Table of Contents
Presentation
Introduction
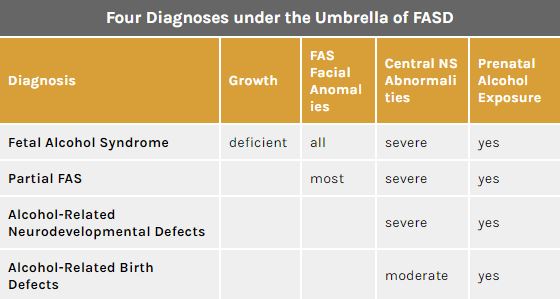
Figure 1: Diagnosis and characteristics under the FASD Umbrella </style>
<style justify> The detrimental effects of drinking during pregnancy have been commonly known for many centuries. However, It wasn’t officially documented until the late 1900s when medical practitioners decided it was necessary to establish formal term to describe these effects.[1] Researchers have since arrived at adopting an overarching term, called Fetal Alcohol Spectrum Disorder (FASD), thereby encompasses all the levels of prenatal alcohol exposure.
Fetal Alcohol Spectrum Disorder (FASD) is an umbrella term that describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy.[2] Such effects could be physical, mental, and/or behavioral. There are four main characteristics of those affected by FASD. They are: growth deficiencies, facial anomalies, central nervous system abnormalities and prenatal alcohol exposure. Notably, the disabilities caused with FASD are irreversible and have lifelong implications to individuals who are affected.[3]
Although FASD is not a diagnosis in itself, four diagnoses fall under its spectrum that increase in seriousness depending on the severity of FASD characteristics (Figure 1)[1]:
- Fetal Alcohol Syndrome (FAS)
- Partial FAS (pFAS)
- Alcohol Related Neurodevelopmental Disorder (ARND)
- Alcohol Related Birth Defects (ARBD)
</style>
Epidemiology
<style justify> Epidemiological studies are central to understanding how health-related conditions, such as fetal alcohol spectrum disorder (FASD), are distributed in human populations and to identifying factors that influence the occurrence of those distributions.
Meta-regressions have estimated that 2.3% of babies are born with FASD globally.[4] However, these rates vary considerably between countries and even within different regions of a nation. It is observed that populations situated within poorer and undeveloped areas experience higher prevalence of FASD. For example, several villages within South Africa can experience rates as high as 32% [5]. In contrast, more wealthy cities in a developed nation like Australia can have prevalence rates as low as 0.1%.[6] Reasons for the discrepancy in rates can lie in systemic problems involving alcohol abuse and poor education.
Canada specifically has over 3,000 babies being born with FASD every year. Furthermore, 300,000 individuals are currently living with a diagnosis under the FASD umbrella. Although the general prevalence rate lies between 1-2%, Aboriginal communities in Canada experience higher incidence of FASD. Studies done in Aboriginal communities in British Columbia found rates as high as 16%.[7]
</style>
Pathophysiology
<style justify> FASD is really hard to detect. The most optimal way is for self-disclosure, otherwise there are tests physicians may carry out in order to determine whether the woman is at risk of drinking.[8] </style>
Signs
<style justify> So the first sign you can see is how much is the intake of alcohol. The average consumption of alcohol for non-pregnant women is defined into three categories – light drinking (1.2 drinks per day), moderate drinking (2.2 drinks per day), and finally heavy drinking (3.5 drinks per day or more).[8] However, the amount of alcohol needed to be consumed to cause potential damage to the offspring is just one drink per day.[8][9] Recent studies have shown that even one drink per day is detrimental to the babies’ health and as such have concluded that establishing a threshold cannot be adequately identified.[8]
As mentioned above the signs for FASD are extremely hard to see. No biological marker is accurate enough to identify FASD. Studies have shown some signs of analysis of meconium and hair samples for fatty acid ethyl esters, but a larger sample size would be needed.[8] Another research that is being seen is γ-glutamyl transferase, hemoglobin-associated acetaldehyde, and carbohydrate-deficit transferrin.[8] Again, the issue lies that this research cannot be proven working to identify drinking in pregnant women.[9] </style>
<style center>
</style>
<style justify> The easiest sign is from the patient themselves. Self-reporting of maternal alcohol is essential to protect the lives of the baby and mother.[8] However, the stigmatization of drinking causes fewer people to discuss with this with their doctor. One thing doctors use as a consensus is the history of the patient. This leads to doctors assuming individuals who did not drink before pregnancy or a minimum amount would stop.[8] As studies showed, this was highly inaccurate as twice as many people admitted to alcohol consumption rather than when compared to medical records.
This is not to say that doctors do not have tools to figuring out women who intake alcohol during pregnancy. For example, one tool is called the T-ACE, and it is a traditional screening test by asking four questions.[8] The results from the analysis typically identify 90% of patients who can be potential risk-drinkers, but false-positives can also be ruled out by asking follow-up questions. Other tests are Tolerance, Worry, Eye-opener, Amnesia, Cut-down (TWEAK), Alcohol Use Disorders Identification Test (AUDIT), and Short Michigan Alcoholism Screen Test (SMAST) are a few tests used to identify potential risk-drinkers.[8] </style>
Symptoms
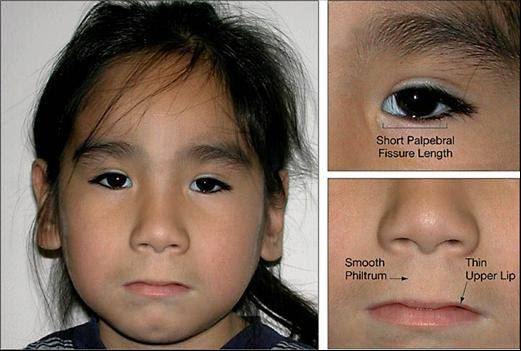
<style justify> The symptoms of individuals who have FAS have often had characteristics of facial dysmorphology, which is midfacial hypoplasia, long smooth philtrum, thin upper lip, small eyes that seem wider apart. Another symptom deals with growth restriction which affects the central nervous system and causes neurodevelopmental abnormalities, like ophthalmic involvement. Habituation to stimuli was most noticeable for neonatally, and much worse symptoms developed at eight months by using two indexes – Bayley Mental Developmental Index, and Psychomotor Developmental Index. Both of these indexes check to see how infant behavioral functioning based on a scale. Additionally, infants were reported to have much slower reaction time when the mother was drinking between low to moderate. Symptoms in Preschool children are much more noticeable, with reaction time towards a task taking much longer, struggling to maintain attention, and hyperactivity. Aside from these effects, learning disabilities, memory deficits, psychiatric problems like mood disorders have been seen in school-aged children who were exposed to moderate drinking levels. </style>
Figure 3: The common symptoms a child with FASD may have </style>
<style justify> Find the symptoms is not an easy thing to do. Many doctors have failed in identifying children with FAS, with one study going as far as out of 19 infants, only one was identified as a possible effect of FAS.[8] However, there were seven who displayed it. About half of family physicians themselves have little confidence in diagnosing for FAS.[8] </style>
Mechanism of Action
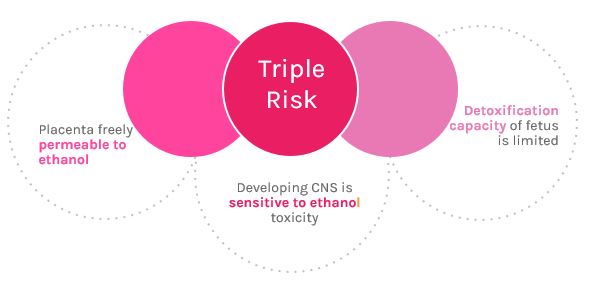
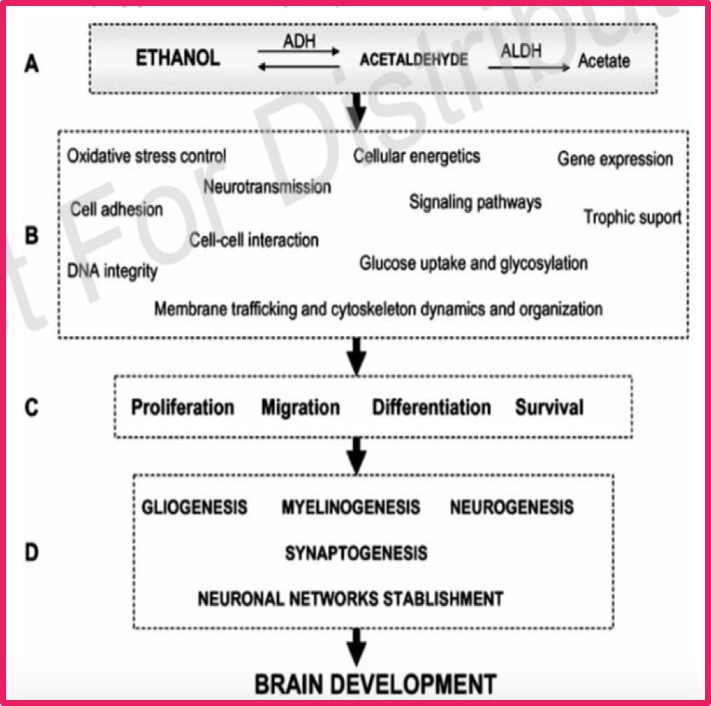
<style justify> The damage caused by ethanol on the development of the fetus depends on the gestational period, amount of alcohol consumed, and chronicity of abuse.[10] The most harm is caused to the fetus during the first gestational period (first 3 months).[11] The human fetus has a triple risk to maternal alcohol consumption. The first risk is that alcohol is able to cross the placenta freely and rapidly reaches the fetus.[12] Secondly the developing nervous system of the fetus is very sensitive to the toxicity of ethanol. The toxicity of ethanol has the ability to comprise all the major processes in the developing central nervous system.[13] Lastly fetal tissues do not have a significant capacity for detoxification of alcohol, and rely on maternal hepatic detoxification.[13] The ethanol remains in the amniotic fluid, and results in the fetus being exposed to the ethanol for a longer period of time.[12]
There has been no clear-cut mechanism of action for the development of FAS, but there have been many different proposed mechanisms that have been tested. Proposed biochemical issues have included abnormal prostaglandin metabolism, chromosomal alterations, placental dysfunction, hypoxia, interference with protein synthesis, alter growth signaling, interferences with neurotransmitter production which can result in neuroendocrine abnormalities and modification of enzymes with control glycogen synthesis and degradation.[14] </style>
Figure 4: The effects of alcohol on the fetus </style>
Growth of Fetus and Malnutrition
<style justify> When ethanol enters the placenta it impairs placenta blood flow to the fetus by constricting blood vessels [10]. This can lead impaired transfer of nutrients and oxygen to the developing fetus which can result in hypoxia and fetal malnutrition.[15] Another mechanism researched associated with fetal malnutrition is that ethanol and acetaldehyde are able to alter the metabolism of proteins, carbohydrates and fats. This results in a decrease of amino acids, glycose, folic acid, zinc and other nutrients being transferred across the placental barrier. Overall the malnutrition of the fetus results in impaired growth.[12] </style>
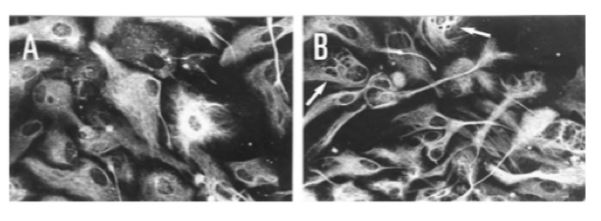
Figure 5: Immunofluorescence showing the effect of pre- natal exposure to ethanol on intermediate filaments of radial glial in primary culture (A) control cells; (B) cell prenatally exposed to alcohol. As shown, exposure to ethanol alter both the intermediate filament pattern (shown with arrows) and the morphological transformation of radial glial cells into astrocytes. </style>
Neural Development
<style justify> Ethanol has multiple cellular targets in the nervous system, which is one of the reason for many different proposed mechanisms.[16] Ethanol exposure causes oxidative injury, induction of apoptosis, suppression of neurogenesis, and disruption of cell-cell interactions.[16] There are certain areas of the developing central nervous system that appear to be more susceptible to defects caused by prenatal alcohol exposure. These areas include the ocular system, corpus callosum, basal ganglia and cerebellum. Ethanol causes damage to the neural stem cell progenitor pools that produce neurons and the supporting glial cells in the CNS. Defects to these cell can result in a decrease in their volumes leading to the structural abnormalities which affects the developing CNS.[15] Oxidative stress is one of the proposed mechanism that plays a part in ethanol-induced cell damage, cell death and nervous system dysfunction that is observed in FAS.[17] Studies have shown that ethanol produces and increase in oxidative stress in the developing brain through two mechanisms: the inductions of reactive oxygen species generations, and decreasing the amount of efficient endogenous-antioxidant systems. </style>
<style center>
</style>
<style justify> Glial cells and their connections with neurons play an essential role during the development of the nervous system. Experimental evidence has shown that in utero ethanol exposure causes structural and functional defects in gliogenesis and in glial-neuronal interactions. This suggests glial cells having a potential role in alcohol- induced abnormalities in the developing brain.[18] Data from Guerri et al. demonstrated that in utero ethanol exposure affects the morphology of radial glia in culture and significantly depresses the expression of glial fibrillary acidic protein in fetal brain development. Other research has helped to support the conclusion that radial glial is among the primary targets for ethanol toxicity. With regards to the structures of the brain, the corpus callosum is initially formed by radial glial that support the growth of axons from one side of the brain to the other. The alterations in the radial glial caused by ethanol exposure would explain the structural abnormalities found in the corpus callosum and why it is one of the areas high susceptible to ethanol toxicity.[18] </style>
Medicinal Treatment
<style justify> Pharmacological treatments should ideally be administered secondary to non-pharmacological (psychological and behavioral) therapies.Currently there are no specific medications for the treatment of FASD, but there are medications that can be used to help treat symptoms.
Stimulants are typically used to treat ADHD, ODD and CD. Stimulants are used to treat symptoms of Inattention, hyperactivity, and impulsivity. Common drugs are an Amphetamine & Dextroamphetamine mix (adderall), LISDEXAMPHETAMINE (vyvanse) ,and METHYLPHENIDATE .[19] Antidepressants are used to treat symptoms such as sad mood, loss of interest, sleep problems, school disruption, and anti-social behaviors.[20] neuroleptics are prescribed to children with developmental disabilities, including FASDs, to address aggression, anxiety, or behavior regulation such as risperidone
A promising new therapy is choline supplementation. Choline is a water-soluble vitamin commonly grouped under vitamin B-complexes. It is known to play a role in normal brain development and nerve function. There has been a recent pilot study conducted by Wozniack and colleagues aimed to assess the feasibility, tolerability and potential adverse side effects of choline supplementation in children (aged 2.5-5 years of age) with FASD.[21] This was a double-blind, placebo-controlled and randomized study with 60 participants who received either 500mg choline or placebo for a period of 9 months.
Results from the study were positive. Choline was well tolerated, the only side effect was a fishy odour.[21] Odour is due to trimethylamine formation by gut bacteria from the metabolism of the choline.[21] There were significant results showing improved memory performance and reduction of learning deficits in the choline group. Additional evaluation of choline supplementation as an intervention for memory functioning in children with FASDs is warranted.[21] Need to establish critical windows (ie. first 4-5 years).Optimal dosages and therapy times need to be determined.[21] </style>
Other Types of Treatment
Introduction
<style justify> Similar to many other disorders those with FASD will be affected very differently thus treatments will have to be very specific to that person. Therefore, a treatment that may have worked for one patient with FASD may not necessarily work for another patient. As of today, there is still no treatment that will cure FASD however, through research it has been found that early intervention treatment services can help with a child’s improvement in development.[22] Early intervention services, such as therapy, are designed to help children at risk or with FASD from birth to 3 years of age, to help the child speak, walk, and interact with others.[23]
</style>
Protective Factors
<style justify> Through studies it has been shown that there are protective factors which help to reduce the effects of FASD and also allow those affected by FASD to reach their full potential.[22] These factors include:
- early diagnosis
- involvement in special education and social services
- having a loving, nurturing, and stable home environment
- absence of violence
All of these factors provide benefits such as: being able to better understand the child, allowing the child and their family to have more positive experiences, and prevent the child from developing secondary conditions (e.g. criminal behaviour, lack of education, and unemployment).[22]
- There are generally five categories of treatment for patients that have FASD, including: medical care, medication, behaviour and education therapy, parent training, and alternative approaches.[22]
</style>
Behaviour and Education Therapy
- Good Buddies: an intervention that uses a group setting to teach children with FASD appropriate social skills for their age group.[23]
- Families Moving Forward (FMF): an intervention intended to provide support for children displaying more severe and significant behaviour problems.[24]
- Math Interactive Learning Experience (MILE): an intervention that helps to improve the child’s mathematical knowledge and skills because it is a consistently reported deficit of children with FASD.[25]
- Parents and Children Together (PACT): an intervention that focuses on improving behaviour regulation and executive functions which include planning, organizing, and understanding.[26]
Although there are multiple therapies these have been scientifically studied and have been effective in children.
Parent Training
- This is often a program offered by therapists to educate parents about their child’s disability and providing different approaches to interact, engage, and teach their children.[22]
- Some tips include: concentrating on the child’s strength, being consistent with everything, and accepting the child’s limitations.[22]
Alternative Approaches
These are untested therapies that are also provided to assist with FASD.[22]
- Biofeedback
- Auditory Training
- Relaxation Therapy
- Creative Art Therapy
- Yoga and Exercise
- Acupuncture and acupressure
- Massage, Reiki and energy healing
- Vitamins, Herbal Supplements, and Homeopathy
- Animal-Assisted Therapy
Conclusion
<style justify> In conclusion, fetal alcohol syndrome disorders have many serious negative implications to thousands of affected individuals globally. These symptoms are irreversible and devastating throughout the entire livelihood of those who are diagnosed.
Research has made great strides forward since the late 1990s, when FASD and its diagnoses were officially recognized. Although there is now a better understanding of the consequences of prenatal alcohol exposure, there is still much to be done. For example, better identification and diagnosis of the full range of FASD are needed, which could be improved with the development of biomarkers that aid in detection and accurate quantification of prenatal alcohol consumption.
Most importantly, there needs to be vast improvement in the scale and efficacy of prevention strategies implemented prior to conception. Fetal alcohol spectrum disorders are entirely preventable, and education and awareness are necessary to dramatically decrease the incidence of FASD. There is no reason for fetal alcohol spectrum disorders to prevail as one of the leading causes of birth defects worldwide. </style>
References
[1] Susan J. Astley. (2004). What is FASD? Retrieved November 2, 2016, from https://depts.washington.edu/fasdpn/htmls/fasd-fas.htm
[2] US Department of Health & Human Services. (2015, April 16). Facts about FASD. Retrieved November 2, 2016, from http://www.cdc.gov/ncbddd/fasd/facts.html
[3] Public Health Agency of Canada Government of Canada. (2010, August 17). Fetal Alcohol Spectrum Disorder (FASD) - Childhood and Adolescence. Retrieved November 2, 2016, from http://www.phac-aspc.gc.ca/hp-ps/dca-dea/prog-ini/fasd-etcaf/index-eng.php
[4] Roozen, S., Peters, G.-J. Y., Kok, G., Townend, D., Nijhuis, J., & Curfs, L. (2016). Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcoholism, Clinical and Experimental Research, 40(1), 18–32. https://doi.org/10.1111/acer.12939
[5] May, P. A., de Vries, M. M., Marais, A.-S., Kalberg, W. O., Adnams, C. M., Hasken, J. M., … Hoyme, H. E. (2016). The continuum of fetal alcohol spectrum disorders in four rural communities in South Africa: Prevalence and characteristics. Drug and Alcohol Dependence, 159, 207–218. https://doi.org/10.1016/j.drugalcdep.2015.12.023
[6] Burns, L., Breen, C., Bower, C., O’ Leary, C., & Elliott, E. J. (2013). Counting fetal alcohol spectrum disorder in Australia: the evidence and the challenges. Drug and Alcohol Review, 32(5), 461–467. https://doi.org/10.1111/dar.12047
[7] Aase, J. M. (1981). The fetal alcohol syndrome in American Indians: a high risk group. Neurobehavioral Toxicology and Teratology, 3(2), 153–156.
[8] Sokol, R. J. (2003). Fetal Alcohol Spectrum Disorder. Jama, 290(22), 2996. doi:10.1001/jama.290.22.2996
[9] Chudley, A. E. (2005). Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Canadian Medical Association Journal,172(5_suppl). doi:10.1503/cmaj.1040302
[10] University of Wisconsin. (2004). Fetal Alcohol Syndrome. Retrieved from http://people.uwec.edu/piercech/fas/fas...htm
[11] Galan, N. (2015, November 9). Fetal Alcohol Syndrome. Retrieved from http://www.healthline.com/health/fetal-alcohol-syndrome#Causes2
[12] Vaux, K. K. (2016, October 22). Fetal Alcohol Syndrome. Retrieved from http://emedicine.medscape.com/article/974016-overview#a5
[13] Wikipedia. (n.d.). Fetal Alcohol Spectrum Disorder. Retrieved from https://en.wikipedia.org/wiki/Fetal_alcohol_spectrum_disorder#Mechanism
[14] Spagnolo, A. (1993). Teratogenesis of alcohol. 29(1), 89-96. Retrieved from http://alleanzacontroilcancro.it/binary/publ/cont/Pag89_96Vol29N11993.pdf
[15] O'Neil, B. E. (2014, January 28). Fetal Alcohol Syndrome. Retrieved from https://embryo.asu.edu/pages/fetal-alcohol-syndrome-fas
[16] Wilkemeyer, M., Chen, S., Menkari, C., Brenneman, D., Sulik, K., & Charness, M. (2003). Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. PNAS,100(14), 8543-8548. Retrieved from http://www.pnas.org/content/100/14/8543.full.pdf
[17] Martinez, S. E., & Egea, G. (2007). Novel Molecular Targets for the Prevention of Fetal Alcohol Syndrome. CNS Drug Discovery, 2, 23-35. Retrieved from http://www.ub.edu/sgr120/Publications/2007-04%20Martinez.pdf
[18] Guerri, C., Pascual, M., & Renau-Piqueras, J. (2001). Glia and Fetal Alcohol Syndrome. Elsevier, 22, 593-599. Retrieved from http://journals1.scholarsportal.info.libaccess.lib.mcmaster.ca/pdf/0161813x/v22i0005/593_gafas.xml
[19] Koren, G. (2015). Pharmacological treatment of disruptive behavior in children with fetal alcohol spectrum disorder. Pediatric Drugs, 17(3), 179-184.
[20] Young, S. J., Absoud, M., Blackburn, C., Branney, P., Colley, B., Farrag, E., … & Plant, M. (2016). Guidelines for identification ad treatment of individuals with Attention Deficit/Hyperactivity Disorder and associated Fetal Alcohol Spectrum disorders based upon expert consensus.
[21] Wozniak, J. R., Fuglestad, A. J., Eckerle, J. K., Fink, B. A., Hoecker, H. L., Boys, C. J., … & Zeisel, S. H. (2015). Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. The American journal of clinical nutrition, 102(5), 1113-1125.
[22] CDC. (2016, October 04). Fetal Alcohol Spectrum Disorders (FASDs). Retrieved from http://www.cdc.gov/ncbddd/fasd/treatments.html
[23] O’Connor MJ, Frankel F, Paley B, et al. A controlled social skills training for children with fetal alcohol spectrum disorders. J Consult Clin Psychol. 2006;74(4):639–648.
[24] Carmichael Olson H, Leavitt S. Families Moving Forward. Platform Presentation on October 23, 2010. FASD Fall Conference at Emory University, Atlanta, GA
[25] Kable JA,Coles CD,Taddeo E. Socio-cognitive habilitation using the math interactive learning experience program for alcohol affected children. Alcohol Clin Exp Res. 2007; 31: 1425-1434. Bertrand J, Floyd L, Chasnoff I, Wells A, Bailey G, et al. Interventions for children with fetal alcohol spectrum disorders (FASDs): Overview of findings for five innovative research projects. Res Dev Disab. 2009;30(5):986–1006.
[26] Wells, A.M., Chasnoff, I.J., Schmidt, C.A., Telford, E., & Schwartz, L. (2012). Neurocognitive habilitation therapy for children with fetal alcohol spectrum disorders: An adaptation of the Alert Program®. American Journal of Occupational Therapy, 66, 24-34.