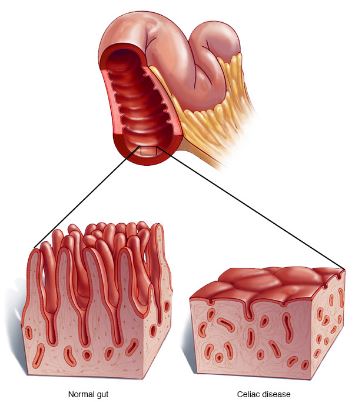
<style justify> Celiac disease (gluten-sensitive enteropathy), which is also referred to as spruce or coeliac is a digestive and autoimmune disease that leads to the destruction of the lining of the small intestine when foods containing gluten are consumed.[1] The immune system of individuals with celiac disease forms antibodies to gluten which attacks the intestinal lining causing inflammation and damage to the villi of the intestine.[2] The damage done to the intestinal lining leads to the inability of the body to absorb nutrients including proteins, fats, carbohydrates vitamins and minerals which are vital for good human health.[1] </style>
Figure 1: Image comparing the intestinal villi of a healthy individual to someone with Celiac Disease </style>
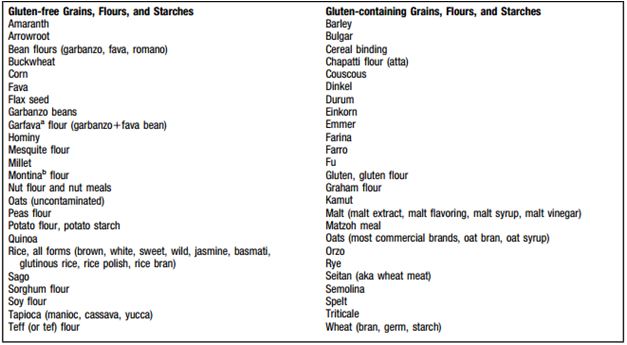
<style justify> Gluten is a protein that can be found in wheat, rye, triticale, barley. Gluten is the ingredient in wheat that assists in holding bread and other baked goods together and keeps them from crumbling.[2] Oats that are made in plants that process other grain products can also be found to contain gluten. Lipsticks, medicines, and vitamins can also contain gluten.[3] </style>
Figure 2: Various product that contain gluten </style>
<style justify> Some long-term health problems that can accompany celiac disease include[1]:
- Osteoporosis- caused by the inability to absorb calcium and vitamin D
- Miscarriage or infertility
- Birth defects (e.g., growth problems)- caused by poor absorption of nutrients including folic acid
- Seizures
- Cancer of the intestine
Individuals with celiac disease may also be more prone to other autoimmune diseases including - Thyroid Disease, type 1 Diabetes, Lupus, Rheumatoid arthritis
It has been estimated that 1 in 133 people in Canada are affected by celiac disease.[2] It is an inherited condition where people have a 1 in 22 chance of developing the disease if their parent or sibling has the condition.[3] Not all individuals that carry the gene for celiac disease will develop the disease.[4] Environmental factors including stress, pregnancy, surgery of an infection can sometimes lead to the onset of the disease.[5] When treated early with a gluten free diet, the damaged tissues are about to heal and help decrease the risk of developing other long-term complications association with celiac disease.[4] </style>
Mechanism of Celiac Disease
There are many mechanisms involved in Celiac Disease. Some are listed below.
Factors Involved in Disease Pathogenesis
<style justify> Celiac disease pathogenesis involves interactions among: environmental factors, genetic factors, and immunological factors. Firstly, in terms of the environmental factors, the specific proteins found in grain products such as wheat, rye, and barley are what contribute to celiac disease. Secondly, the MHC class II HLA-DQ alleles are the key genetic factors that interact with the environmental factors to produce the negative consequences of CVD. Finally, tissue TGase is a critical compound that leads to the cascade of events that lead to the symptoms of celiac disease.[6] </style>
Environmental Factors: Gluten
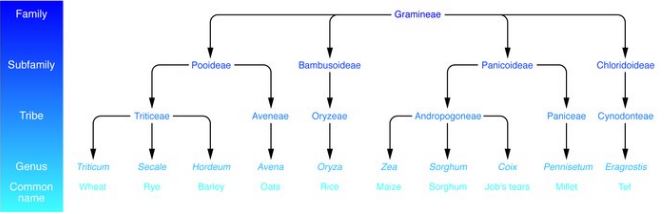
Figure 3: Taxonomy of dietary grains. It is shown that grains like wheat, rye, and barley which are most commonly associated with celiac disease are also very closely related. Whereas, grains like oats and rice which are rarely contribute to celiac disease are more distantly related to wheat. </style>
<style justify>
As shown in figure 3 celiac disease is activated by proteins in wheat, rye, and barley. These proteins are popularly referred to as “gluten”, however, it is important to note that gluten only represents the specific proteins found in wheat. There are two major types of gluten which are known as gliadins and glutenins. These have a high proline and glutamine content. A high proline content causes these proteins to be resistant to complete proteolytic digestion by enzymes in the human intestine. This leads to the accumulation of large peptide fragments with a high proline and glutamine content in the intestines. In individuals with celiac disease this accumulation leads to epithelial cell brush border injury and pancreatic dysfunction.[6]
</style>
Genetic Factors: MHC class II HLA-DQ alleles
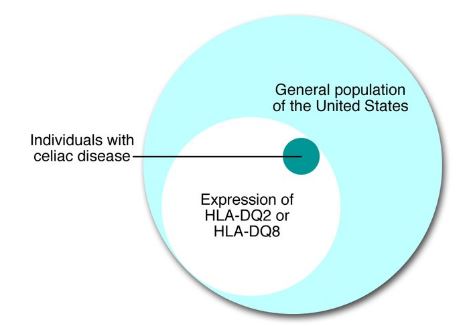
Figure 4: This venn diagram shows that almost all individuals with celiac disease will also express the alleles HLA-DQ2 or HLA-DQ8 </style>
<style justify>
Pathogenesis of celiac disease is rooted by host genetic factors and this evident in monozygotic twins who have a concordance rate of about 75%. Celiac disease is related to specific MHC class II alleles mapping the HLA-DQ locus. The presence of HLA-DQ is necessary for the phenotypic expression of celiac disease, in all individuals affected by celiac disease, regardless of where they are from, they express HLA-DQ alleles which encode HLA-DQ2 or HLA-DQ8 heterodimers which leads to the susceptibility of celiac disease. The HLA-DQ2 allele can be inherited in cis (on 1 parental chromosome) or trans (on 1 chromosome from each parent). Usually, the prevalence of CD is higher in individuals where 50 or 100% of heterodimers of HLA-DQ are HLA-DQ2 than those with only 25% of HLA-DQ heterodimers being HLA-DQ2. HLA-DQ2 on APCs (antigen presenting cells) can bind and present “gluten” peptides to populations of CD4+T cells in the lamina propria of the small intestine. The binding of HLA-DQ2 on APCs to CD4+ T cells usually occurs with the help of the tissue TGase.[6]
</style>
Immunological Factors: Tissue TGase
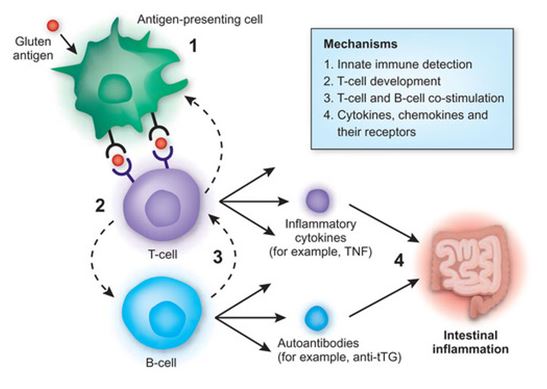
Figure 5: Tissue TGase helps mediate the binding of HLA-DQ2 to APCs (antigen presenting cells) which eventually lead to the cascade of events in which the end result is intestinal inflammation. </style>
<style justify> Tissue TGase is primarily released in the intestinal mucosa usually during tissue injury. It plays a role in tissue repair and cross-links proteins by forming isopeptide bonds between glutamine and lysine residues. During conditions of low pH it can deamidate glutamine and convert it into neutral glutamine into a negatively charged glutamic acid which then increases the binding affinity of HLA-DQ2 molecules which favour binding negatively charged residues. Once the HLA-DQ2 molecules bind, they form the gluten-peptide-HLA-DQ complex which then meet a population of CD4+ T cells that are HLA-DQ2 restricted. Upon meeting, these T cells which recognize the complex become activated in the small intestine. These T cells lead to the production of IFN-γ which plays a major role in the downstream initiation of mucosal damage. This was found by neutralizing IFN-γ in mucosa that was affected by celiac disease which resulted in the prevention of mucosal damage.[6] </style>
Pathogenesis of Celiac Disease
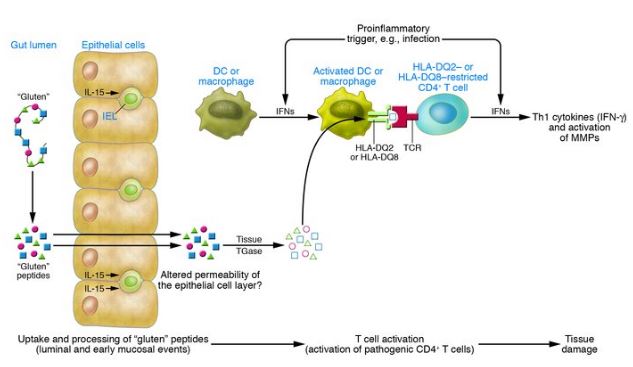
Figure 6: This image represents the three major events that eventually lead to intestinal tissue damage, specifically targeting the villi in the small intestine. The three events include: luminal and early mucosal events, activation of pathogenic CD4+ T cells and tissue damage. </style>
<style justify> There are three major events that eventually lead to the symptoms that represent celiac disease. These include: luminal and early mucosal events, activation of pathogenic CD4+T cells, and subsequent events that lead to tissue damage. The start of luminal and mucosal events occurs when an individual that is genetically susceptible to celiac disease ingests gluten. Gluten can’t be digested by this individual due to high levels of proline. This leads to a large amount of undigested gluten peptides. These peptides then cross the epithelial barriers and enter the lamina propria where they encounter tissue TGase and APCs. These APCs express HLA-DQ2 heterodimers that bind to proline-rich peptides that contain negatively glutamic acid residues; this conversion was done by tissue TGase which caused the glutamine in the peptides to undergo deamidation. Once this occur APCs present the peptides to HLA-DQ2 restricted populations of CD4+ T cells which are then activated. This activations leads to the release of mediators which are what eventually cause tissue damage.[6] </style>
Unknowns of Pathogenesis
<style justify> The mechanism of celiac disease is very complex as it involves various different compounds and processes that eventually lead to the primary characteristic of celiac disease, mucosal damage. As a results there are multiple unknowns such as: how gluten crosses the epithelial cell barrier, role of immunity, role of IFNs in pathogenesis of celiac disease, how tissue TGase leads to deamidation, and sequence of CD4+T cell responses.[6] </style>
Treatment of Celiac Disease with a Gluten-Free Diet
<style justify> As we already know celiac disease is a condition in which the absorptive surface of the small intestines is damaged by certain substances we intake, primarily gluten. Celiac disease cannot be cured but can be effectively treated and controlled. One treatment is a strict adherence to a gluten-free diet (for life). </style>
Figure 7: Shows what products have gluten </style> <style justify> With the complete removal of a gluten from the diet, the patient will result in symptomatic, serologic, and histologic remission. For children, the growth and development returns to normal following the gluten-free diet. While in adults, many complications are often avoided following this. It usually takes around two weeks for improvement in symptoms after starting the gluten free diet. Additionally, many reported that with a strict dietary control the antibody levels may revert to normal during 6-12 months after initiating the diet. </style>
Problems
<style justify> In a study conducted it was seen that small intestinal recovery and resolution of the symptoms is incomplete. This maybe on account that the form of celiac disease is more complicated like refractory celiac disease. In cases like this it was found some patients may not fully respond to the gluten free diet.
Nutrition is an important thing to look at if a patient has celiac disease. The nutritional status of a person diagnosed will depend on the length of time the person lived with an active but undiagnosed disease, as well as the damage to the GI tract, and degree of malabsorption. The most common things noted in patients who start the gluten free diet is that there is substantial weight loss, anemia, and vitamin/mineral deficiencies. The malabsorption of iron, folate, and calcium is common with patients having celiac disease as these nutrients are absorbed in the proximal small bowel. If the malabsorption is further along the intestine then it will affect the absorption of carbohydrates, fat, and vitamins (fat-soluble) like A, D, E, and K. Anemia can be treated by intaking iron, folate, or vitamin B-12 supplements but it depends on the origin of the anemia. Nonetheless, studies have shown 78% to 94% have recovered from anemia due to being on a gluten free diet. </style>
Case Studies
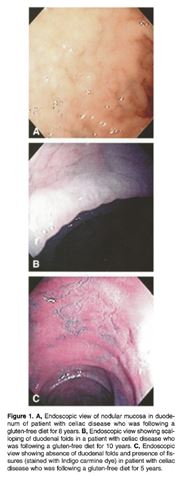
Figure 8: Shows the problems that have been found in the duodenum </style>
<style justify> One treatment that is often used for patients having celiac disease is going on a gluten-free diet for life. This is because people who have celiac disease are ‘gluten sensitive’ thus avoiding it can often be a treatment. As discussed gluten is a protein that is found in wheat, barley, rye, and other products. An individual who consumes gluten who has celiac disease would have symptoms such as dermatitis hepetiformis (an itchy skin rash), fatigue, gastrointestinal distress (e.g. diarrhea, constipation, gas, bloating, etc.), and many more. Most of this can be avoided by sticking with a gluten free diet.
While Gluten Free Diet is a treatment for celiac disease there are some potential setbacks. A study conducted with patients having celiac disease and the effects of what a gluten-free diet would do. Malnutrition is something often under looked, and a huge problem for individuals who undertake this diet. In a study that had two groups one that followed strict dietetic prescriptions and another group having gluten-containing food. It was noted that the dietary levels of calcium, fiber, were low. This is further exacerbated within females, with iron being low as well.
Another study conducted on individuals who were on a gluten-free diet examined the endoscopic and histopathologic appearance of the duodenum. With over 39 adult patients in the study, with an average age of 52, who had been on a gluten-free diet (average person was on for about 8.5 years). The results showed that only 9 patients had their endoscopic appearance as normal. The other patients had at least one abnormality, while 18 had multiple abnormalities. The two most common abnormality was reduced folds and mucosal fissures which were in 46% and 44% of patients. This was then followed by scalloping of folds and nodularity of the mucosa in 33%. The study concludes that despite adherence to a gluten-free diet, there still is endoscopic and histopathologic abnormalities found in the duodenum in most patients. </style>
References
[1] WebMD. (n.d.). Celiac Disease Causes, Symptoms, Treatments, Tests, & More. Retrieved from http://www.webmd.com/digestive-disorders/celiac-disease/celiac-disease#
[2] Celiac Disease Association. (n.d.). About Celiac Disease. Retrieved from http://www.celiac.ca/?page_id=882
[3] Lights, V., & Boskey, E. (2016, February 19). Celiac Disease (Gluten Intolerance). Retrieved from http://www.healthline.com/health/celiac-disease-sprue
[4] Health Canada. (2016, January 11). Celiac Disease. Retrieved from http://www.hc-sc.gc.ca/fn-an/securit/allerg/cel-coe/index-eng.php
[5] Celiac Disease Canada. (n.d.). Celiac Disease. Retrieved from http://www.diabetes.ca/diabetes-and-you/complications/celiac-disease
[6] Kagnoff, M. F. (2007). Celiac disease: pathogenesis of a model immunogenetic disease. Journal of Clinical Investigation, 117(1), 41–49. http://doi.org/10.1172/JCI30253