Table of Contents
PRESENTATION SLIDES
INTRODUCTION
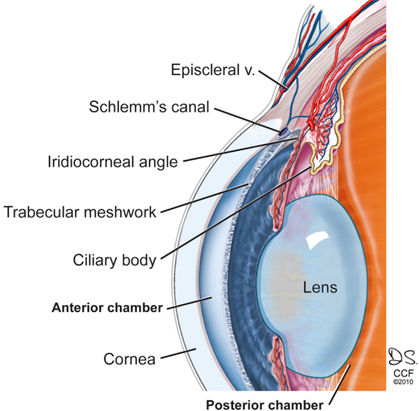
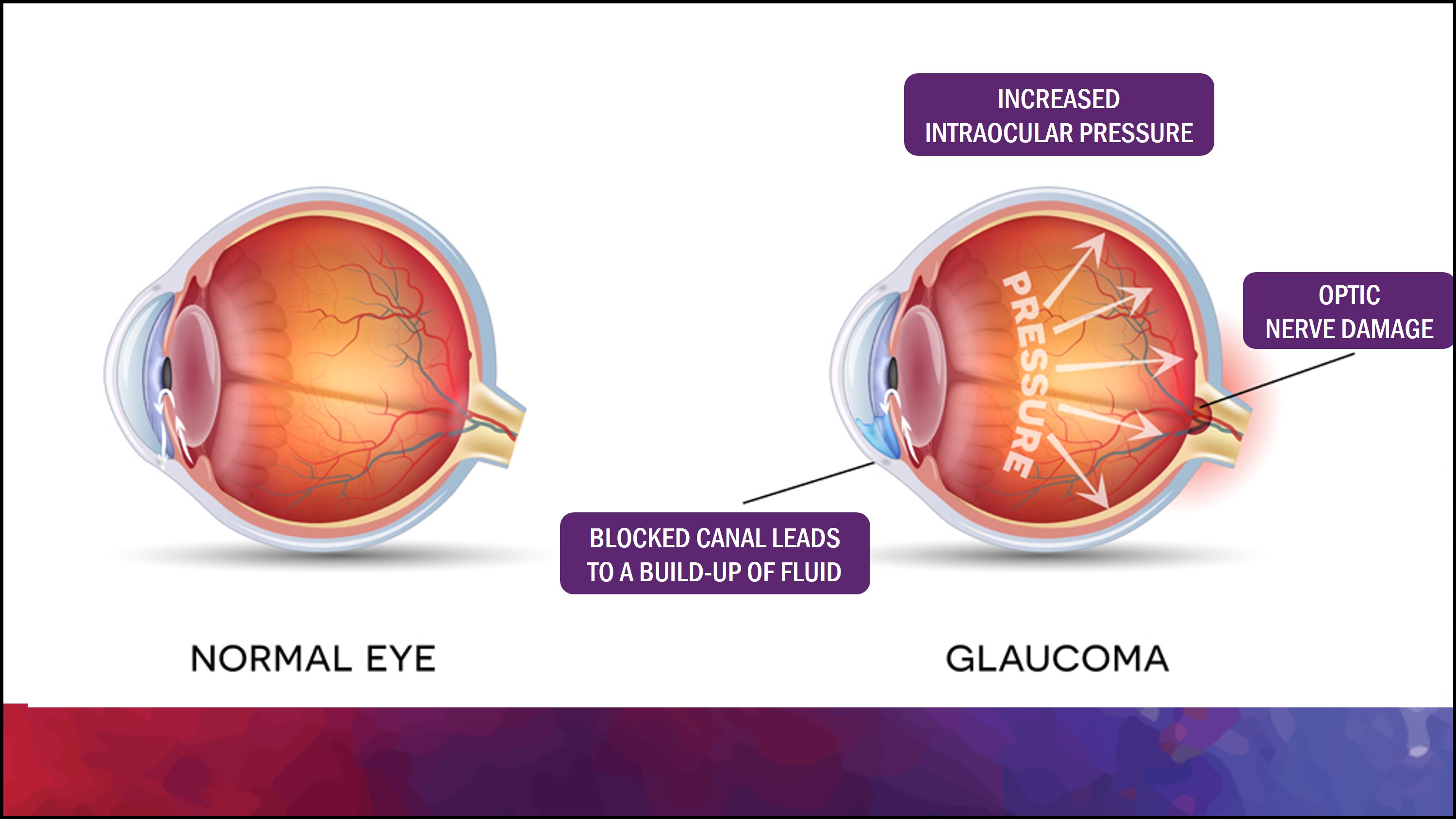
Glaucoma is a common cause of blindness around the world (Harada et al., 2019). It results from the buildup of aqueous humour fluid in the anterior chamber of the eye. Aqueous humor is produced by the ciliary body and it flows from the posterior chamber through the pupil into the anterior chamber of the eye (Civan & Macknight, 2004). Normally, this fluid then flows out through a spongy tissue at the front of the eye called the trabecular meshwork and into a drainage canal called Schlemm’s canal (Civan & Macknight, 2004). The aqueous humour plays an essential role in the health of your eye. As well as nourishing the cornea and the lens by supplying nutrients such as amino acids and glucose, the aqueous humour plays an important role in maintaining intraocular pressure (Civan & Macknight, 2004). Individuals with glaucoma are unable to drain out the aqueous humour fluid through the trabecular meshwork because it gets blocked, leading to an increase in intraocular pressure. This buildup of pressure in the eyes can eventually begin leading to irreversible damage to the optic nerve which can impair your vision. Many forms of glaucoma have no warning signs. The effect is so gradual that you may not notice a change in vision until the condition is at an advanced stage. Early treatment of the condition can, however, prevent the condition from leading to blindness. Treatment typically involves prescription eye drops, oral medications, laser treatment, surgery or a combination of any of these.
HISTORY
The word “glaucoma” is derived from the Ancient Greek word glaukos which means blue, green, or gray. The term was first used by Hippocrates in Greece in 400 BC to describe a dimming of vision (Realini, 2011). For hundreds of years the condition of glaucoma was known, yet treatment was unavailable because there was not much of an understanding of its underlying cause (Realini, 2011). In fact, there was a misconception that glaucoma is caused by the opacification of the vitreous humour in the eye. It was not until 1622, that ophthalmologist Richard Bannister linked glaucoma to an increase in intraocular pressure (Realini, 2011). A clear understanding of the condition did not progress much further until the mathematician Charles Babbage and Hermann von Hemholtz created the first ophthalmoscope in 1851, which was an instrument used to view the interior of the eye. After this invention, Albrecht Von Graefe, a German physician, began a series of clinical tests that solidified the anatomical characteristics associated with glaucoma (Realini, 2011).
EPIDEMIOLOGY
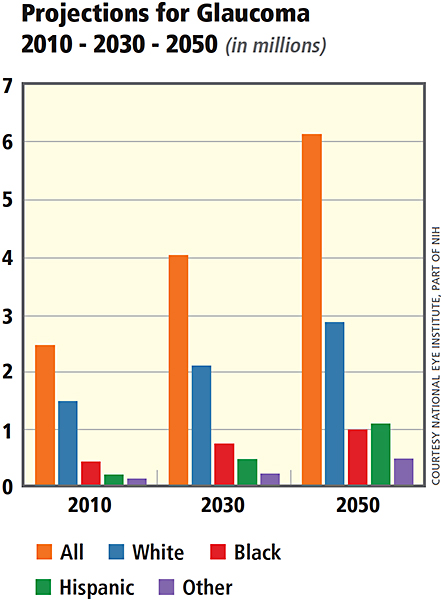
It is estimated that there were 60 million people with glaucoma and 8.4 million people who are blind due to glaucoma in 2012 (Cook & Foster, 2012). Specifically, it is estimated that 4.5 million people are blind due to open-angle glaucoma and 3.9 million are blind due to angle-closure glaucoma. In open-angle glaucoma, the angle in your eye where the iris meets the cornea is as wide and open as it should be, but the eye's drainage canals become clogged over time, causing an increase in internal eye pressure and subsequent damage to the optic nerve. With angle-closure glaucoma, the block takes place at the angle of the anterior chamber formed by its junction of the cornea with the iris, again leading to a sudden (acute) or slowly progressive (chronic) blockage of the normal circulation of fluid within the eye. By 2020, it is estimated that 80 million people will be affected by glaucoma, with 5.9 million and 5.4 million people blind due to open-angle and angle-closure glaucoma, respectively (Quigley & Broman, 2006).
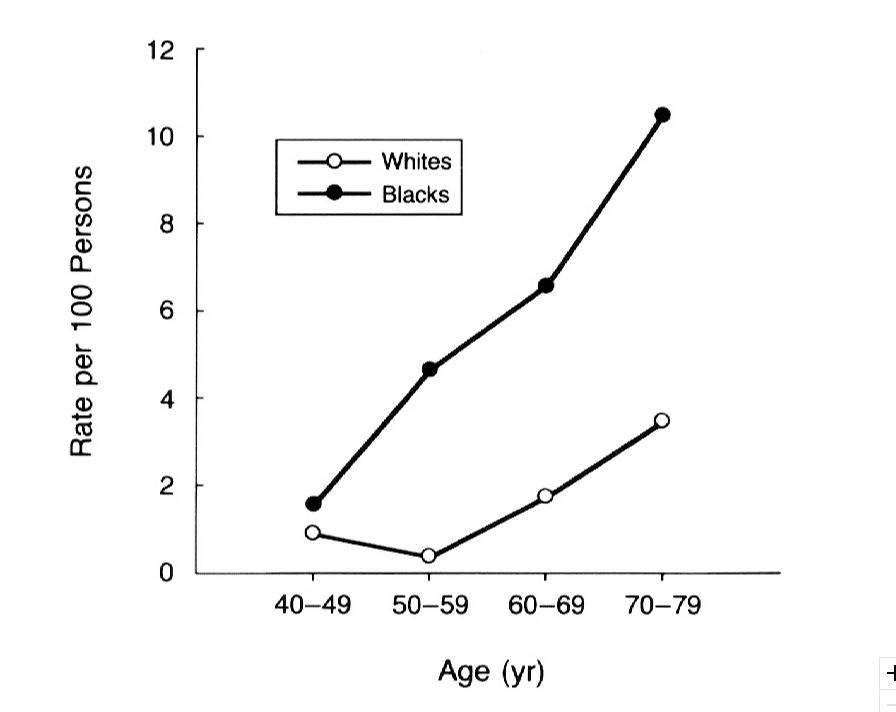
Prevalence of glaucoma also varies among different racial group. Asians are the largest group affected, comprising 47% of the total affected, with all types of glaucoma, and 87% of primary angle-closure glaucoma (Cook & Foster, 2012). On the other hand, Africans have the highest prevalence of primary open-angle glaucoma. Prevalence of primary open-angle glaucoma is similar between white Europeans, Americans, and Australians. Additionally, women are more affected than men, making up 59% of all glaucoma (Cook & Foster, 2012).
There are also risk factors associated with primary open-angle glaucoma. African people have a prevalence up to 5 times higher than other ethnic groups of developing glaucoma (Cook & Foster, 2012). You risk of developing glaucoma exponentially increases with age. Particularly, Hispanics have a more pronounced increased risk of developing glaucoma with age compared to other ethnic groups (Cook & Foster, 2012). Additionally, having a thin cornea and/or a large optic disc diameter has shown to increase one's risk for glaucoma. Other factors associated with higher risk is low physical activity, having cardiovascular disease, and hypertension (Cook & Foster, 2012).
SYMPTOMS
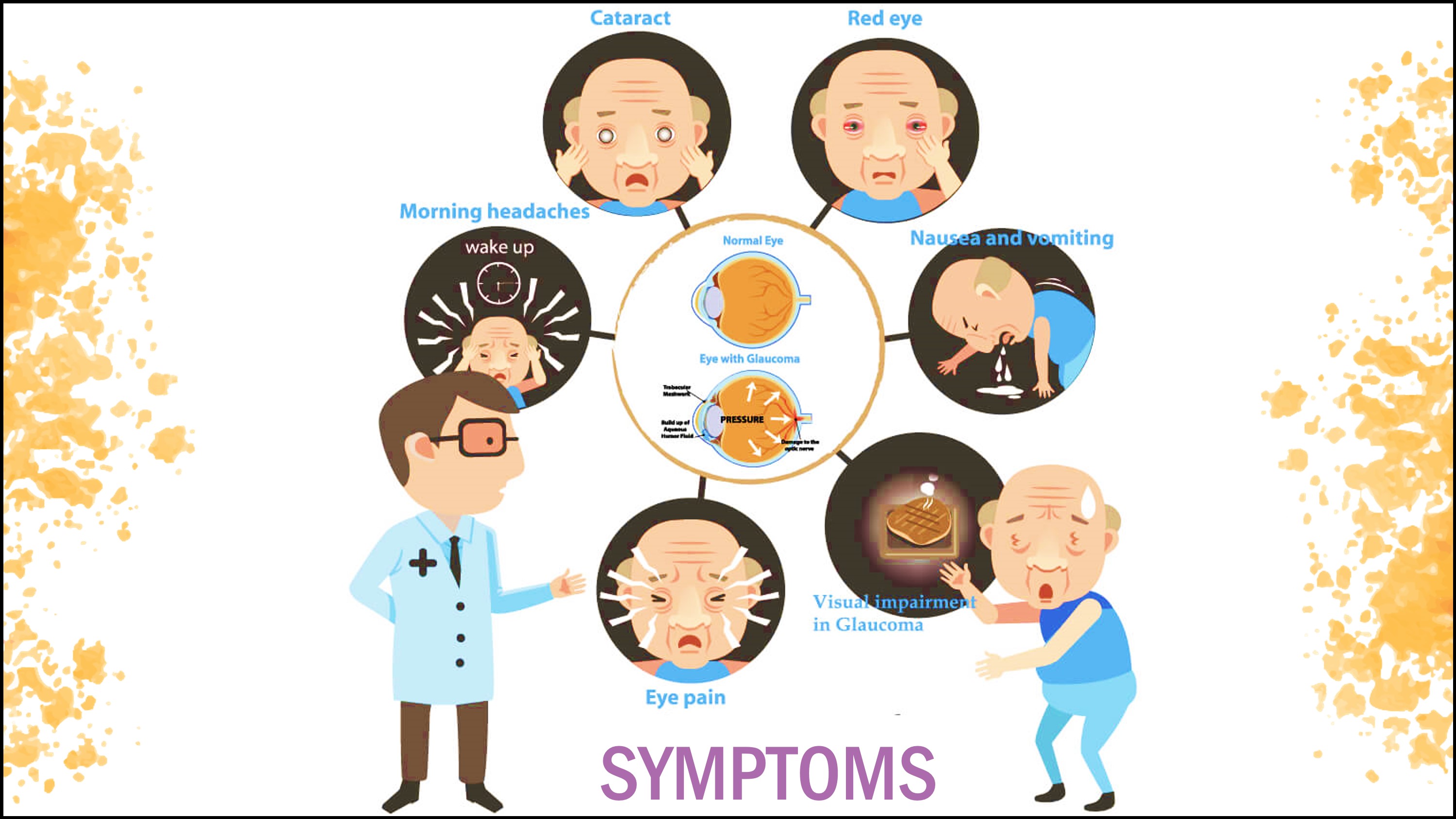
Glaucoma, also known as the “silent thief” of sight, rarely causes symptoms until optic-nerve-fiber damages occurs, creating scotomas. The condition can develop in one or both eyes. Clinical features vary with the form of glaucoma. At first, there are no symptoms. Vision stays normal, and there is no pain. As the disease progresses, a person may notice the side vision gradually failing. That is, objects in front may still be seen clearly, but objects to the side may be missed. As glaucoma remains untreated, the person may miss objects to the side and out of the corner of their eye. Without treatment, the person will slowly lose their peripheral side vision. Over time, straight-ahead vision may decrease until no vision remains. The following are the most common features seen in most forms of glaucoma (Naklha, 2007).
Pain and Redness: Rapid increase in pressure to very high levels leads to eye pain and redness.
Blurred Vision/Visual Field Loss: This occurs as a result of damages to the retinal nerve fibers leading to arcuate scotoma, an inferior nerve-fiber-bundle defect. Central vision is spared initially and the person is unable to notice the defect. Vision may still be perfect even at the terminal stage of glaucomatous field loss (tunnel vision) (Naklha, 2007).
Elevated Interaocular Pressure: Pressure and severity of glaucomatous damage determines the rate at which elevated intraocular oressure causes optic nerve damage. In general, pressures of 20 to 30mm Hg usually causes damage over several years, but pressures of 40 to 50mm Hg can lead to rapid visual loss and also deteriorate retinovascular occlusion (Naklha, 2007).
Rainbow-Colored Rings or Halos Perceived Around Lights and Cloudy Cornea: Endothelial cells are continuously removing fluid to keep the cornea transparent. When pressure rises, the cornea becomes waterlogged, causing visual acuity to decrease and forming halos around lights (Naklha, 2007).
Other clinical symptoms that have been observed in association with the different forms of glaucoma due to the rapid buildup of intraocular pressure include nausea and vomiting, headaches, cataract, photophobia, blepharospasm (involuntary blinking or spasm of the eyelids), strabismus (misalignment of the eyes), epiphoria (excessive tearing), and amblyopia (lazy eye) (Naklha, 2007).
CAUSES
One of the main causes of Glaucoma is the failure of the eye to maintain a balance between aqueous production inside the eye and aqueous drainage out of the eye through the trabecular meshwork (Weinreb & Khaw, 2004). This imbalance can affect the intraocular pressure of the eye. The normal intraocular pressure is 10-21mm Hg but in the case of glaucoma, the intraocular pressure could increase up to 70mm Hg (Weinreb & Khaw, 2004). The deterioration of the optic nerve fibers leads to blind spots eventually leading to symptoms of glaucoma. Some of the less common causes of glaucoma include chemical injury to the eye, blocked blood vessels inside the eye and inflammatory conditions. There are also some genetic risk factors associated with glaucoma that could increase the chance of an individual developing the disease. Family history is a well- established risk factor. Mutations in the MYOC gene is primarily responsible for open-angle glaucoma, this gene provides information to code for a protein called myocilin (Leske, 2007). Myocilin is found in the trabecular meshwork and in the ciliary body that regulates intraocular pressure. In addition, mutations in the CYP1B1 gene is often detected in people with primary congenital glaucoma. This gene codes for a cytochrome P40 protein which like myocilin is found in the trabecular meshwork and ciliary body and regulates intraocular pressure (Leske, 2007). Diabetes and high blood pressure can also be risk factors for glaucoma.
DIAGNOSIS
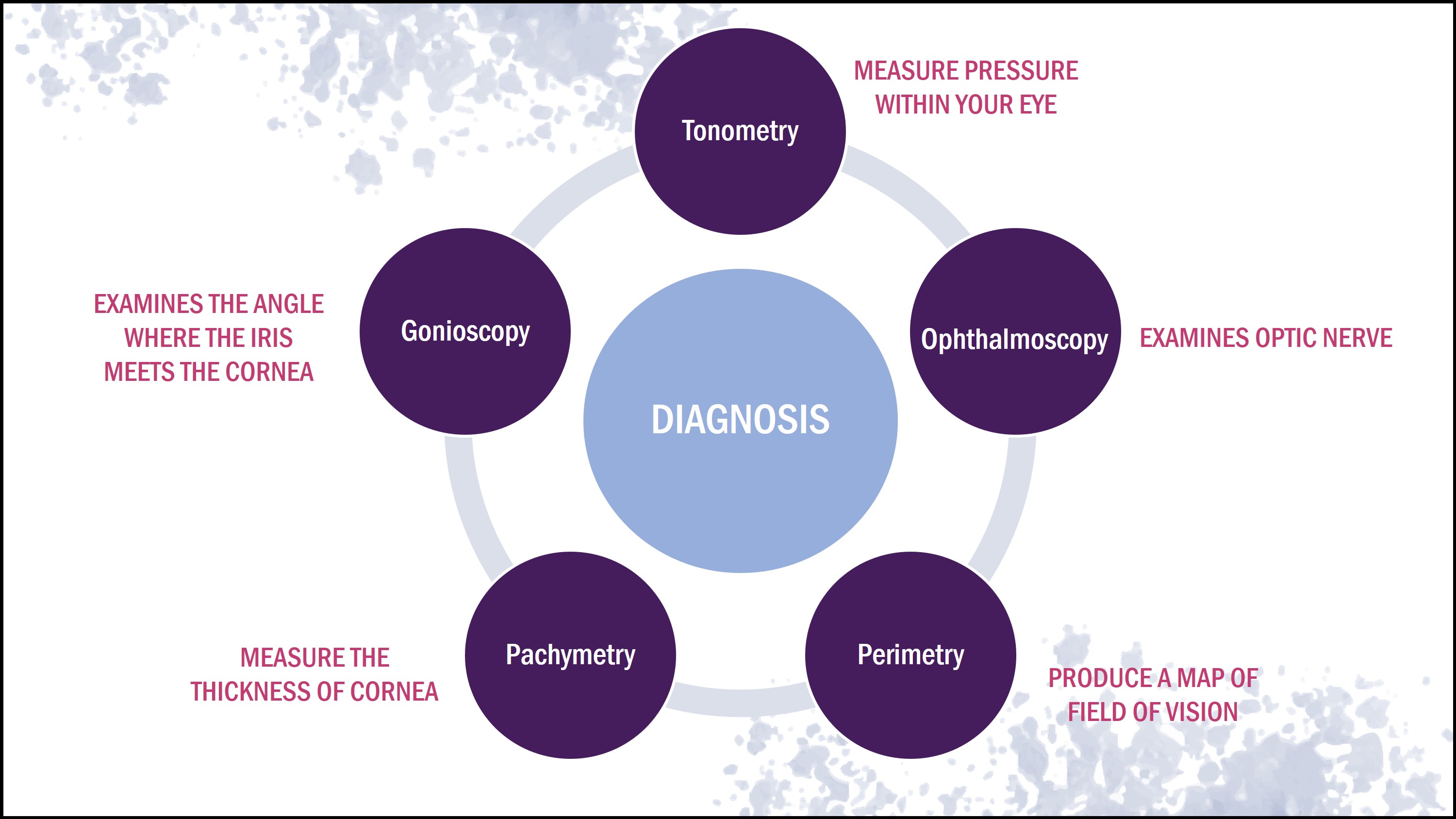
Early diagnosis for glaucoma would include an examination of the optic discs, retinal nerve fiber layer, and visual field (Weinreb & Khaw, 2004). New imaging and psychological tests can not only detect the disease but also can monitor the progression of the disease (Weinreb & Khaw, 2004). There are five main methods to diagnose an individual who may have the disease.
Tonometry
Tonometry measures the pressure within your eye using a device called the Tonometer. Eyedrops are used on the patient prior to the test (Eddy et al., 1983). The device applies a small amount of pressure to the eye by the puff of air. The range for normal pressure is between 10-20 mm Hg, but individuals exceeding 20 mm Hg are diagnosed with glaucoma (Singh et al., 2001).
Ophthalmoscopy
Ophthalmoscopy is used to examine the optic nerve in order to check for glaucoma-induced damage (Wolf et al., 1993). Eyedrops are used to dilate the pupil so that it is easier for the physician to examine the shape and color of the optic nerve (Wolf et al., 1993). A small device with a light source on the end is then used to light and magnify the optic nerve. Irregularities in the intraocular pressure and the optic nerve may lead to further glaucoma tests to ensure the patient truly has the disease (Wolf et al., 1993).
Perimetry
Perimetry is a visual field test that produces a map of your complete field of vision (Johnson & Samuels, 1997). This helps determine if your vision has been affected by glaucoma. The procedure for this test includes looking straight ahead and indicating whether you see a moving light in your peripheral side vision (Bengtsson & Heijl, 2000). This test might be repeated multiple times to make sure the results are accurate. Afterwards, a visual test can be done once or twice a year if glaucoma is diagnosed to test the progression of the disease (Bengtsson & Heijl, 2000).
Pachymetry
Pachymetry measures the thickness of the cornea. Central corneal thickness is an important parameter to diagnose glaucoma (Realini et al., 2003). A probe called a pachymeter is placed on the front of the eye to measure its thickness (Brandt, 2004). Corneal thickness can be used to interpret eye pressure. A thicker than average cornea can lead to a high pressure reading and a thinner cornea can lead to a low pressure reading (Ehlers & Hansen, 1974)
Gonioscopy
This procedure help dictates whether the angle where the iris meets the cornea is open and wide or narrow and closed. It can help determine the type of glaucoma the patient has (Barken, 1955). Eye drops are provided to the patient to numb the eye, then a handheld contact is placed on the eye (Hsai et al.,2000). The contact lens has a mirror that shows the doctor is the angle between the iris is closed a blocked which is a possible sign of angle- closure or acute glaucoma and wide and open which can be a sign of open angle and chronic glaucoma (Hsai et al.,2000).
PATHOPHYSIOLOGY
Glaucoma is a multifactorial disease. The death of retinal ganglion cells can be induced by a plethora of factors, which directly affect retinal cell bodies and axons. The primary factors that lead to the development of glaucomatous symptoms are increased intraocular pressure and vascular dysregulation. These two factors lead to the early stages of cell atrophy observed in the development of glaucoma and act by impeding axoplasmic flow in the retinal ganglion cell axons located predominantly at the lamina cribrosa. These factors are also involved in changes to the microcirculation of the optic nerve. Additionally, secondary influencing factors are primarily focused around excitotoxic damage caused by the amino acids glutamate and glycine, which are excreted by damaged neurons affected by persistent oxidative damage. The oxidative damage stems from the excessive production of the compound nitric acid as well as other excitotoxic damage inducing compounds. The net impact of these primary and secondary factors is retinal ganglion cell death resulting in permanent loss of vision. The progression of glaucomatous symptoms associated with cell death is divided into two phases. The initial stages feature cell loss exclusively linked to apoptosis, whereas the second stage is impacted more heavily by toxic effects of cells lost during the initial stage as well as long-term exposure to intraocular pressure (Agarwal et al., 2009).
Apoptosis Induced Atrophy
During the development of glaucoma, the head of the optic nerve undergoes a transformation near the optic disk. The optic disc is the point of exit for ganglion cell axons leaving the eye. This transformation leads to the cupping of the optic disk, a site known for the loss of ganglion cell axons. The death of both ganglion cell bodies and axon terminals located in the retina and lateral geniculate body respectively are both involved in the aforementioned loss of cell axons at the site of cupping. All of the cell loss experienced during the development of glaucoma, specifically the death of retinal ganglion cells, occurs via a mechanism known as apoptosis. The mechanism of apoptosis, also referred to as programmed cell death, is a process by which a cell induces its own destruction. The characteristic features of this process include chromosome clumping, cell shrinkage, DNA fragmentation, and membrane blebs. Once the nucleus has induced the aforementioned features, the succeeding step is the division of the cell into smaller vesicles. These small membrane-bound vesicles are then taken into surrounding cells via a mechanism known as phagocytosis. Apoptosis is the predominant process of retinal cell death during the initial stages of glaucoma development but is complemented by the addition of necrotic cell death during later stages of glaucoma development (Agarwal et al., 2009). The process of apoptosis is regulated by a family of proteases known as caspases. These caspases are better classified as cysteine aspartyl-specific proteases and are integral to the regulation of programmed cell death. Initially existing in an inactive zymogenic state, the caspases are activated during glaucoma development and are involved in cell death. The caspases cause a cascade of proteolytic events in the cytosol of retinal ganglion cells, eventually leading to cell death. Ligand influenced extrinsic activation of these caspases occurs via binding of proapoptotic receptors located on the surface of the cells. On the other hand, the intrinsic regulation of these caspases occurs via mitochondrial release of proapoptotic substances. One study observed that 34% of retinal ganglion cells survived inevitable apoptosis linked to the inhibition of caspases (Agarwal et al., 2009).
Elevated Intraocular Pressure
Increase in intraocular pressure stems from an obstruction in the drainage of aqueous humour. Specifically, the trabecular meshwork undergoes changes which impedes the drainage of aqueous humour. There is a strong positive correlation associated with a gradual increase in intraocular pressure being linked to retinal ganglion cell death. Several researchers have observed this correlation through studies conducted on rats, which measured the elevation in intraocular pressure, retinal ganglion cell death, and overall progression of glaucoma. The loss of retinal ganglion cells in association with an elevation in intraocular pressure is divided into two stages. The primary stage features an accelerated loss of retinal ganglion cells at approximately 12% per week. This stage is succeeded by a slower stage of cell death (Agarwal et al., 2009).
TREATMENTS
There is currently no cure for glaucoma. Treatment focuses on lowering the intraocular pressure to a level that is less likely to cause further optic nerve damage. Depending on your situation, your options may include prescription eye drops, oral medications, laser treatment, surgery or a combination of any of these.
Eye drops/Oral Medications
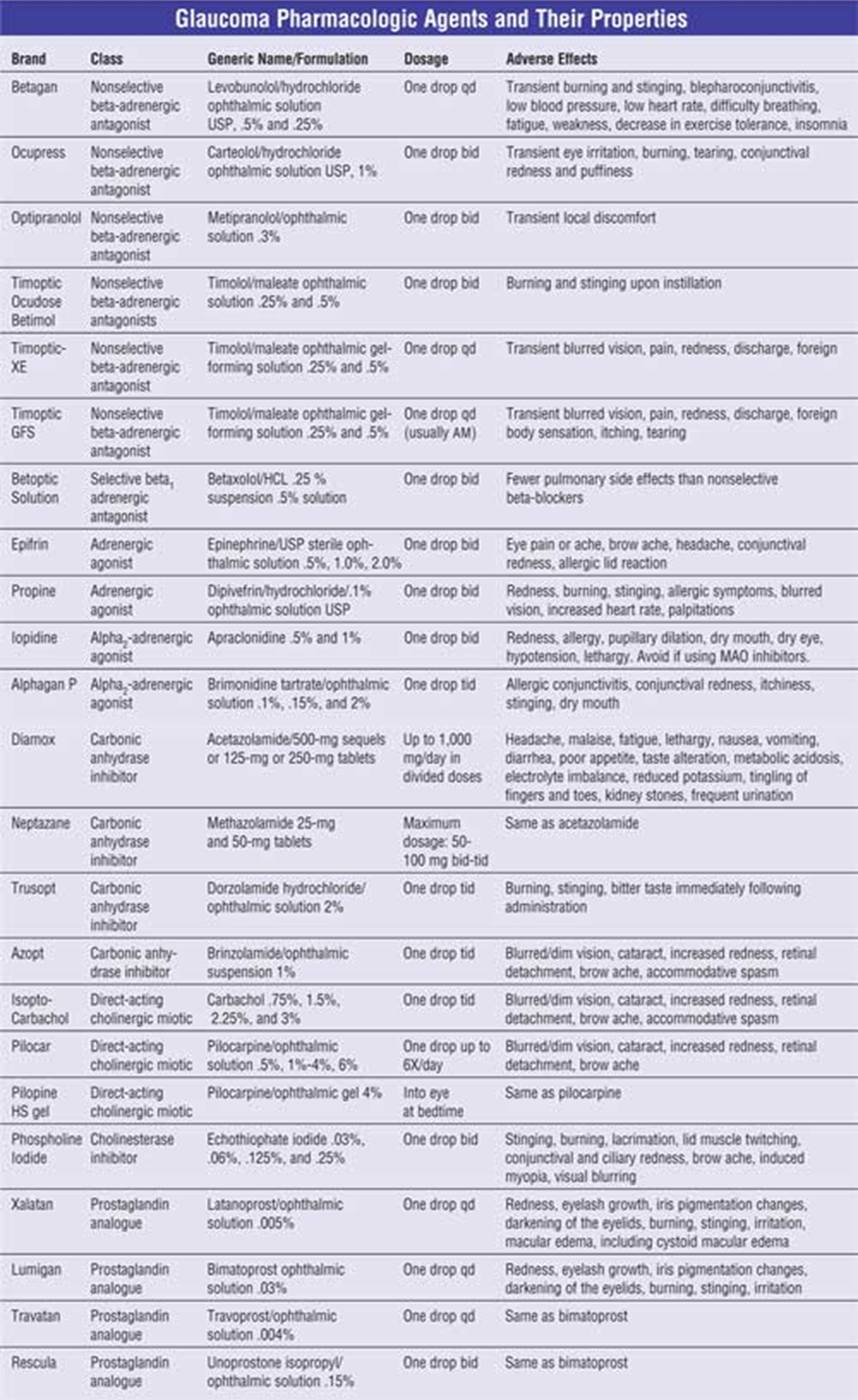
In the early stages of its development, glaucoma can be treated with eye drops that decrease eye pressure by either increasing aqueous humour outflow of reducing the production of the fluid. One or more prescription eye drop medication can be prescribed to an individual with the condition (Sleath et al., 2011). In addition, these drugs are typically taken once a day, or sometimes twice per day for life (Sleath et al., 2011). Examples of the types of drugs that may be prescribed to an individual include prostaglandins, beta-blockers (also available in pill form), alpha-adrenergic agonists, carbonic anhydrase inhibitors (also available in pill form), rho kinase inhibitors, and cholinergic agents (Mayo Clinic, n.d.). If eye drops alone are insufficient in lowering eye pressure, individuals may also be prescribed oral medications (pills), yet these are less often used (Mayo Clinic, n.d.).
Laser/Intrusive Treatments
Treatment may also involve laser therapy or surgery to improve fluid drainage within the eye as a means of lowering eye pressure. The most common forms include:
- Selective Laser Trabeculoplasty (SLT): Involves using a laser beam to open clogged channels in the trabecular meshwork (Latina & Tumbocon, 2012). This procedure is used when eye drop medications are not lowering the eye pressure enough or are causing significant side effects. It can also be used as an initial treatment for glaucoma.
- Laser Peripheral Iridotomy (LPI): Involves a laser being used to create an opening in the iris so that fluid can leave the angle of the eye more easily (He et al., 2007). LPI is the preferred procedure for treating glaucoma caused by a relative or absolute block of aqueous humour flow. LPI eliminates pupillary block by allowing the aqueous to pass directly from the posterior chamber into the anterior chamber, bypassing the pupil.
- Trabeculectomy: Involves creating a small channel is to drain fluid from the eye into a reservoir located underneath the eye’s natural lining, the conjunctiva. This reservoir, known as a bleb, may appear like a blister-like bump on the white of the eye. Regarded as the “gold standard” in glaucoma surgery (Brandão & Grieshaber, 2013; Skuta & Parrish, 1987). Trabeculectomy is a standard surgery for lowering pressure inside the eye when medical treatments or laser surgery have failed to bring the eye pressure low enough. In this operation, a small hole is made in the wall of the eye, and a “trapdoor” or “flap” is created over this hole to allow fluid to escape the eye in a controlled fashion. The fluid is shunted from inside the eye, bypassing the obstructed trabecular meshwork, through the small hole and “trapdoor,” while remaining underneath the outer clear membrane of the eye (conjunctiva). This forms a small blister or “bleb” underneath the upper eyelid. Normally, no one will be able to see the “bleb” just by looking at the eyes.
- Tube-Shunt Surgery (Seton glaucoma surgery): Involves small tubes being inserted into the eye to drain away excess fluid and lower eye pressure (Eid & Augsburger, 1997). This type of surgery is usually done after a trabeculectomy has failed.
Future Treatment: Gene Therapy
There has been a lot of recent research on the use of gene therapy as a means of treating glaucoma. The idea behind gene therapy is to introduce an exogenous gene into a cell in order to modify its activity. Gene therapy is not used to fix defective genes, but to introduce a new gene which down or up-regulates a function of the receiving cell. This is accomplished through the use of inactivated (i.e., they do not express any other activity except the one of the gene of interest and its promoter) vectors (typically, viruses) that are unable to replicate (Fogagnolo & Rossetti, 2011).
The idea behind gene therapy is to introduce an exogenous gene into a cell in order to modify its activity. Gene therapy is not used to fix defective genes, but to introduce a new gene which down or up-regulates a function of the receiving cell. This is accomplished through the use of inactivated (i.e., they do not express any other activity except the one of the gene of interest and its promoter) vectors (typically, viruses) that are unable to replicate (Fogagnolo & Rossetti, 2011).
In a study conducted by Borrás and colleagues (2001), they investigated the effects of treating the eyes of monkeys with a protein, adenoviral vector expressing rat non-muscle caldesmon fused to green fluorescent protein. After 24-48 hours, trabecular meshwork cells had a change in activity and GFP-caldesmon was hyper-expressed. As a result, aqueous humour outflow was increased by 50%. In another study by Borrás and colleagues (2010), they evaluated the effects of an adenoviral vector carrying the gene of metalloproteinase 1 when injected on living sheep before and after the induction of corticosteroid ocular hypertension leading to intraocular pressure. Sheep were chosen because they have a high corticosteroid response on intraocular pressure. In sheep with high eye pressure, the injection achieved pressure reductions up to 70% in 24 hours. In eyes with normal pressure, pre-injection protected against the increase in IOP which was induced by the continuous application of the corticosteroid for 5 days. While the research on gene therapy treatment for glaucoma is growing and has been investigated with animal models, many important issues such as the long term efficacy and safety of using it as a mechanism of treatment need to be considered before beginning in vivo human studies.
REFERENCES
Agarwal, R., Gupta, S. K., Agarwal, P., Saxena, R., & Agrawal, S. S. (2009). Current concepts in the pathophysiology of glaucoma. Indian Journal of Ophthalmology, 57(4), 257.
Barkan, O. (1955). Pathogenesis of congenital glaucoma: gonioscopic and anatomic observation of the angle of the anterior chamber in the normal eye and in congenital glaucoma. American Journal of Ophthalmology, 40(1), 1-11.
Bengtsson, B., & Heijl, A. (2000). False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability?. Investigative Ophthalmology & Visual Science, 41(8), 2201-2204.
Brandt, J. D. (2004). Corneal thickness in glaucoma screening, diagnosis, and management. Current Opinion in Ophthalmology, 15(2), 85-89.’
Bollinger, K. E., Crabb, J. S., Yuan, X., Putliwala, T., Clark, A. F., & Crabb, J. W. (2012). Proteomic similarities in steroid responsiveness in normal and glaucomatous trabecular meshwork cells. Molecular Vision, 18, 2001.
Borrás, T., Gabelt, B. A. T., Klintworth, G. K., Peterson, J. C., & Kaufman, P. L. (2001). Non‐invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. The Journal of Gene Medicine: A Cross‐Disciplinary Journal for Research on the Science of Gene Transfer and Its Clinical Applications, 3(5), 437-449.
Brandão, L. M., & Grieshaber, M. C. (2013). Update on minimally invasive glaucoma surgery (MIGS) and new implants. Journal of Ophthalmology, 2013.
Civan, M. M., & Macknight, A. D. (2004). The ins and outs of aqueous humour secretion. Experimental Eye Research, 78(3), 625-631.
Cook, C., & Foster, P. (2012). Epidemiology of glaucoma: what’s new? Canadian Journal of Ophthalmology, 47, 223–226. https://doi.org/10.1016/j.jcjo.2012.02.003
Eddy, D. M., Sanders, L. E., & Eddy, J. F. (1983). The value of screening for glaucoma with tonometry. Survey of Ophthalmology, 28(3), 194-205.
Ehlers, N., & Hasen, F. K. (1974). Central corneal thickness in low‐tension glaucoma. Acta Ophthalmologica, 52(5), 740-746
Eid, T. E., Katz, L. J., Spaeth, G. L., & Augsburger, J. J. (1997). Tube-shunt surgery versus neodymium: YAG cyclophotocoagulation in the management of neovascular glaucoma. Ophthalmology, 104(10), 1692-1700.
Fogagnolo, P., & Rossetti, L. (2011). Medical treatment of glaucoma: present and future. Expert Opinion on Investigational Drugs, 20(7), 947-959.
Gerometta, R., Spiga, M. G., Borrás, T., & Candia, O. A. (2010). Treatment of sheep steroid–induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Investigative Ophthalmology & Visual Science, 51(6), 3042-3048.
Glaucoma. (n.d.). Retrieved from https://fleetwoodfamilyeyecare.com/glaucoma/
Glaucoma: Open-angle. (2019). National Institutes of Health. Retrieved from: https://nei.nih.gov/eyedata/glaucoma
Harada, C., Kimura, A., Guo, X., Namekata, K., & Harada, T. (2019). Recent advances in genetically modified animal models of glaucoma and their roles in drug repositioning. The British Journal of Ophthalmology, 103(2), 161-166.
He, M., Friedman, D. S., Ge, J., Huang, W., Jin, C., Cai, X., … & Foster, P. J. (2007). Laser peripheral iridotomy in eyes with narrow drainage angles: ultrasound biomicroscopy outcomes. The Liwan Eye Study. Ophthalmology, 114(8), 1513-1519.
Hsia, J. C., Melamed, S., & Lowery, J. A. (2000). U.S. Patent No. 6,059,772. Washington, DC: U.S. Patent and Trademark Office.
Johnson, C. A., & Samuels, S. J. (1997). Screening for glaucomatous visual field loss with frequency-doubling perimetry. Investigative Ophthalmology & Visual Science, 38(2), 413-425.
Latina, M. A., & Tumbocon, J. A. (2012). Selective laser trabeculoplasty. Innovations in Primary Open Angle Glaucoma, 2, 1-9.
Leske, M. C. (2007). Open-Angle Glaucoma—An Epidemiologic Overview. Ophthalmic Epidemiology, 14(4), 166–172. doi:10.1080/09286580701501931
Mayo Clinic. (n.d.). Glaucoma. Retrieved from https://www.mayoclinic.org/diseases-conditions/glaucoma/diagnosis-treatment/drc-20372846
MD Supplies. (n.d.). Betaxolol is a selective beta1 receptor blocker used in the treatment of hypertension and glaucoma. Retrieved from https://www.mdsupplies.com/medical-supplies-Betoptic-BETAXOLOL-HYDROCHLORIDE-Betaxolol-Ophthalmic-Solution-USP-05-5-mL-RLRDT6NWED.html
Naklha, E. (2007). Therapeutic options for glaucoma. Journal of Modern Pharmacy, 14(9), 14. Retrieved from: https://www.uspharmacist.com/article/therapeutic-options-for-glaucoma
Patel, S. C., & Spaeth, G. L. (1995). Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surgery, Lasers and Imaging Retina, 26(3), 233-236.
Quigley, H. A., & Broman, A. T. (2006). The number of people with glaucoma worldwide in 2010 and 2020. The British Journal of Ophthalmology, 90(3), 262–267. https://doi.org/10.1136/bjo.2005.081224
Realini, T. (2011). A History of Glaucoma Pharmacology. Optometry and Vision Science, 88(1), 36–38. https://doi.org/10.1097/OPX.0b013e3182058ead
Singh, R. P., Goldberg, I., Graham, S. L., Sharma, A., & Mohsin, M. (2001). Central corneal thickness, tonometry, and ocular dimensions in glaucoma and ocular hypertension. Journal of Glaucoma, 10(3), 206-210
Skuta, G. L., & Parrish II, R. K. (1987). Wound healing in glaucoma filtering surgery. Survey of Ophthalmology, 32(3), 149-170.
Sleath, B., Blalock, S., Covert, D., Stone, J. L., Skinner, A. C., Muir, K., & Robin, A. L. (2011). The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology, 118(12), 2398-2402.
Weinreb, R. N., & Khaw, P. T. (2004). Primary open-angle glaucoma. The Lancet, 363(9422), 1711-1720.
Wolf, S., Arend, O., Sponsel, W. E., Schulte, K., Cantor, L. B., & Reim, M. (1993). Retinal hemodynamics using scanning laser ophthalmoscopy and hemorheology in chronic open-angle glaucoma. Ophthalmology, 100(10), 1561-1566.