This is an old revision of the document!
Table of Contents
Introduction
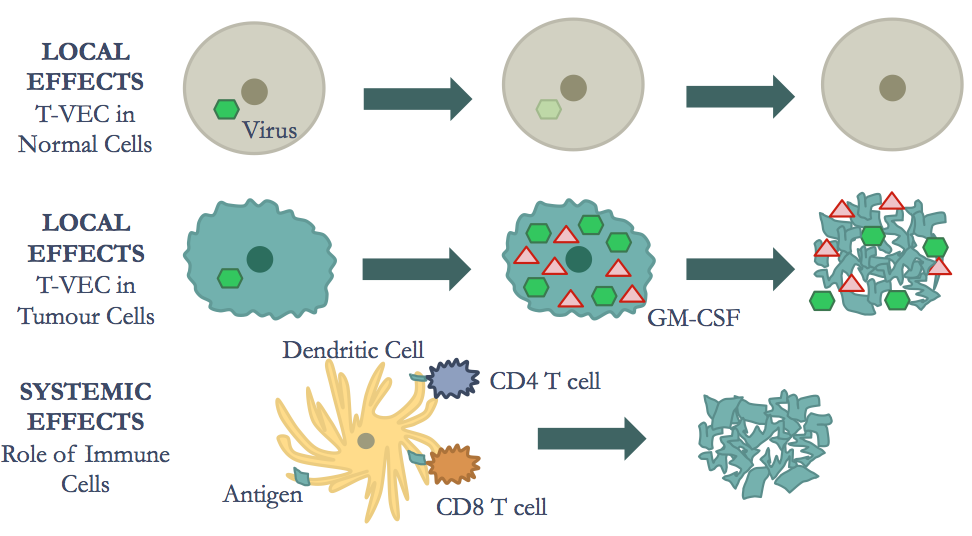
The mechanisms of oncolytic immunotherapy can be broken down into two major effects: the local effects and the systemic effects.1 The local effects consist of tumour cell lysis.1 The oncolytic virus (OV) must first selectively target cancer cells and replicate.1 Next, the tumour cells lyse, thus representing the oncolytic effect. 1 Furthermore, the systemic effects include the tumour-specific immune response, which activates antigen presenting immune cells. 1 This primes the adaptive immune response and leads to a series of downstream effects that result in death of distant cancerous cells.2
Oncolytic Immunotherapy
Advancements in the knowledge of mechanisms of viral entry, replication, induction and/or suppression of immune responses, and lytic versus latent infections, have led to increased interest in utilizing viruses for the treatment of human diseases.3 In particular, for the treatment of specific types of cancers.3
The ability of viruses to kill cancer cells has been recognized for nearly a century, but only over the past decade have clinical trials documented a beneficial effect in patients with cancer.3 Oncolytic immunotherapy is a therapeutic approach to cancer treatment, which utilizes genetically modified oncolytic viruses to kill tumour cells, and induce a systemic anti-tumour immune response.4 It achieves this through a dual mechanism of action:
1. Oncolysis (the destruction of tumour cells)5: The modified oncolytic virus selectively infects, and replicates in tumour cells, resulting in tumour cell lysis. This releases a new progeny of viruses that can then infect, replicate in, and lyse other tumour cells. Thus producing a direct cytotoxic effect.4
2. Immunotherapy: In addition to releasing newly synthesized viruses, oncolysis also releases tumour antigens, exposing them to various immune cells. The virus may also be modified to express and release specific cytokines that enhance the body's immune response. Together, the tumour antigens and genetic modifications, elicit a robust systemic anti-tumour adaptive immune response, that is both patient and tumour specific.4 When systemic immunity is fully engaged, therapeutic responses may be seen in both locally injected tumours and at distant sites of un-infected tumour growth.3
Oncolytic Viruses
An oncolytic virus is a type of virus that selectively infects and lyses (breaks down) cancer cells.6 Oncolytic viruses can occur naturally, or they can be made in the laboratory through genetic modifications of other viruses.6 They have defined a new class of therapeutic agents that promote anti-tumour responses through a dual mechanism of action: selective tumour cell killing and the induction of systemic anti-tumour immunity.3 Many viruses have been proposed as vectors for oncolytic virus immunotherapy, and considerable work has been done to optimize viral vectors—through genetic modifications—by enhancing their potency, selectivity, and the permanency of their anti-cancer effect.3
Genetic Modifications
Recombinant engineering has allowed the development of a number of strategies to enhance the effectiveness of oncolytic viruses. Oncolytic viruses are live viral particles, as such, the genetic modifications made to the viruses used for immunotherapy must alter them such that, the virus selectively targets tumour cells and the viral pathogenicity is attenuated.3 The modifications must also consider approaches to limit viral immunogenicity (the body’s immune response to the virus), while also promoting tumour cell killing and immunogenicity (the body’s immune response to tumour cells).3
Targeting Oncolytic Viruses to Cancer Cells
Many oncolytic viruses that are currently in development for immunotherapy, already have a natural tendency to bind to cell surface proteins that are expressed by cancer cells. For example, the naturally occurring herpes simplex virus-1 (HSV-1), utilizes the herpesvirus entry mediator (HVEM) and certain nectins to enter healthy host cells. These receptors are also overexpressed on the surface of some cancer cells, including melanoma and various carcinomas.3 Thus, naturally occurring HSV-1 is able to enter cancer cells without modification. Oncolytic viruses without this natural tendency, can be engineered to directly target unique cell surface receptors expressed by cancer cells. For example, adenovirus can be modified to selectively bind to receptors highly expressed on ovarian cancer cells.3
Along with targeting oncolytic viruses to cancer cells, genetic modifications also reduce the ability of these viruses to replicate in healthy, non-cancerous cells.4 This is accomplished by deleting or inactivating genes in the virus that are critical for replication in healthy cells, but are not necessary for replication in tumour cells.4 For example, in normal healthy cells, naturally occurring HSV-1 is able to enter the cell and begin producing viral RNAs. This activates the host cell’s antiviral defense mechanism, the protein kinase R (PKR) pathway. Activated PKR inhibits protein translation, preventing the production of viral particles and stopping the spread of the virus. HSV-1 relies on the neuro-virulence factor ICP34.5 to overcome the PKR pathway.3,4 Therefore, by inactivating the gene in the virus that encodes this factor, the virus will no longer be able to replicate in healthy cells. This results in preferential lysis of tumour cells compared to normal cells, because the virus can now only replicate in cells with defective PKR signalling.3
Attenuating Viral Pathogenesis
As mentioned previously, oncolytic viruses are live viruses that have the potential to cause acute toxicity and, in some cases, latent infection and chronic disease. The potential pathogenicity depends on the virus itself, the presence of natural or engineered attenuation factors, and the host immune response. In order to prevent acute and long-term toxicity, most clinically relevant oncolytic viruses utilize attenuated vectors, which are altered so that they become harmless, or less virulent.3 Oncolytic viruses used for oncolytic immunotherapy may be attenuated by deleting pathogenic viral genes, or the expression of their virulence genes may be restricted to tumour tissues through incorporation of regulatory promoters.3
Augmenting Anti-Tumour Immunity
It is crucial to engineer oncolytic viruses so that they can more effectively stimulate anti-tumour immune responses.3 For example, anti-tumour immune responses can be enhanced through viral expression of pro-inflammatory cytokines, and/or T-cell co-stimulatory molecules. Granulocyte Macrophage – Colony Stimulating Factor (GM-CSF) is a cytokine that promotes dendritic cell accumulation and maturation. It is often engineered into the viral genome of oncolytic viruses used for oncolytic immunotherapy, as GM-CSF improves tumour antigen presentation and stimulates strong T-cell responses.3
Enhancing Lytic Activity
The inclusion of ‘suicide genes’ (genes that make cells more sensitive to apoptosis, or therapy with other drugs) into oncolytic viruses can enhance their ability to directly kill cancer cells.3
Enhancing Virus Bioavailability
This type of modification aims to increase viral penetrance into therapeutic target areas. For example, to avoid physical limitations, viral penetrance can be enhanced by pre-treatment of the tumour microenvironment with proteolytic enzymes. These enzymes break down the barrier presented by the extracellular matrix (ECM) of tumour cells, allowing viruses to enter the cells more effectively.3
Oncolytic Viruses in Clinical Development: Herpes Simplex Virus-1 (HSV-1)
HSV-1 is a double-stranded DNA virus with a large genome, in which a significant portion encodes genes that are not essential for viral infection. HSV-1 replication occurs in the nucleus, but does not affect the host cell’s DNA. These properties make HSV-1 an attractive candidate for oncolytic virus development. However, it is a major human pathogen that causes skin lesions and rashes. It can also infect peripheral nerves and enter a latent stage.3
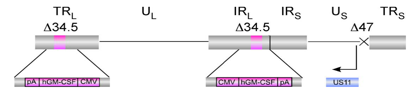
HSV-1 is an example of a naturally occurring virus that has been engineered into an attenuated oncolytic virus. It is the basis of the newly FDA approved oncolytic immunotherapeutic, talimogen laherparepvec (T-VEC), also known as, ImlygicTM.3 For its use as an oncolytic immunotherapeutic, HSV-1 has been modified to reduce its pathogenesis, while enhancing its selective tumour cell infectivity. It contains deletions of the neuro-virulence gene ICP34.5 (limiting toxicity), and the inhibitor of antigen presentation ICP47 (enhancing the immune response).3,4
As previously mentioned, ICP34.5 is critical for blocking the host antiviral PKR response, allowing the progression of viral replication in healthy, non-cancerous cells. In HSV-1 based T-VEC, two ICP34.5 genes are deleted, improving cancer cell selectivity and preventing the infection of healthy cells, thereby significantly reducing the overall pathogenesis of HSV-1.3 In the naturally occurring HSV-1, ICP47 blocks antigen processing, and thus prevents infected cells from presenting antigens to immune cells, which is necessary for induction of systemic immunity. Therefore, its deletion in HSV-1 based T-VEC, leads to the immune-mediated destruction of cancer cells that selectively propagate oncolytic HSV-1.3 The deletion of ICP47 also induces the early activation of the unique short-11 (US11) promoter. This prevents cancer cells from undergoing abortive apoptosis when infected, increasing oncolytic therapeutic activity. Finally, the gene encoding GM-CSF has been engineered into the viral genome to improve the induction of anti-tumour immunity.3
Local and Systemic Effects
Local Effects
Selective Viral Replication in Cancer Cells
Several viruses have been used in oncolytic immunotherapy, however, Herpes Simplex Virus-1 (HSV-1) and Adenovirus have most commonly been genetically modified to become oncolytic viruses 7 In oncolytic immunotherapy used to treat melanoma, genetically engineered HSV-1 has been used to target the malignant cells. The HSV-1 OV can enter both cancerous and non-cancerous cells, however, it is engineered to be susceptible to antiviral defenses in non-cancerous cells 8. Therefore, the OV cannot replicate and produce viral progeny in non-cancerous cells.8
Oncolysis of Cancer Cells
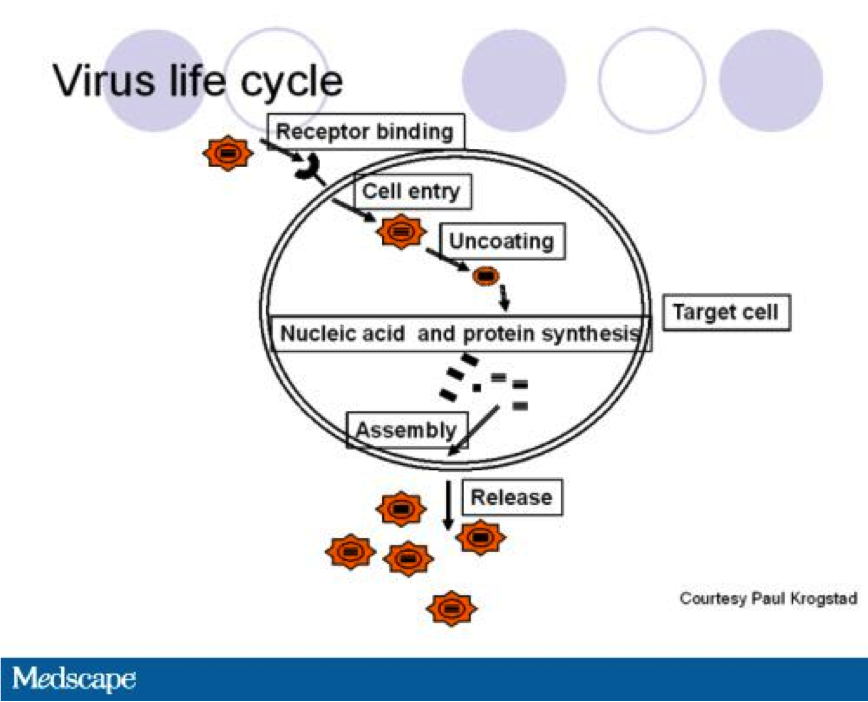
The HSV-1 OV enters a malignant tumour cell through interaction of viral envelop glycoproteins and glycosaminoglycan components of the heparin sulfate polysaccharide on the cell surface. 9 The viral envelope then fuses to the cell membrane after glycoprotein gD binds to cell surface receptors and the capsid is transported to the nuclear core of the cell through the microtubule network. 9 At this time, the double stranded DNA is released via channels controlled by the tegument proteins. 9 Transcription is then facilitated by by RNA polymerase II enabling replication of the virus. The capsid is then assembled with the genomic viral DNA in nucleus. 9 This rapid replication cycle, in turn, leads to cell lysis. 9 The progeny virions that are produced can then go to infect nearby cancerous cells. 1 Moreover, the HSV-1 OV encodes a granulocyte macrophage colony stimulating factor (GMCSF) immunostimulatory transgene which produces the granulocyte macrophage colony stimulating factor (GM-CSF).2 GM-CSF is a cytokine that plays a role in the differentiation of monocytes to antigen presenting dendritic cells (DC). Therefore, the combination of viral particles, tumour cell debris and GM-CSF that acts to recruit immune cells. 1
Systemic Immune Response
Role of GM-CSF in Dendritic Cell Differentiation
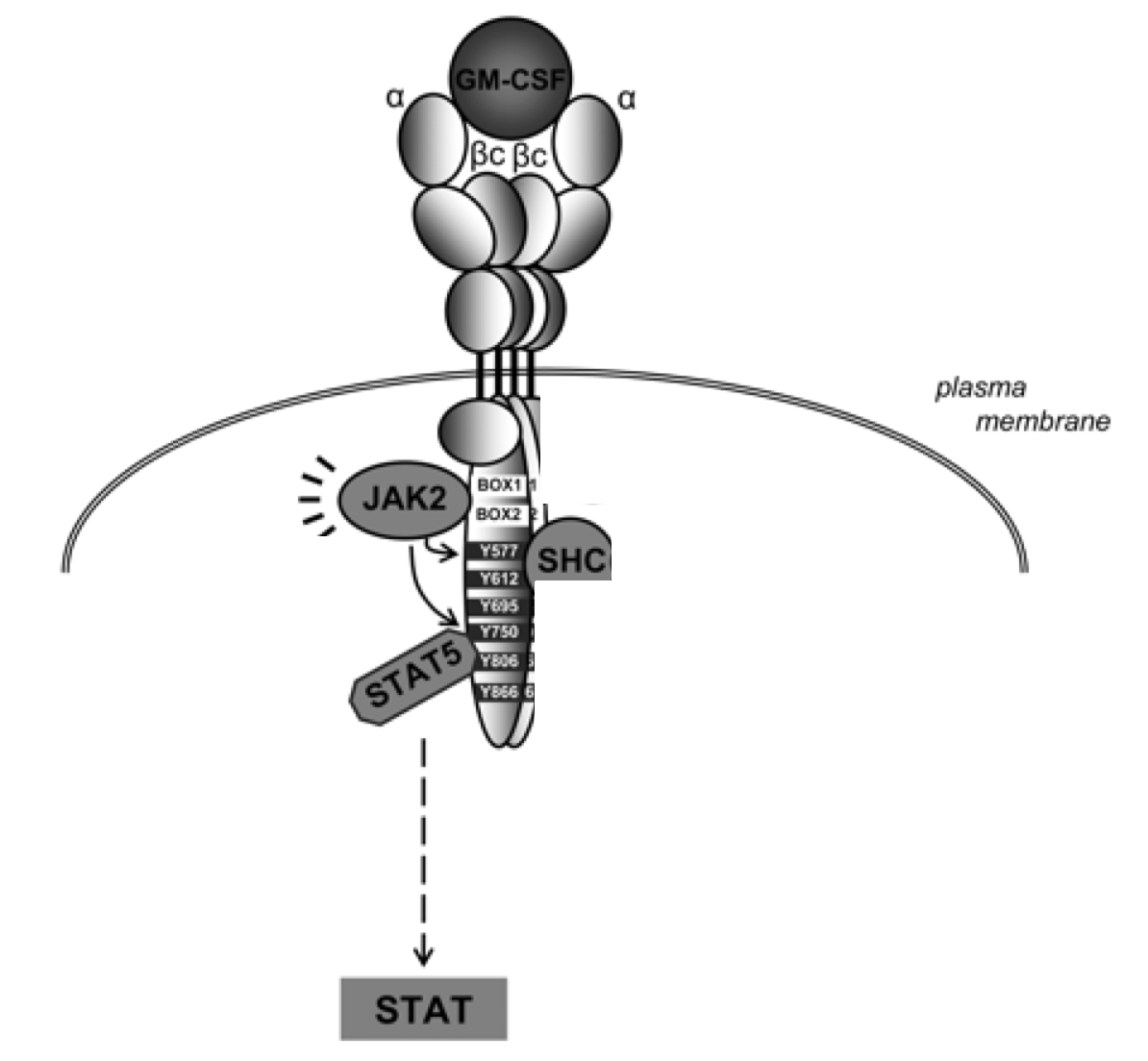
In order to induce systemic immunity, antigen presenting cells (APCs), such as DCs, must process and present the tumour antigens to T-cell. Therefore, GM-CSF plays an important role in the differentiation of DCs, ensuring a sufficient population of these immune cells. Research conducted by Mach et al. (2000) shows that injection of irradiated GM-CSF secreting melanoma tumour cells in mice leads to increased infiltration of DCs in the tumour tissue. 10 These increased levels can be attributed to the expression of the GM-CSF, which stimulates the production and differentiation of monocytes into DCs. This differentiation process is facilitated through the Janus kinases (JAK). Activated signal transducers and activators of transcription (STAT) pathway. 11 In this process, GM-CSF binds to α chain of the the GM-CSF receptor (GM-CSFR), causing association with the βc dimer. 11 This leads to the formation of a hexamer comprised of 2 GM-CSF molecules, 2 GM-CSFRα chains and 2 βc chains. 11 The dimerization of 2 of these hexamers allows for the transactivation of JAK2. 11 Next, STAT5 and Src kinases are recruited via interaction between the their SH2 domains and the phosphorylated tyrosine domains. 11 These STAT proteins are phosphorylated at tyrosine residues by JAK2 and which results in homodimers or heterodimers that translocate to the nucleus. 11 Importantly, these dimers act like transcription factors, allowing for the differentiation of monocytes into DCs. 11
Antigen Processing and Presentation by Dendritic Cells
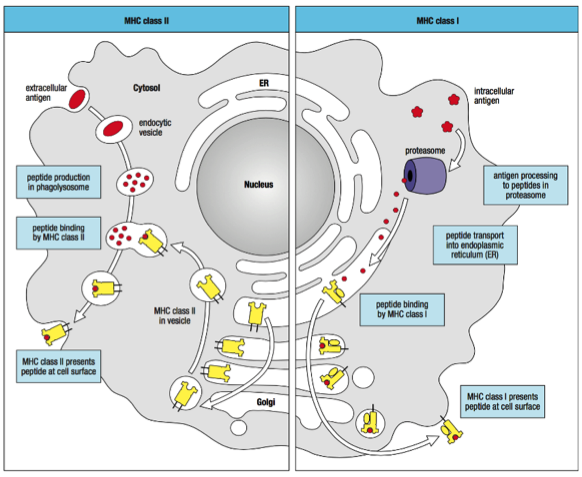
To induce adaptive immunity, the DCs must process the tumour antigens and then present them on their cell surface to activate CD8+ effector T-cells and CD4 T helper cells.12 The tumour antigens can either be endogenous (intracellular) or exogenous (extracellular).13 The endogenous antigens are located in the cytosol of the cell and are broken down into small peptides by proteasomes (Alexandrescu et al., 2010). The peptides are transported to the endoplasmic reticulum (ER) where they bind to a Major Histocompatibility Complex (MHC) Class I molecule.13 MHC Class I molecules are essential for the binding and presentation of these peptides on the cell surface, therefore, the peptide:MHC Class I complex is transported to the surface of the DC through the Golgi apparatus.13 The exogenous antigens, however, must be taken up by endocytosis or phagocytosis and broken down into smaller peptides by proteases in the vesicles.13 These peptides then bind to MHC Class II molecules which have been transported to the vesicle containing the peptide through the endoplasmic reticulum and Golgi apparatus.13 The peptide:MHC Class II complex is then transported and presented on the surface of the DC as well.13 Antigen presentation on MHC Class I or II molecules subsequently completes the maturation process, allowing for priming of T-cells.13
T Cell Response
The maturation of the DCs and their migration to the lymph nodes allows for induction of effector functions against tumour cells.13 CD4 and cytotoxic CD8 T-cells play important roles in the systemic immune response of oncolytic immunotherapy.13
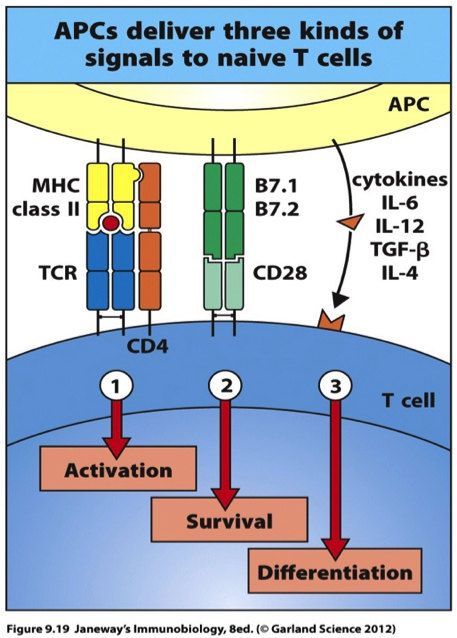
Activation of CD4 T-Cells
In the lymph nodes, the mature DC encounters a CD4 cell. In the activation processes, the CD4 cell receptor and the CD4 cell co-receptor binds to the peptide:MHC Class II complex.13 In addition to this, a co-stimulatory signal is required to allow the CD4 cell to divide and survive.13 CD28, a co-stimulatory receptor located on the cell surface of the naïve CD4 cell binds to the co-stimulatory ligand, B7, on the DC.13 In addition, the DC secretes cytokines, such as interleukin (IL) -2, -4, -6 and transforming growth factor (TGF) -β which allows for optimal proliferation and differentiation of CD4 cells. Therefore, the combination of these signals allows the CD4 cell to differentiate into effector cells.13 These cells become important in the activation of cytotoxic CD8 cells, which travel throughout the body to kill distance tumour cells.
Activation of Cytotoxic CD8 T-Cells
The activation of cytotoxic CD8 cells occurs through two methods. The first method of activation of CD8 cells occurs in a very similar manner to the CD4 cells. In this case, the CD8 cell co-receptor binds to the peptide:MHC Class I complex.13 The binding of the B7 co-stimulatory ligand to the CD28 co-stimulatory receptor is required for CD8 cell activation as well.13 When CD8 cells are activated by these intracellular interaction, they synthesize interleukin (IL) -2, which is a cytokine, and the IL-2 receptor.13 This subsequent interaction allows for proliferation and differentiation of CD8 cells.13 If this process does not provide sufficient co-stimulation, the CD4 effector cells can help to activate naïve virus-specific CD8 cells as well.13 In this case, the DC interacts with both CD4 and CD8 T-cells through interactions with the peptide:MHC Class II complex and the peptide:MHC Class I complex, respectively.13 In addition, the IL-2 cytokine that is secreted by the CD4 cell binds to the IL-2 receptor on the CD8 cell, which leads to proliferation and differentiation of the CD8 cell.13 The activated cytotoxic CD8 cells can now exit the lymph nodes and travel throughout the body to kill distant tumour cells.
Cytotoxicity of CD8 Cells
Cytotoxic T-cells can induce apoptosis in tumour cells through releasing cytotoxins such as perforin and granzyme.14 Perforin molecules are released from lytic granules and polymerize to form pores in the membrane of the cancerous cell, allowing granzymes, which are serine proteases to enter the cytoplasm of the cell.14 This leads to the activation of nucleases in the cell, which effectively grade the cell’s DNA.14 The nucleus also ruptures and membrane integrity decrease, resulting in cell death.14
Studies on Melanoma Patients Using T-VEC
What is Melanoma?
Before discussing the studies that were conducted using T-VEC on melanoma patients, it is important to define what melanoma is and give a background on various stages of this cancer. Melanoma is a tumour that starts in melanocytes which is malignant in nature, meaning it is able to spread to other parts of the body. Melanocytes are a type of cell responsible for producing melanin, the pigment that your skin and eyes their respective colors.25 The skin itself is part of the integumentary system, and is the largest organ in mammals.25 It covers the entirety of the body, protecting it from external damage via infection, ultraviolet damage and injury. Moreover, it produces vitamin D and maintains body temperature in addition to storing water and fat.25 Human skin is composed of two primary layers, the dermis and the epidermis. The latter is the outermost layer of the skin, creating a protective barrier over the body’s surface. It is also made up of three types of cells, one of which is melanocytes, found inferior to both squamous and basal cells respectively, at the bottom of the epidermis.25 Melanoma is the most dangerous form of skin cancer. Interestingly enough, when skin cancer starts in squamous or basal cells, it is called non-melanoma skin cancer. The primary cause of melanoma is ultraviolet light exposure (ex. excessive exposure to sunlight) in those with low levels of melanin.25 However, unusual moles, health history and poor immune function can also affect the risk of melanoma. Up to 25% of melanoma cases develop directly from moles.25 Diagnosis occurs through a biopsy of any skin lesions showing symptoms of melanoma. Due to a variety of factors, cells in the skin are often altered and no longer function or grow normally. When melanocytes become atypical they often go undetected because they display precancerous conditions despite not being cancerous.25 However, there is an increased chance these changes may become cancerous, such as in the presence of an abnormal mole or dysplastic nevus. The most common form of melanoma is superficial spreading melanoma, which occurs on the skin.25 However, it is possible that melanoma can occur in other parts of the body where it is present such as internal organs (mucosal lentiginous melanoma).25
Stages of Melanoma
Stage 0: in situ melanoma (99.9% survival)
- The melanoma is only found in the epidermis and have not started to spread into deeper layers.
Stage IA: Invasive melanoma (89-95% survival)
- The melanoma is less than 1mm thick and the covering layer of skin over the tumour has not been broken or ulcerated.
Stage 1B: Invasive melanoma (89-95% survival)
- The melanoma is less than 1mm thick and the skin has ulcerated OR they are 1-2mm and not ulcerated.
Stage IIA: High risk melanoma (45-79% survival)
- The melanoma is 1-2mm thick and ulcerated or 2-4mm and is not ulcerated.
Stage IIB: High risk melanoma (45-79% survival)
- The melanoma is between 2 and 4mm thick and is ulcerated or it is thicker than 4mm and is not ulcerated.
Stage IIC: High risk melanoma (45-79% survival)
- The melanoma is thicker than 4mm and is ulcerated.
Stage III (A/B/C): Regional metastasis (24–70% survival)
- In stage 3 the melanoma may be a variety of thicknesses with or without ulceration. However, one or more of the following is true. Firstly, the cancer has spread to one or more lymph nodes. Secondly, the lymph nodes are matted or joined together. Third, the cancer is in a lymph vessel between the primary tumour and nearby lymph nodes. Fourthly, the cancer is more than 2 centimeters away from the primary tumour. Finally, very small tumours can be located on or underneath the skin, less than 2 centimeters away from the primary tumour.
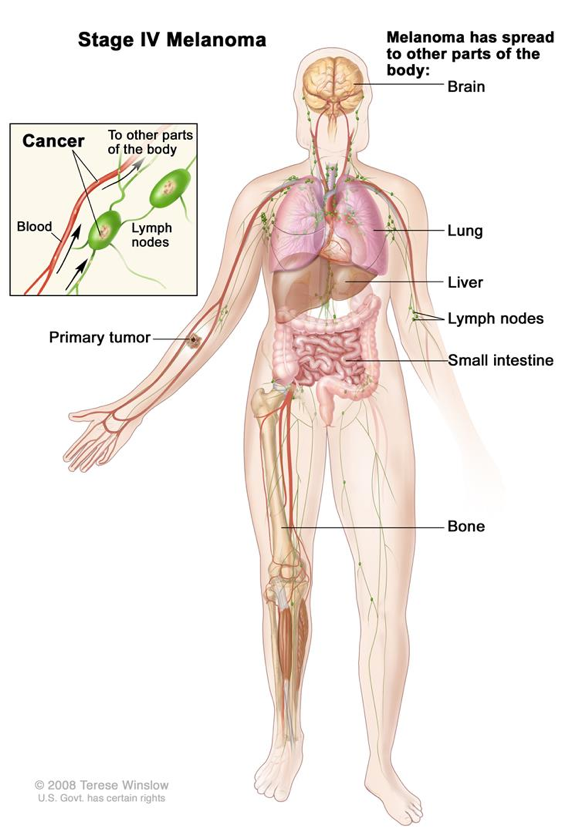
Stage IV: Distant metastasis (7–19% survival)
- In the fourth and final stage of melanoma, the cancer has spread to other parts of the body away from the primary site and the nearby lymph nodes. In most cases, it is common for the cancer to spread to the lungs, liver, and brain or to distant lymph nodes or areas of the skin. However, it is not unheard of for the cancer to spread to places far away from its origin.
Study 1: Local and Systemic Immune Response to T-VEC
Objective and Study Design
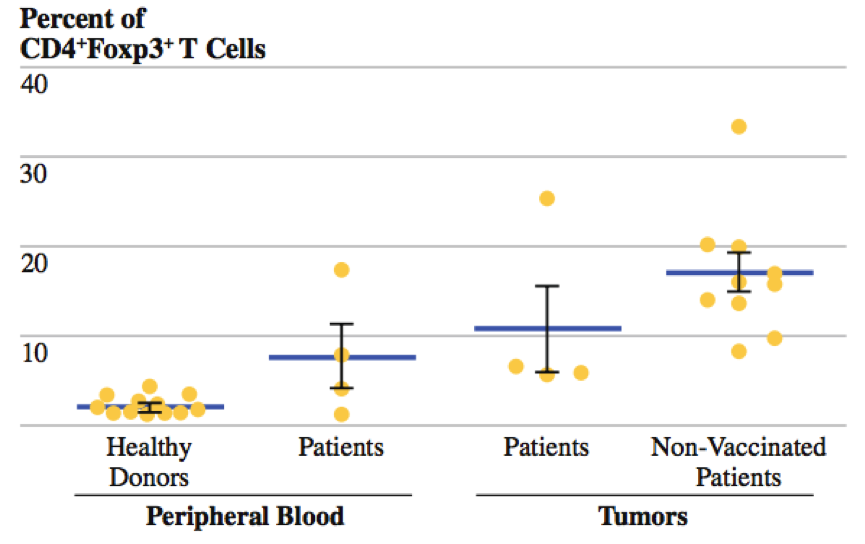
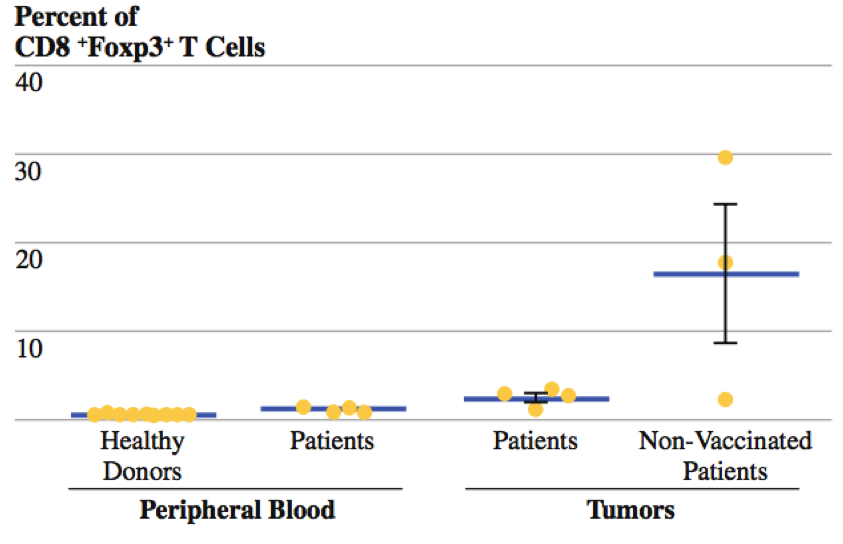
Kaufman and colleagues (2010) conducted a phase II clinical trial using T-VEC on patients who had Stage IIIc and IV melanoma.12 The researchers sought out to determine the local and distant effects of injecting T-VEC into the lesions of these patients.12 To accomplish this, the patients received one intratumoural injection in a single lesion every two weeks for a total 24 injections.12 Peripheral blood and tumour tissues were also collected for analysis of CD4+FoxP3+ regulatory T cells (Treg) and CD8+FoxP3+ suppressor T-cells (Ts).12 In these experiments, forehead box P3 (FOXP3) nuclear transcription factor was used since it is a marker of CD4 regulatory and CD8 suppressor T-cells.12 The researchers analyzed the frequency of Treg cells since they block effector T-cell, such as CD4 cells, and induce a tolerance to activated T-cells.12 In addition, they also analyzed frequency of Ts cells since they inhibit cytotoxicity of CD8 cells.12
Findings and Conclusions
Researchers compared the frequency of CD4+FoxP3+ regulatory T cells in both the peripheral blood and the tumour microenvironment.12 In the peripheral blood, there was a higher frequency of Treg cells among patients who were treated with T-VEC compared to healthy donors who did not have melanoma.12 In addition, there was also a higher frequency of T-reg cells in patients who were treated with T-VEC compared to patients who were not treated with T-VEC in the tumour microenvironment.12 Therefore, by decreasing the frequency of Treg cells, T-VEC acts to decrease the inhibitory functions these cells on CD4 cells in the tumour microenvironment.12 Researchers also compared the frequency of CD8+FoxP3+ suppressor T cells in both the peripheral blood and the tumour microenvironment.12 Again, similar results were found, as there was a higher frequency of Ts cells among patients who were treated with T-VEC compared to healthy donors who did not have melanoma in the peripheral blood.12 In addition, there was also a higher frequency of Ts cells in patients who were treated with T-VEC compared to patients who were not treated with T-VEC in the tumour microenvironment.12 Therefore, by decreasing the frequency of Ts cells, T-VEC acts to decrease the inhibitory effects of on the cytotoxicity of CD8 cells in the tumour microenvironment.12 In addition, to determine the systemic immune effects of T-VEC, the researchers analyzed the frequency of Treg cells in the lesions that received the T-VEC injection and in the lesions that did not receive the injection in the same patients.12 They then compared the Treg frequencies in lesions of non-vaccinated patients.12 The results indicated that there was a lower frequency of Treg cells in non-target lesions in vaccinated patients than in lesions of non-vaccinated patients.12 This ultimately provides evidence that T-VEC is able to decrease the frequency of Treg cells in distant lesions that are not injected.12 Ultimately, the collective findings of the experiments provided evidence of antigen-specific immunity in both locally injected lesions and distant non-injected tumors.12 More importantly, these findings are consistent with priming of systemic anti-tumour immunity and suggest that T-VEC is capable of inducing systemic immunity.12
Study 2: Phase II Clinical Trial
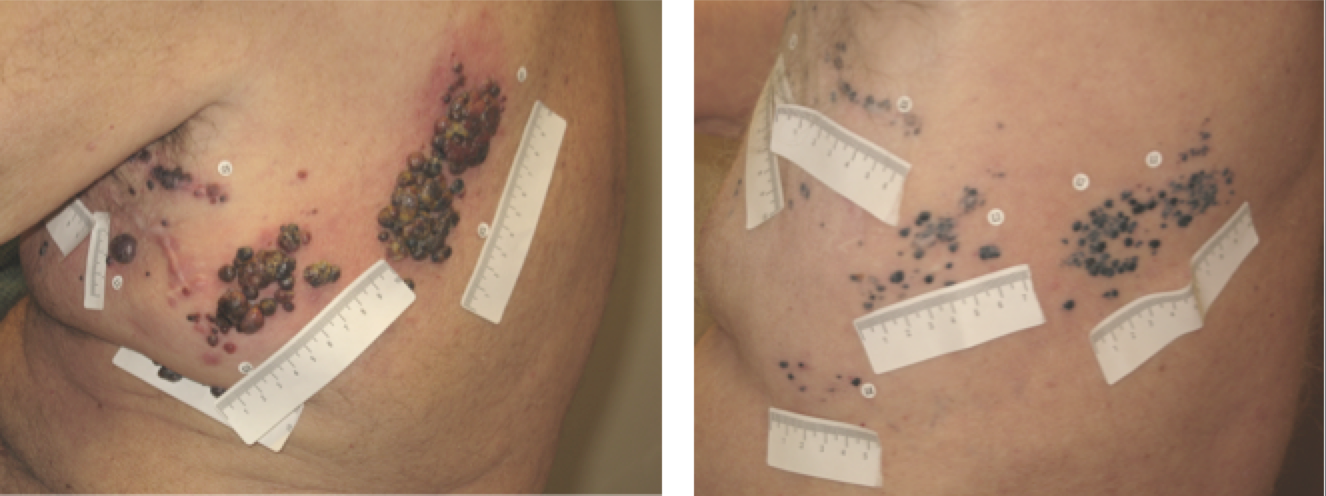
In 2009, 50 patients with advanced melanoma were treated with T-VEC and evaluated via RECIST (Response Evaluation Criteria In Solid Tumours).26 This is a set of published rules that define when tumours in cancer patients improve (“respond”), stay the same (“stabilize”), or worsen (“progress”) during treatment. Following treatment, the following results were observed. Approximately16% of patients experienced a complete response, indicated by a disappearance of all target lesions.26 An additional 10% experienced a partial response, characterized by a minimum 30% decrease in the size of target lesions, taking as reference the baseline longest diameter (LD) for an overall response rate of 26%.26 Furthermore, 20% of the patients, a small but critical portion, maintained a stable condition for 20 months.26They experienced neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease. Overall, 54% of patients survived until Year 1 while 52% survived until Year 2 under treatment with T-VEC.26 Additionally, a few patients exhibited initial disease progression before eventually responding by generating the full immune response. Finally, responses were observed in both injected and uninjected tumours (including those in visceral organs), demonstrating systemic immunotherapeutic effects.
Study 3: Phase III OPTiM Clinical Trial
In a global, open-label trial, 430 patients with unresectable stage IIIB, IIIC or IV melanoma were treated with either T-VEC or subcutaneously administered GM-CSF.27 Patients underwent outcome-adaptive randomization via a 2:1 fixed ratio, to create twice as many patients randomly assigned to T-VEC treatment. The primary endpoint was durable response rate (DRR), defined as a complete or partial tumor response lasting at least 6 months and starting within 12 months of treatment.27 The median time to respond was 4.1 months, and more than half of the patients experienced approximately a 25% or greater increase in the size of lesions or appearance of new lesions before achieving a response.27 This pseudo pattern is consistent with other immunotherapy's and illustrates the importance of continuing treatment in clinically stable patients even if individual lesions increase in size or new lesions develop. T-VEC showed superior benefits to metastatic melanoma as outlined by the DRR achieved in 16% of patients receiving T-VEC compared with only 2% in the GM-CSF control group.27 The greatest benefit was seen in stage IIIB or IIIC melanoma, with 33% of T-VEC patients maintaining a DRR in comparison to 0% with GM-CSF. The objective response rate (any response) with T-VEC was 32%, with 17% of patients experiencing a complete response, characterized by a complete disappearance of melanoma throughout the body.27 Ultimately, this showed that T-VEC has a systemic immune effect that destroys distant, uninjected tumours.
Negative Implications of Oncolytic Immunotherapy
Oncolytic immunotherapy is a promising immunological therapy that has the potential to prevent and treat numerous types of cancer. However, despite its favourable risk-benefit ratio, there are some negative aspects and limitations regarding the clinical use and application of the therapy.3 Theoretically, oncolytic immunology utilizes an engineered virus to attack the specific tumour within a body but the oncolytic virus raises some concern in clinical trials. For example, patients diagnosed with cancer also have an onset of other critical health issues. Immunocompromised patients may not be good candidates to receive this therapy since the oncolytic virus-mediated anti-tumour may introduce unwanted immune reactions.3 Tumour immunity could be compromised in these patients. The introduction of a novel drug has to undergo extensive clinical trials in order to identify the biological activity, the safety, the efficacy, the correct dosage and potential side effects if any.3 In addition, some of the safety concerns for manufacturing a live viral product include pre-clinical safety challenges, manufacturing bio-safety and assuring viral safety of the product by designing and constructing a safe virus.3 There are two main categories in assessing the challenges in administering oncolytic immunotherapy; the possible side-effects or adverse events and issues with utilizing oncolytic agents such as TVEC.
Adverse Events in Clinical Trials
The Food and Drug Administration has recently approved the use of Talimogene laherparepvec (TVEC), also known as Imlygic in October 2015.15 This is the first oncolytic immunotherapy approved and it is readily used in the treatment of melanoma. After undergoing repeated testing of TVEC in clinical trials, there were some adverse events that were documented. Imlygic was reported to have a generally acceptable tolerability profile in patients with malignant melanoma however, in phase III OPTiM, the overall incidence of at least one adverse event of any grade was 99%.15 The incidence of treatment-related grade 3 or 4 adverse effect was 11%. 15 The rate of discontinuation as a result of adverse events was 4%, with disease progression being the main reason for patient dropout.2 Overall, there were 438 people who participated in the phase III OPTiM trial and 295 of those individuals received TVEC treatment whereas the other patients received the standard of care.2 In addition, patients from the trials reported adverse events of chills (49%), pyrexia (43%), injection-site pain (28%), nausea (36%), influenza-like illness (30%) and fatigue (50%).15 The side effects observed in the TVEC group compared to the control were significantly higher. The only grade 3 or 4 adverse events that occurred in greater than 2% of TVEC treated patients were cellulitis (2.1%). The treatment was not fatal to any of the participants. There were many side effects that causes discomfort, however none of the adverse events were fatal. Nevertheless, oncolytic viruses have been associated with a very favourable risk-benefit ratio. The adverse events that the participants experienced are insignificant compared to the potential reward of reducing the malignant tumour size.
Issues in the Development and Utilization of Oncolytic Agents
There are many challenges to a successful systemic delivery of viruses. The host defences limit most oncolytic viruses’ ability to infect tumours after administration of the virus. There are other factors that contribute to limit the virus’ ability to replicate in the host body. Blood cells, antibodies and antiviral cytokines are always in circulation in order to detect foreign pathogens.16 It may incorrectly identify the engineered virus as a foreign particle and remove it from the system before it is able to target the tumour. Non-specific uptake by other tissues such as lung, liver and spleen may also pose a problem by reducing the concentration of the viral agent, reducing its effectiveness.16 Tissue-resident macrophages and poor virus escape from vascular compartment are also main barriers to systemic delivery of oncolytic viruses.16 After intravenous injection of the virus, pre-existing antibodies and other immune responses will neutralize it. Evidently, in order for the virus to be effective, it must maintain its circulation without depletion or degradation, while also selectively targeting tumour cells.
In addition, there are some safety and efficacy developmental issues. To develop a safe viral oncolytic agent, a certain criteria should include cancer specificity, chances of regaining pathogenicity and possibility of transmission to healthy individual.17 A possible solution for this is to use a non-human virus in oncolytic immunotherapy. To avoid any efficacy problems, approaches to evade antiviral mechanisms in the host body should be considered. Techniques such as serotype switching and polymer coating should be employed so that antibodies cannot recognize the virus particle.4 There are also some limitations to more advanced patients with extensive visceral disease meaning the tumor is not located cutaneously or subcutaneously.17 This is because TVEC is injected directly into lesions rather than given orally or intravenously. As a result, a single agent of an oncolytic virus such as TVEC will not be effective. However, there is a possible solution for this limitation. Researchers are currently conducting trials to address this limitation through combination therapy with checkpoint blockade drug agents.17 Despite having a few side-effects and limitations, the continued development of this novel therapy should be anticipated, especially in combination approaches. Oncolytic immunotherapy is very promising approach and introduces a new method of treating people with cancer.
Future Applications of Oncolytic Immunotherapy
Most cases of oncolytic immunotherapies generally show successful growth when utilized in combination therapy. An oncolytic virus whose promising future essentially lies in combination therapy is T-VEC, which goes by the brand name of Imlygic.18 Many other companies have seen the potential success and growth of Imlygic and have decided to invest in its future.18 One company that recognizes Imlygic’s potential is Merck whom Amgen is collaborating with on a study pairing its treatment with the PD-1-blocking Keytruda in melanoma and head and neck.1 Another company that is following the same trend is ROCHE who have signed on to match its atezolizumab, a PD-L1 therapy, with T-VEC in breast and colon cancer.18 Due to its many benefits in treating melanoma, it is clear as to why there is great success in T-VEC as an oncolytic immunotherapeutics. However, this is just one of the many different cancers that can be treated by oncolytic immunotherapy such as ovarian, prostate and renal cancers.
Ovarian Cancer
One of the many cancers that have treatments via oncolytic immunotherapy is ovarian cancer. Oncolytic viruses, like Vaccinia Virus, have been employed in virotherapy and have proven to work by lysing tumour cells directly to battle against ovarian cancer.19 However, the key to the success viral oncolysis in ovarian cancer is repeated treatments to boost the immune response.20 Based on this, a study was done by Zhang et al. 2010, to test the limitations of repeated vaccinations by using two different viruses, Semliki Forest Virus (SFV) and Vaccinia Virus (VV) to boost immune response. These mice were infected with VV then infected with SFV, or SFV followed by VV, showing an increase in antitumor effects against murine ovarian surface epithelia carcinoma (MOSEC). More tests were done involving the infection with VV-ovalbumin (OVA) then the injection of an infection with SFV-OVA or vice versa. The results of these test showed an increase OVA-specific CD8+ T-cell immune response. Also, the infections of SFV-OVA followed by VV-OVA infections lead to an increase in antitumor effects in vivo as well as an increase in tumour killing in vitro via the combination of viral oncolysis and antigen-specific immunity.20
Prostate Cancer
In April 2010, the FDA approved Provenge (sipuleucel-T), the first ever FDA approved immunotherapy vaccine, a cell-based immunotherapy for advanced prostate cancer.21 Provenge showed enhanced survival in advanced prostate cancer patients in 2010 which led to its approval by the FDA.22 Initially, Provenge was expected to be an autologous dendritic-cell-based vaccine; but it was found to actually be comprised of a complex mixture of peripheral blood mononuclear cells added with a cytokine and tumour-derived differentiation antigen.22 Through many tests it was found that treatments involving Provenge were lacking the mitigation of tumours as well as a lack of sufficient vaccines for the target population.21 These factors indicate that there is a lot of work that needs to be done to create a successful oncolytic immunotherapeutics to treat prostate cancer effectively especially in larger quantities.22
Renal Cancer
Renal cancer has several different possible treatments with multiple oncolytical immunotherapy vaccines such as, Interleukin-2 (IL-2), Interferon-α and Sunitinib (sdsad). IL-2 is a recombinant cytokine which is not only used to treat renal cell cancer, but also melanoma. IL-2 is a T-cell growth factor that is proven to induce objective tumour regression.23 Interferon-α is another cytokine agent that is used to treat melanoma and renal cell cancer. As an oncolytic immunotherapy vaccine associated with low response rates and high-dose toxicity, Interferon-α still shows an impressive survival response reflecting antitumor immunity.20 Lastly, Sunitinib is a receptor tyrosine kinase inhibitor that has also been approved for the treatment of renal cancer.7 It acts to increase the growth of oncolytic versions of vesicular stomatitis virus (VSV) in order to have anti-angiogenic properties or to increase antitumour immunity.7 Similarly, to Imylgic, Sunitinib also undergoes combination therapy with Pexa-Vec as they synergize to treat renal cancer by increasing the growth of oncolytic VSV.7
Summary
Conclusively, the local and long term systemic effects of oncolytic immunotherapy provide a promising treatment for patients who are suffering from melanoma. In addition, there is also evidence that oncolytic immunotherapy can be a possible treatment for other types of cancers. However, as with any novel cancer treatment, there is the potential risk of some mild adverse events. Additionally, there are also various challenges with genetically engineering viruses to have specificity for only cancerous cells. Therefore, further studies need to be conducted on the safety and long term effects of using oncolytic immunotherapy.
References
1. Lawler, S. E., & Chiocca, E. A. (2015). Oncolytic Virus-Mediated Immunotherapy: A Combinatorial Approach for Cancer Treatment. Journal of Clinical Oncology, 33(25), 2812-2814.
2. Kaufman, H. L., & Bines, S. D. (2010). OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future oncology, 6(6), 941-949.
3. Kaufman, H. L., Kohlhapp, F. J., & Zloza, A. (2015). Oncolytic viruses: A new class of immunotherapy drugs. Nature Reviews Drug Discovery Nat Rev Drug Discov, 14(9), 642-662. Retrieved February 14, 2016.
4. Amgen Oncology. (2012). Oncolytic Immunotherapy: A Local and Systemic Antitumour Approach. Retrieved February 14, 2016, from http://www.amgenoncology-international.com/pdfs/TVEC2X0014_A_ASCO_Booklet.pdf
5. Merriam Webster. (n.d.). Medical Definition of Oncolysis. Retrieved February 14, 2016, from http://www.merriam-webster.com/medical/oncolysis
6. National Cancer Institute. (n.d.). NCI Dictionary of Cancer Terms. Retrieved February 14, 2016, from http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=615577
7. Lichty, B. D., Breitbach, C. J., Stojdl, D. F., & Bell, J. C. (2014). Going viral with cancer immunotherapy. Nature Reviews Cancer.
8. Seymour, L. W., & Fisher, K. D. (2016). Oncolytic viruses: finally delivering.British journal of cancer.
9. Shen, Y., & Nemunaitis, J. (2006). Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer gene therapy, 13(11), 975-992.
10. Mach, N., Gillessen, S., Wilson, S. B., Sheehan, C., Mihm, M., & Dranoff, G. (2000). Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer research, 60(12), 3239-3246.
11. van de Laar, L., Coffer, P. J., & Woltman, A. M. (2012). Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood, 119(15), 3383-3393.
12. Kaufman, H. L., Kim, D. W., DeRaffele, G., Mitcham, J., Coffin, R. S., & Kim-Schulze, S. (2010). Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Annals of surgical oncology, 17(3), 718-730.
13. Alexandrescu, D. T., Ichim, T. E., Riordan, N. H., Marincola, F. M., Nardo, A. D., Kabigting, F. D., & Dasanu, C. A. (2010). Immunotherapy for Melanoma: Current Status and Perspectives. Journal of Immunotherapy (Hagerstown, Md. : 1997), 33(6), 570–590.
14. Janeway, C. A., Travers, P., & Walport, M. (2005). Immunobiology: the immune system in health and disease. New York, NY: Garland Science
15. Andtbacka, R. H., Kaufman, H. L., Collichio, F., Amatruda, T., Senzer, N., Chesney, J., … & Milhem, M. (2015). Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. Journal of Clinical Oncology, JCO-2014.
16. Ferguson, M. S., Lemoine, N. R., & Wang, Y. (2012). Systemic delivery of oncolytic viruses: hopes and hurdles. Advances in virology, 2012.
17. Coffin, R. (2016). Interview with Robert Coffin, inventor of T-VEC: the first oncolytic immunotherapy approved for the treatment of cancer.Immunotherapy, (0).
18. Sheridan, C. (2015). First oncolytic virus edges towards approval in surprise vote. Nature biotechnology, 33(6), 569-570.
19. Zhang, Y. Q., Tsai, Y. C., Monie, A., Wu, T. C., & Hung, C. F. (2010). Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy.Molecular Therapy, 18(4), 692-699.
20. Galanis, E., Hartmann, L. C., Cliby, W. A., Long, H. J., Peethambaram, P. P., Barrette, B. A., … & Sloan, J. A. (2010). Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer research,70(3), 875-882.
21. Mellman, I., Coukos, G., & Dranoff, G. (2011). Cancer immunotherapy comes of age. Nature, 480(7378), 480-489.
22. Bartlett, D. L., Liu, Z., Sathaiah, M., Ravindranathan, R., Guo, Z., He, Y., & Guo, Z. S. (2013). Oncolytic viruses as therapeutic cancer vaccines.Molecular cancer, 12(1), 1.
23. Lesterhuis, W. J., Haanen, J. B., & Punt, C. J. (2011). Cancer immunotherapy–revisited. Nature reviews Drug discovery, 10(8), 591-600.
24. Melanoma Treatment. (2015, September 3). Retrieved March 11, 2016, from http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001853/
25. What is melanoma? - Canadian Cancer Society. (2011, July 17). Retrieved March 11, 2016, from http://www.cancer.ca/en/cancer-information/cancer-type/skin-melanoma/melanoma/?region=on
26.Senzer, N. N., Kaufman, H. L., Amatruda, T., Nemunaitis, M., Reid, T., Daniels, G., . . . Nemunaitis, J. J. (2009). Phase II Clinical Trial of a Granulocyte-Macrophage Colony-Stimulating Factor-Encoding, Second-Generation Oncolytic Herpesvirus in Patients With Unresectable Metastatic Melanoma. Journal of Clinical Oncology, 27(34), 5763-5771. Retrieved March 11, 2016.
27.Andtbacka, R. H., Collichio, F. A., Amatruda, T., Senzer, N., Chesney, J., Delman, K., . . . Kaufman, H. (2014). Final planned overall survival (OS) from OPTiM, a randomized Phase III trial of talimogene laherparepvec (T-VEC) versus GM-CSF for the treatment of unresected stage IIIB/C/IV melanoma (NCT00769704). Journal for ImmunoTherapy of Cancer J Immunother Cancer, 2(Suppl 3). Retrieved March 11, 2016.