This is an old revision of the document!
Table of Contents
Parkinson's Disease Powerpoint
Parkinson's disease
Parkinson’s disease (PD) is a neurodegenerative disorder which affects the substantia nigra, a region within the brain.[1] PD can also lead to various cognitive impairment, autonomic dysfunction, sleep disorders, and depression which further affect those living with the illness.[1]
History
Traditionally, descriptions of PD can be found as early as 1000 BC for Indian texts, in addition to those within ancient Chinese sources.[2] PD was initially described by James Parkinson in 1817 through this work in “Essay on the shaking palsy”.[3] This was originally thought to be a movement disorder with three fundamental signs: bradykinesia, rigidity, and tremors.[3] Parkinson at that time described the disease as: “involuntary tremulous motion, with lessened muscular power, in parts not in action and even when supported; with a propensity to bend the trunk forward, and to pass from a walking to a running pace: the senses and intellects being uninjured”.[2] With time, postural changes and instability were included as a fourth fundamental sign.[4]
Jean-Martin Charcot was the first to suggest describing the illness as, “Parkinson’s disease” whereby dismissing earlier choice of names such as paralysis agitates or shaking palsy.[2] This was due to the fact that he observed that the individuals affected with PD were not distinctly weak nor had tremors.[2] Continuing the work on PD, further descriptions and studies related to changes in the pathology of the disease were mainly reported by the French neurologic school.[2] Furthermore, in 1895, Richer and Beige shared details on the clinical and morphological stages related to the progression of PD.[2] To further enhance knowledge on the illness, Babinski noted the irregular motor fluctuations whereas Brissaud was the first to relate PD to the damage of the substantia nigra.[2] Trétiakoff, Foix, and Nicolesco further created pathological studies to better understand the relationship of PD to the midbrain.[2] It was in 1953 however, that Greenfield and Bosanquet created the most comprehensive pathological analysis of the illness.[2] Therefore, literature shows how many people played a role in shaping the meaning of PD.
Epidemiology
PD is described as the most common movement disorder and the second most common degenerative disease of the central nervous system.[3] It affects 1-2 people for every 1000 of the population at any given time.[5] Additionally, it is also observed that PD is slightly more prevalent in males when compared to females.[3] Studies show that it is rare for individuals who are under the age of 50 to be affected by PD however, this prevalence increases with increasing age.[6,7,8] As such, PD affects 1% of the population over the age of 60 years and reaches a prevalence of 4% in the oldest age group.[6,7,8] Particularly within the the United States of America, the prevalence of PD was observed using tools such as the Kaiser Permanete Northern California Integrated Health Care System, Ontario Rochester Epidemiology Project, and the California Parkinson’s disease Registry-Pilot Project data.[9] It was observed that the combined prevalence for men and women over the age of 45 was 572 per 100,000 where females were measured at approximately 488 per 100,000 and males at 667 per 100,000.9 Currently, using meta-estimates in accordance with the 2010 census for the US population, 680,000 cases of PD are expected.[9] Through further analysis with the projected population structure, this number is expected to rise to 930,000 in 2020 and 1,238,000 by 2030.[9] Furthermore, individuals from Asia aged 70-79 years were observed to have significantly lower frequency of PD cases, 646 per 100,000, when compared to populations from Europe, North America, and Australia for the same age range, 1602 per 100,000.[9]
With this data however, it important to keep in mind that values can vary noticeably due to a variety of reasons.[3] It is possible that differences in methodological practices such as ascertainment and diagnostic criteria could affect these measurements.3 Moreover, the data would also be affected by the under-diagnosis of PD which has been known to occur within older adults.[3]
Causes
PD is a result of decreased neuronal activity. These neurons in the brain begin to deteriorate or die down over a period of time.10 The neurons that are primarily degraded in PD produce dopamine for the body. This decrease in dopamine leads to symptoms such as tremors, rigid muscles, impaired balance, etc. There are also certain changes that can occur in the overall physical structure of the brain. This is primarily through the abundance of Lewy bodies which contain an accumulation of alpha-synuclein protein.11 These clumps of Lewy bodies will interfere with neuronal function and can accelerate the progression of PD.11 This disease will primarily manifest from genetic mutations, which are rare, but if present will affect the brain makeup and dopamine levels. The familial mutations include changes in the LRRK2, PARK7, PINK1, PRKN, or SNCA genes.12 These mutations are not the only changes that PD could potentially affect ; however, these are often present in a lot of the cases. Overall, family history only plays a 15% role in the inheritance of this disease.12 PD can be triggered through environmental toxins, particularly certain chemical substances..10 Furthermore, PD can also be dependent on age and sex such that is is more common in older adults and males.10
Pathophysiology
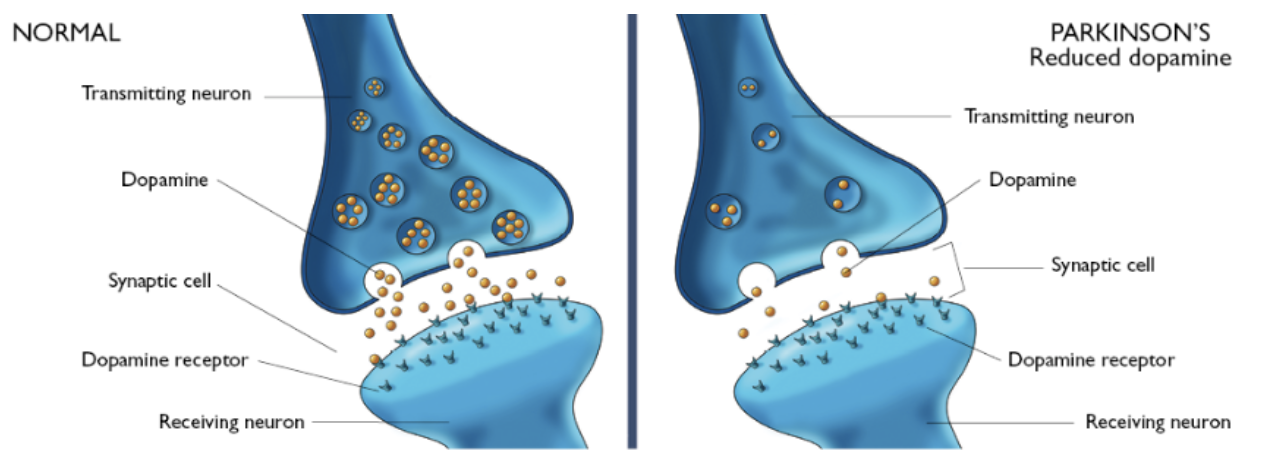
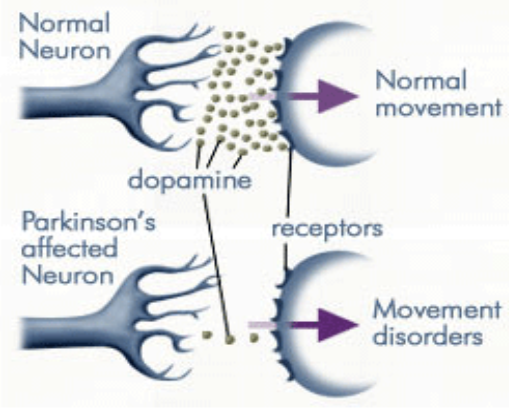
PD is caused by a deficiency of the neurotransmitter dopamine due to the degeneration of substantia nigra in the basal ganglia.[13] The substantia nigra contains dopamine secreting neurons whereas the basal ganglia is part of the brain which controls coordinated movement.[14]
The role of dopamine
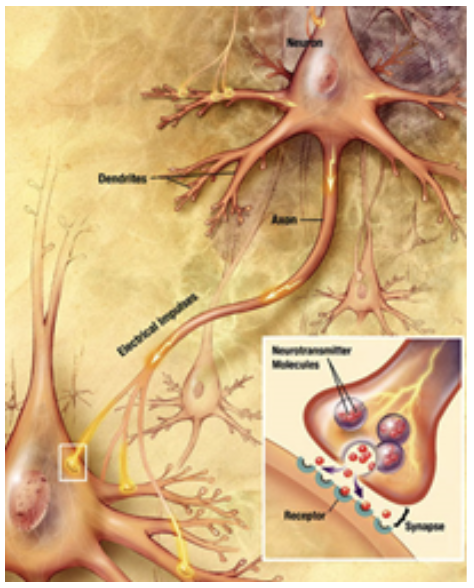
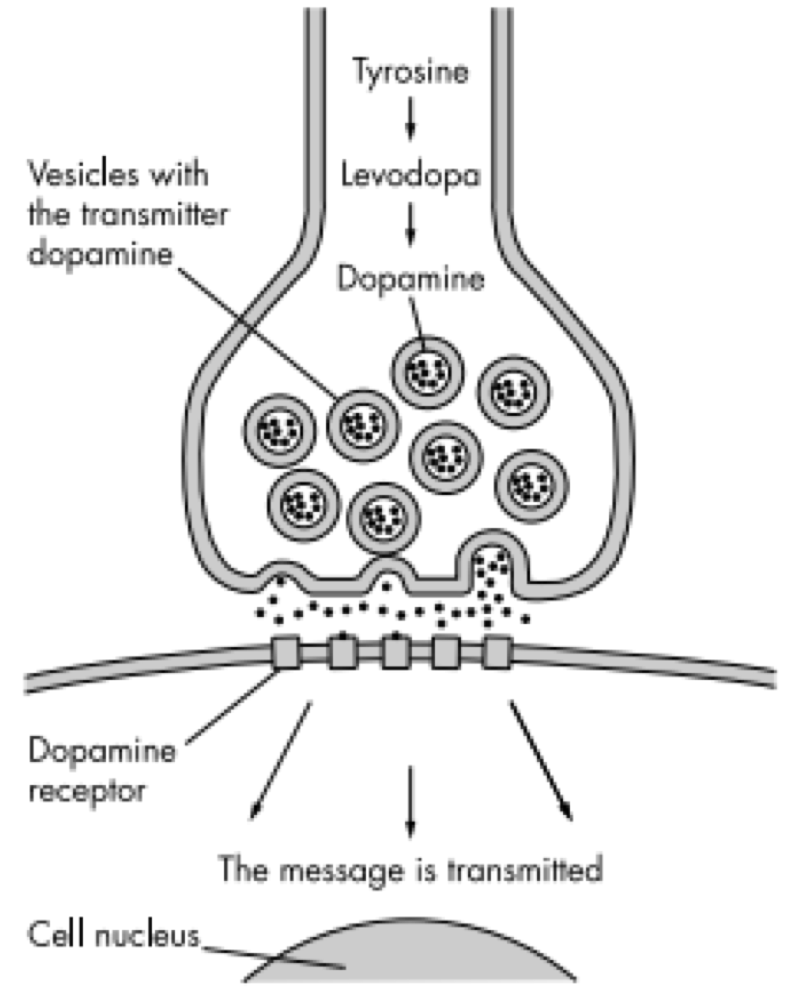
Dopamine is an excitatory and inhibitory neurotransmitter within our body. It is the main neuron responsible for regulating the reward and pleasure pathway in the brain, and it also plays a role in motor function and control.[15] Dopamine moves through the body via synaptic transmission, where it moves from the presynaptic cell through the synapse released via vesicles towards the postsynaptic cell. Dopamine plays a role in the basal ganglia function and is seen in much lower levels in PD patients.[16]
The progressive loss of dopamine
As PD progresses, dopaminergic neurons will continue to deteriorate, affecting the excitatory response and inhibitory response from the thalamus. This has been found to disrupt voluntary motor control leading to the characteristic symptoms of PD.[16]
Nigrostriatal Pathway
The loss of dopamine neurons in the substantia nigra, effects the Nigrostriatal Pathway. This is the bundle of neurons between the substantia nigra to the striatum. The substantia nigra will increase the amount of gamma-Aminobutyric acid (GABA), which is a neurotransmitter released from striatum, affecting the thalamus. The thalamus is responsible.[18] In PD, the substantia nigra is depleted of around 80% of its dopaminergic neurons and is unable to adequately stimulate the striatum from releasing GABA to Globus Pallidus Internal leading to unwanted and uninitiated movement.[19]
Lewy bodies
In patients with PD, Lewy bodies are present in many of the remaining cells in the substantia nigra. Lewy bodies mainly consists of the alpha-synuclein proteins. It has been found that alpha synuclein plays a role in the progression of PD however, the way in which the Lewy bodies affect the neurons are not known.[11] Nonetheless, there has been evidence suggesting that they act as toxins. [20]
Symptoms
PD can present both motor and non-motor symptoms. Motor symptoms are those that affect your movement, while non-motor symptoms are symptoms that are not visibly seen.
Motor Symptoms
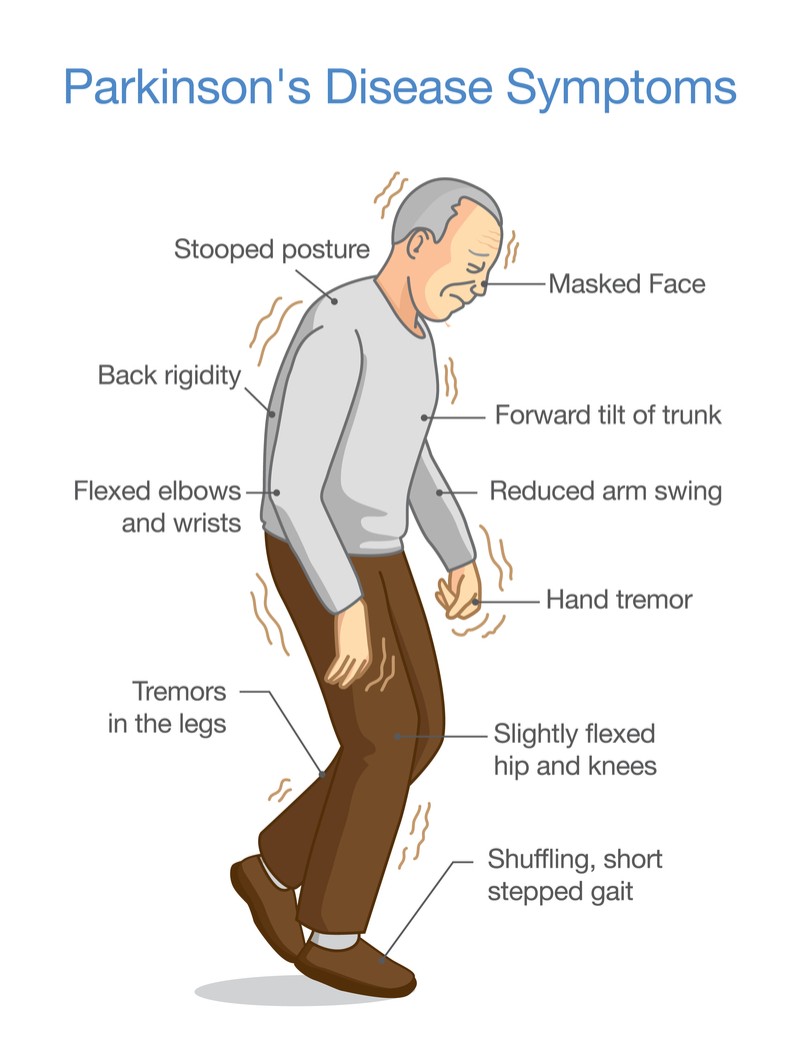
There are four main motor symptoms associated with PD: resting tremors, bradykinesia, rigidity and postural instability.[21] Tremors at rest usually start very occasionally, slowly spreading out to other limbs on the upper body.[22] Postural instability occurs five years after the disease, which also leads to another symptom: likelihood of falling.[22] Other motor symptoms can include freezing and muscle cramps.[21]
Non-Motor Symptoms
Non-motor symptoms include neuropsychiatric disorders, sleep disorders, and autonomic dysfunction.[23] This includes: REM sleep disorders, insomnia and excessive sleeping during the daytime, depression, dementia, anxiety, bladder problems, constipation and pain as well as others that present as a result of motor symptoms.[23]
Symptoms worsen over time as the disease progresses, and severe disability is typically seen at around 10 years after its onset.[23]
Diagnosis
Currently, there is no definitive test to determine whether or not someone has PD however, medical history and clinical tests are used to test for symptoms of PD.[24]
Misdiagnosis for PD is quite common since symptoms overlap with a number of other diseases, resulting in either a lack of diagnosis or inaccurate diagnosis of PD.[23] The following tests are currently used to assess presence of PD:
UK Brain Bank Criteria
This is a three step process that first requires determining whether the patient has parkinsonian syndrome, which includes bradykinesia (slowed movement) and at least one of: muscular rigidity, 4-6 Hz rest tremor, and postural instability.[25] The next step is excluding criteria that is not relevant with PD, such as history of repeated head injury.[25] The final step is determining whether there is any supportive criteria in relation to step 1, such as unilateral onset, progressive disorder or a positive response to levodopa.[25]
MDS-UPDRS
The Movement Disorders Society - Unified Parkinson’s Disease Rating Scale is used to test onset of non-motor symptoms in a more reliable manner. It is a questionnaire ranking levels of discomfort and certain events from 0 - 4.[24]
Neuroimaging
While imaging isn’t useful for determining presence of PD, it is useful in ruling out other diseases that might present similarly to PD and differentiating between typical PD and atypical PD.[22] This includes getting an MRI, CT scan, PET scan or SPECT.[22]
Drugs and Medication
Currently there is no cure for PD but various drugs and medication can provide relief from the symptoms. Over the recent years, extensive research has been conducted allowing for significant results in treatments for PD. Although there are numerous medications available, the use of these drugs are dependent on the disease stage and the age of onset. The main groups of drugs used for treating PD, especially for the coordination of movement, are: levodopa, MAO-B inhibitors, and dopamine agonists.
Levodopa
One of the most prominent drugs used to treat Parkinson’s is Levodopa (L-dopa). Patients with PD have a decreased number of dopamine producing cells in their substantia nigra. These dopaminergic cells help process information pertaining to controlling movements. L-dopa is an amino acid that is involved in dopamine producing pathway as a crucial metabolic intermediate.[26] Levodopa is quickly decarboxylated in the brain to dopamine by L-amino amino acid decarboxylase.[26] Once dopamine is replenished in the basal ganglia, information is processed and sent back to the cortex which is responsible for coordinating movement. Levodopa has various side effects such as nausea, involuntary movement, confusion, mood swings and sleepiness.
Dopamine Agonist
This family of drugs are an alternative agent for treating PD. Dopamine agonist (DA) was first discovered for its antiparkinsonian effects by Calne and colleagues, in the early 1970’s.[26] Since the time of the first discovery, scientist have developed several dopamine agonists that have been approved and marketed as treatments for PD patients. Dopamine agonist differ from L-dopa, due to their direct interaction with dopamine receptors. Stimulation of the postsynaptic dopamine receptors allows to bypass the degenerating dopaminergic pathway in the substantia nigra.[26] Furthermore, dopamine agonist does not need decorboxylase enzymes for the conversion of dopamine. Although the pharmacodynamic properties differ between the various types of DA, most do not produce potentially toxic metabolites or free radicals.[26]
One of the most commonly used dopamine agonist is ropinirole. Ropinirole is an agonist that binds to dopamine receptors to produce the same effects as dopamine. Moreover, it induces the classical dopaminergic reactions such as nausea, vomiting, hypotension and psychosis. Furthermore, PD patients with dementia are prevented from taking dopamine agonists due to the drugs susceptibility to produce hallucinations.[27]
MAO-B Inhibitors
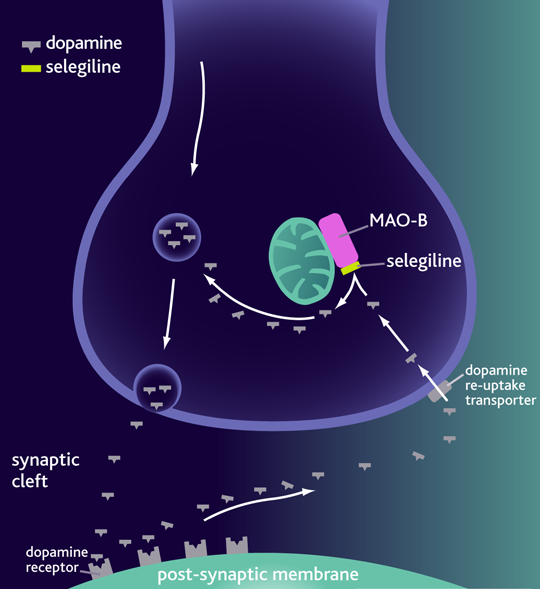
Monoamine oxidase B (MAO-B) is an enzyme that is present in the neurons and involved in dopamine metabolism. MAO-B inhibitors blocks the metabolism of dopamine and therefore increases the dopamine levels in the basal ganglia. By preventing dopamine degradation, there are more internal stores of dopamine available for release. Furthermore, MAO-B inhibitors block the conversion of MPTP to its active metabolic MPP+. MPP+ is a substantia nigra neurotoxin therefore MAO-B inhibitors has neuroprotective properties.[26]
Selegiline was the first MAO-B inhibitor to be used for individuals living with PD.[26] This is a drug with a mild to moderate benefit and is used as a monotherapy in early onset of PD. Once the disease is progressive it is used as an adjunctive therapeutic with either levodopa or dopamine agonist.[26] Side effects of this drug include headache, nausea, loss of balance, and gastrointestinal symptoms.
Future Therapeutics
A2A Antagonists
This is class of drugs similar to dopaminergic receptors that are currently available. These drugs are highly effective in reducing off time.[28] Off time refers to the period where the medications are not working and is often the most troubling times for the patients.[28] Medications in the past would often address the concern of off time; however, they had generally caused dyskinesia. This is the abnormality or impairment of voluntary movement.[28] These new A2a antagonist drugs will help to address symptoms of dyskinesia making life more comfortable for the patient.
Gene therapy
This is a new technology that uses gene editing to help fix any impairments. There are two ways that gene therapy could prove to be effective in helping Parkinson’s patients. The first being helping neurturin cross the blood brain barrier. This protein is a neurotropic factor that will help with development, survival and functionality of neurons.[29] Gene therapy can be used to adjust the CERE-120 vector to help allow for neurturin to enter into the brain and promote the regeneration of neurons that have been degraded.[29] Patients were given these bilateral injections and the therapy was found to safely reduce off time and help with dyskinesia symptoms.[29] The second way that gene therapy can be used to help moderate the progression of Parkinson’s is by addressing dopamine reduction. By adjusting the Glutamic Acid Decarboxylase there is an increase in synthesis of gamma-aminobutyric acid (GABA). [29] This will help to reduce the inhibitory outputs to Globus pallidus internal that are dramatically increased in Parkinson’s patients.[29] It will also help to increase the output of excitatory signals, which will help return neurons and dopamine levels to a moderate state.[29] Gene therapy in these cases was shown to improve symptoms of 33% relative to the control. Both of these gene therapy alternatives have been tested in a lab setting, but are still far from being used in practise.[29]
Levodopa formulations
There are two primary variations of levodopa that have shown to be effective in helping patients with PD. The first being levodopa/carbidopa, which has been shown to reduce off time hours by more than 4.6 when compared to the control.[30] This drug, similar to the levodopa on its own, will help to increase dopamine levels in the body. However, the carbidopa is what allows more levodopa to cross the blood brain barrier.[29] This will help to further increase dopamine concentrations. This drug is still in clinical trials, but has shown to be very effective when compared to levodopa alone. This drug also exhibits similar benefits to deep brain stimulation, which enables individuals with PD to have an oral alternative to actual therapy.[30] The next variation of the drug is IPX066, which is a slow release form of levodopa/carbidopa.[29] In this form the drug can be given at lower doses and requires less pills. This drug is still in the early stages, but has shown statistically significant improvement over the levodopa/carbidopa alternative.
AFQ056
This is selective antagonist of the mGluR5 receptor which can help mediate symptoms of dyskinesia. This drug is particularly effective at reducing the amount of dyskinesia as a direct result of levodopa.[31] This drug had statically significant impact on patients that were on levodopa treatment.[31] This drug is too given in conjecture with traditional treatments and can dramatically reduce certain symptoms. It is still early in the testing process, but has shown excellent progression thus far.
References
[1] Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nature reviews Disease primers. 2017 Mar 23;3:17013.
[2] Goetz CG. The history of Parkinson's disease: early clinical descriptions and neurological therapies. Cold Spring Harbor perspectives in medicine. 2011 Sep 1;1(1):a008862.
[3] Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. Journal of Neural Transmission. 2017 Aug 1;124(8):901-5.
[4] Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Archives of neurology. 1999 Jan 1;56(1):33-9.
[5] von Campenhausen S, Bornschein B, Wick R, Bötzel K, Sampaio C, Poewe W, Oertel W, Siebert U, Berger K, Dodel R. Prevalence and incidence of Parkinson's disease in Europe. European Neuropsychopharmacology. 2005 Aug 1;15(4):473-90.
[6] De Lau LM, Breteler MM. Epidemiology of Parkinson's disease. The Lancet Neurology. 2006 Jun 1;5(6):525-35.
[7] De Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, Van der Meche FG, Hofman A. Prevalence of Parkinson's disease in the elderly The Rotterdam Study. Neurology. 1995 Dec 1;45(12):2143-6.
[8] Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, Hofman A. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S21-3.
[9] Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, Abbott RD, Savica R, Van Den Eeden SK, Willis AW, Tanner CM. Prevalence of Parkinson’s disease across North America. NPJ Parkinson's disease. 2018 Jul 10;4(1):21.
[10] Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson's disease. Movement disorders. 2011 May;26(6):1049-55.
[11] Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, Widner H. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature medicine. 2008 May;14(5):501.
[12] Camargos ST, Dornas LO, Momeni P, Lees A, Hardy J, Singleton A, Cardoso F. Familial Parkinsonism and early onset Parkinson's disease in a Brazilian movement disorders clinic: phenotypic characterization and frequency of SNCA, PRKN, PINK1, and LRRK2 mutations. Movement Disorders. 2009 Apr 15;24(5):662-6.
[13] Filion M. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain research. 1991 Apr 26;547(1):140-4.
[14] DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in neurosciences. 1990 Jul 1;13(7):281-5.
[15] Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neuroscience & biobehavioral reviews. 2000 Jan 1;24(1):125-32.
[16] Ribrault C, Sekimoto K, Triller A. From the stochasticity of molecular processes to the variability of synaptic transmission. Nature Reviews Neuroscience. 2011 Jul;12(7):375.
[17] Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Archives of neurology. 2003 Mar 1;60(3):337-41.
[18] Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Experimental neurology. 2002 Jun 1;175(2):303-17.
[19] Greenwood CE, Tatton WG, Seniuk NA, Biddle FG. Increased dopamine synthesis in aging substantia nigra neurons. Neurobiology of aging. 1991 Sep 1;12(5):557-65.
[20] Auluck PK, Chan HE, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002 Feb 1;295(5556):865-8.
[21] Jankovic J. Parkinson’s disease: clinical features and diagnosis. Journal of neurology, neurosurgery & psychiatry. 2008 Apr 1;79(4):368-76.
[22] Reichmann H. Clinical criteria for the diagnosis of Parkinson’s disease. Neurodegenerative diseases. 2010;7(5):284-90.
[23] Weintraub D, Comella CL, Horn S. Parkinson's disease–Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care. 2008 Mar 1;14(2 Suppl):S40-8.
[24] Marsili L, Rizzo G, Colosimo C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Frontiers in neurology. 2018 Mar 23;9:156.
[25] Davie CA. A review of Parkinson's disease. British medical bulletin. 2008 Apr 8;86(1):109-27.
[26] Lang AE, Lees A. Management of Parkinson’s disease: an evidence-based review. Mov Disord. 2002 Jan 1;17(Suppl 4):S1-66.
[27] Nutt JG, Wooten GF. Diagnosis and initial management of Parkinson's disease. New England Journal of Medicine. 2005 Sep 8;353(10):1021-7.
[28] van Rensburg HJ, Legoabe L, Terre’Blanche G, Van der Walt M. 2–Benzylidene–1–Indanone Analogues as Dual Adenosine A1/A2a Receptor Antagonists for the Potential Treatment of Neurological Conditions. Drug research. 2019 Jan 7.
[29] Palmer C, Coronel R, Bernabeu-Zornoza A, Liste I. Therapeutic Application of Stem Cell and Gene Therapy in Parkinson’s Disease. InPathology, Prevention and Therapeutics of Neurodegenerative Disease 2019 (pp. 159-171). Springer, Singapore.
[30] Anang JB. Levodopa-Carbidopa-Related Rash in Parkinson’s Disease: A Case Series. Canadian Journal of Neurological Sciences. 2018 Sep;45(5):588-9.
[31] Jones T, Murray R. Current research in and development of treatments for Parkinson’s disease. Pathophysiology. 2018 Dec 11;14:00.