This is an old revision of the document!
Table of Contents
PRESENTATION SLIDES
INTRODUCTION
Lactose intolerance is the onset of symptoms caused by the inability to digest lactose, or in other words, lactose malabsorption (Shaukat et al., 2010). Lactase deficiency leads to lactose malabsorption, which causes lactose intolerance. Lactase is the enzyme responsible for breaking down lactose into glucose and galactose (Vesa et al., 2000). Lactose intolerance prevalence varies by region, affecting approximately 75% of the world population (Mattar et al., 2012). The impact of lactose intolerance varies in populations around the world. Some populations have grown to develop lactase persistence, which is a gene mutation that enables individuals to continue producing lactase throughout their life, allowing them to be able to consume dairy (Mattar et al., 2012). Diagnosis for this condition is primarily conducted via removing dairy products from an individual's diet and assessing whether the symptoms have been resolved, or by conducting either a hydrogen breath test or stool acidity test (Shaukat et al., 2010). Management of this condition is predominantly achieved via dietary modifications including dairy exclusion and substitution, lactase supplements, and colonic bacteria adaptation (Shaukat et al., 2010). Lactose intolerance exists in three forms: primary hypolactasia (primary lactose intolerance), secondary hypolactasia (secondary lactose intolerance), and congenital alactasia (congenital lactose intolerance) (Mattar et al., 2012). Primary and secondary hypolactasia is associated with a reduction in lactase enzyme compared to the general population (Mattar et al., 2012). On the other hand, congenital alactasia is a rare form of this condition associated with a complete lack of the lactase enzyme (Mattar et al., 2012). Symptoms of lactose intolerance arise from the buildup of lactose in the small intestine, stemming from lactose malabsorption (Mattar et al., 2012). The most common symptoms of lactose intolerance include flatulence and diarrhea.
HISTORY
Lactose intolerance was not viewed as the norm until the 1960s. The condition was described as an allergy to dairy products or being caused by pathogens. This was because studies were mostly conducted on European populations. Their culture of cattle-farming and consumption of dairy over many generations allowed the population to gain lactase persistence, which was standardized as the norm (Simoons, F. J., 1969). As a result, these studies were conducted in populations where tolerance was considered normal.
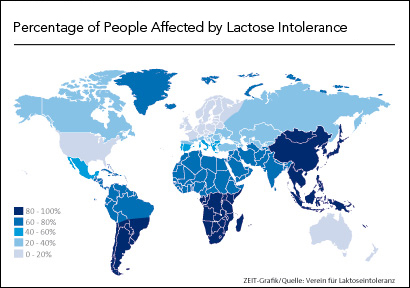
Compilations of studies around the world show that lactose intolerance is more common globally, such as in regions of South East Asia, Australia, and Africa (Itan et al., 2010). The wide variation in the prevalence of lactase persistence into adult life was hypothesized to be due to a mutation, and that intolerance may actually have been the historic natural state (Itan et al., 2010). Indeed, the Northern European populations contain a mutation that has provided them with a selective advantage by being able to digest lactose (Itan et al., 2010). In fact, even in other mammals, lactase gene expression declines dramatically as animals stop drinking their mothers’ milk (Fang et al., 2012). A single genetic variant is strongly associated with lactase persistence and appears to have been favoured by natural selection in the last 10,000 years (Itan et al., 2009). In fact, presently, most adults do not produce the enzyme lactase (Itan et al., 2009). It is believed that the ability to digest milk lactose first evolved in dairy farming communities in Europe. As a result of coevolution with the cultural practice of dairying, Europe and other populations can produce lactase throughout their life (lactase persistence) (Itan et al., 2009).
EPIDEMIOLOGY
All humans lose about 90 to 95% of birth lactase levels after weaning and continue to experience a dramatic decrease throughout their life. However, different ethnic backgrounds vary in the amount of lactase decline.
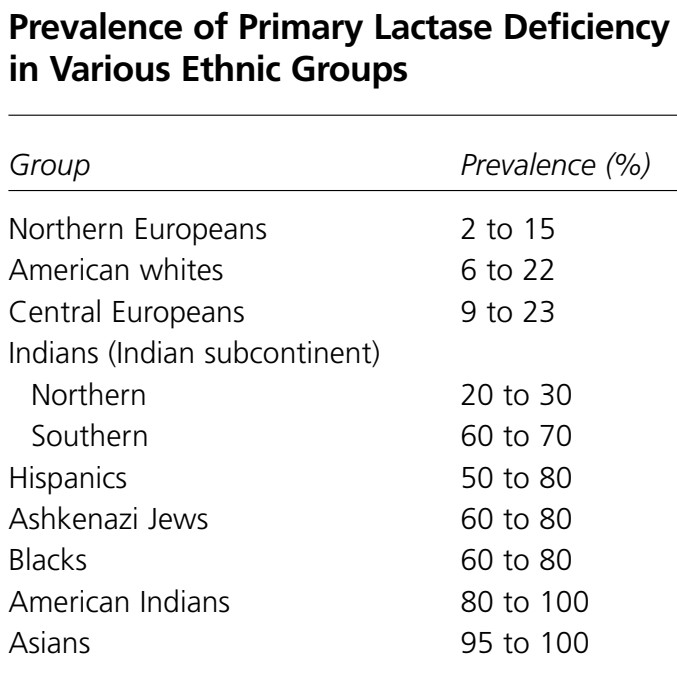
There is high polarity between certain ethnic backgrounds in terms of lactose intolerance. Some cultures seem to be dramatically more lactose intolerant than others. It is estimated that only 2% of Northern Europeans have the condition, whereas nearly 100% of adult Asians and American Indians cannot digest lactose (eMedicine, 2017). Africans and Jews have a prevalence of 60 to 80% lactose intolerance, and Latinos have a prevalence of 50 to 80% (eMedicine, 2017).
Cultural factors and lifestyles may also play a role. For example, the upper-class population of Japan are able to digest more lactose than the lower-class (Food-intolerance, 2013). The African Tuareg and Massai tribes who live in southern regions and practice intensive cattle-farming and consumption of milk are also able to digest lactose (Food-intolerance, 2013). This suggests that those who have grown up in an environment where access to dairy is readily available are not often affected by lactose intolerance.
SYMPTOMS
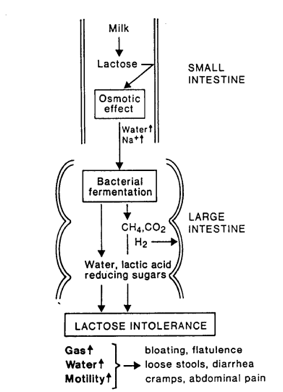
Lactose intolerance does not cause any disturbance in the body if lactose-containing food is not consumed (Mattar et al., 2012). Symptoms tend to show after 30 minutes to 2 hours after consumption of foods that contain lactose (Rusynyk & Still, 2001). As the undigested lactose settles in the small intestine, it eventually gets thick. The thickened lactose is metabolized by colonic bacteria and leads to the production of hydrogen gas, methane, and carbon dioxide. These byproducts lead to abdominal pain, bloating and flatulence (Mattar et al., 2012). Lactose intolerance can also decrease stool pH due to the production of lactic acid and short chain fatty acids from the fermentation of lactose by colonic bacteria (Mattar et al., 2012). The undigested lactose acidifies the colon and increases the osmotic load which leads to loose stool and diarrhea (Mattar et al., 2012). Some patients can experience constipation due to the slower transit time and decreased intestinal motility. Some patients also showed systemic symptoms like headaches, vertigo, memory impairment, muscle and joint pain (Mattar et al., 2012). This is caused by toxic by-products formed in the intestines such as acetaldehyde, ethanol, and peptides which affect cell- signaling mechanism (Mattar et al., 2012).
DIAGNOSIS
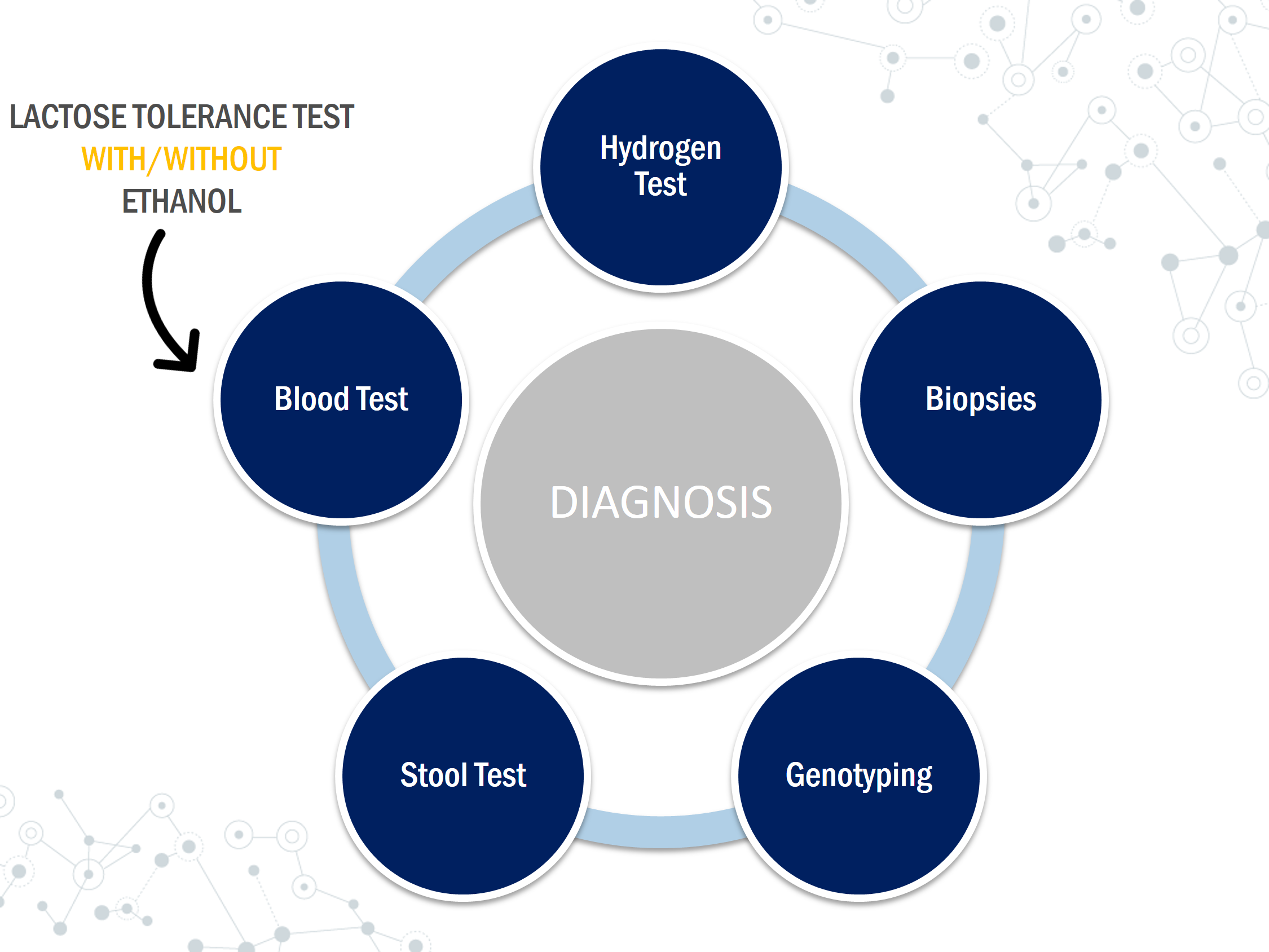
There are several ways to test for lactose intolerance including breath tests, blood tests, internal biopsies, and stool tests. When diagnosing, if the symptoms described line up with those of lactose intolerance, a lactose- free diet can be implemented. The diet can last up to 2 weeks and must not include any sources of lactose, especially from hidden sources. If the symptoms appear to be subtle, then further testing can be done using the techniques listed below.
Hydrogen Test
Hydrogen test is a non-invasive, non-labor-intensive and inexpensive breath analysis. Hydrogen analysis uses thermal conductivity gas chromatography and interval collection of expired air (Solomon et al., 1980). The breath test detects the hydrogen produced as bacteria ferment unabsorbed lactose in the expiratory air (Patel & Minocha, 2000). The test is based on the fact that there is no source of hydrogen gas in the body other than bacteria metabolism of carbohydrates (Simren & Stotzer, 2006). Expired breath is measured following ingestion of 25-50g of lactose which can correlate to 400ml- 1000 ml of milk (Simren & Stotzer, 2006). Hydrogen concentration for expired breath is measured every 15-30 minutes for 4-5 hours (Simren & Stotzer, 2006). A mean rise of 10-20 ppm of hydrogen levels from the basal level would signify a positive test (Simren & Stotzer, 2006). The change in hydrogen levels would suggest the presence of symptoms during the recorded time (Solomon et al., 1980).
Small Biopsy
Testing lactase activity can be done through mucosal biopsies. A sample is taken from the lining of the small intestine using an endoscope and is tested to see the amount of lactase. A small amount of the enzyme lactase means the patient is lactose intolerant. This procedure can be invasive and requires detailed biochemical analysis. Sometimes it might lack accuracy if the lactase deficiency is focal or patchy therefore it is rarely performed (Rusynyk & Still, 2001)
Genotyping
Genetic tests can be useful to identify lactase persistence since primary lactose intolerance (the most common form) is an autosomal recessive trait (Ridfelt & Håkansson, 2005). Lactose intolerance is caused due to variance in a gene called the MCM6 gene which controls the production of lactase. Lactase is the enzyme that breaks down lactose and is produced in the intestinal epithelial cells. The two variants of the MCM6 genes are CC variant which means you are lactose tolerant and TT variant which means you are unable to digest lactose.
Stool Test
This test is most popular for infants and small children. It is mostly used by pediatricians since it is easy and convenient (Arola, 1994). Stool pH can be determined using an indicator paper. Low pH levels can be caused by the fermentation of lactose by bacteria which leads to the formation of lactic acid and short chain fatty acids (Arola, 1994). Stool pH results which are less than 6 can indicate the existence of lactose intolerance (Arola, 1994).
Lactose Tolerance Test
The lactose tolerance test relies on an increase in the blood glucose levels following a lactose load of 50g (Misselwitz et al., 2013). This test can be inaccurate for patients with impaired glucose tolerance or diabetes (Misselwitz et al., 2013). Symptoms during the test period are also recorded (Patel & Minocha, 2000). This test requires frequent blood samples and the results can be affected by GI motility, diabetes, and other malabsorption syndromes. A rise in blood glucose levels less than 1.1 mmol/l can be indicative of lactose intolerance while an increase of more than 1.7 mmol/L can be considered normal (Arola, 1994). This test can be ineffective for patients with diabetes and glucose levels can easily be affected by hormonal changes.
Lactose Tolerance Test with Ethanol
This is similar to the lactose tolerance test but instead, it measures galactose instead of glucose levels. In a lactose tolerant individual, when lactose is ingested and broken down to glucose and galactose, the galactose in the blood is converted to glucose inside the liver (Villako & Maaroos, 1994). Ethanol delays the clearance of galactose from the circulation (Villako & Maaroos, 1994). A doctor will administer 500 mg of ethanol/kg body weight 10 min before providing the 50g lactose load to the patient (Villako & Maaroos, 1994). Galactose concentrations are measured at 0, 20, 40, 60 and 90 minutes (Patel & Minocha, 2000). If the patient has lactase deficiency and is unable to hydrolyze lactose and absorb its monosaccharides, the blood galactose levels do not usually increase.
PATHOPHYSIOLOGY & CAUSES
Lactose is a disaccharide found in milk and as a result, many processed foods such as cheese, chocolate, bread, and even sausages (Srinivasan & Minocha, 1998). Lactose gives milk its partially sweet taste but has no special nutritional importance for adults (Vesa et al., 2000). However, for infants, it is the most important source of energy, especially in the first year of their life when their primary source of food is their mothers’ milk. In fact, for infants, lactose provides almost half the total energy requirement (Vesa et al., 2000). Lactose is composed of the two monosaccharides, glucose and galactose (Vesa et al., 2000). Lactose is one third as sweet as the disaccharide sucrose (found in table sugar), which is made of glucose and fructose (Vesa et al., 2000).
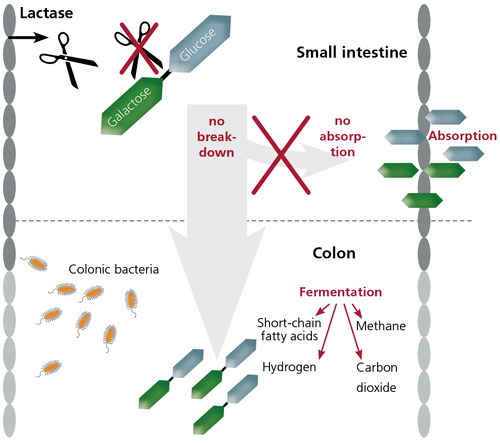
To break down lactose into its constituent simple sugar monomers, the enzyme β-galactosidase, lactase-phloritzin hydrolase (EC 3.2.1.23/26), generally called lactase is involved (Vesa et al., 2000). Lactase is an enzyme located in the brush border (microvilli) of the small intestine cells (Swagerty et al., 2002). Specifically, lactase is found in the jejunum, the beginning portion of the small intestine (Swagerty et al., 2002). The small intestine is typically the final step in the digestion of dietary carbohydrates and proteins. Brush border enzymes such as lactase are tethered as integral membrane proteins in the plasma membrane of the intestinal cells (enterocytes) (Louvard et al., 1992). The enterocyte plasma membrane contains small projections called microvilli where lactase is located. Lactose is too large to penetrate through the small intestine and enter the blood to be used and metabolized by the body, which is why the enzyme is necessary. When lactase hydrolyzes (breaks down with water) lactose into glucose and galactose, these monomers can easily be absorbed. Glucose and galactose can then be used for energy by the body through cellular respiration.
Lactose intolerance is the result of an inadequate amount of lactase production or none at all. This prevents the hydrolysis of lactose in the small intestine. The unabsorbed sugar will subsequently continue moving into the large intestine. The abundance of lactose sugar will osmotically attract water and electrolytes (from blood) into the intestine (Swagerty et al., 2002). Under normal body conditions, the osmolarity in the intestinal lumen is higher than the osmolarity of the blood (Swagerty et al., 2002). As a result, the fluid influx into the intestines of a lactose intolerant individual (after ingesting a lactose-rich meal) is approximately triple the predicted amount based on the osmolarity of the lactose by itself (Swagerty et al., 2002). This abundance of water results in diarrhea (Swagerty et al., 2002). In addition, lactose entering the colon is fermented (metabolized) by bacteria to produce short-chain fatty acids and gases (hydrogen, carbon dioxide, and methane). This leads to flatulence, bloating, pain, and even cramping (Srinivasan & Minocha, 1998).
Types of Lactose Intolerance
While characterized by the inability to breakdown lactose due to lactase deficiency, the condition has three main types: primary, secondary, and congenital lactase deficiency.
Primary lactose intolerance is the most common type (Swagerty et al., 2002). In this form, individuals are born with the ability to produce normal and adequate levels of lactase (Swagerty et al., 2002). This allows them to be capable of drinking their mothers’ milk when they are infants with no complications. As babies stop weaning and begin to replace milk with other foods, their lactase production decreases (Swagerty et al., 2002). Often individuals with this form of the condition will have continuously decreasing levels of lactase with age, and develop symptoms of lactose intolerance by adulthood, as their lactase production decreases sharply at that point (lactase non-persistence) (Vesa et al., 2000). From an evolutionary perspective, this is believed to be how the grand majority of humans were prior to dairy farming. Lactase persistence (tolerance) is inherited as a dominant trait. Lactose intolerance is the result of being homozygous for the recessive lactase allele that is poorly expressed after early childhood (Swallow, 2003). It has been found that heterozygotes can manifest partial intolerance, indicating that this is an incompletely dominant gene (Swallow, 2003).
Secondary lactose intolerance occurs when there is a decrease in lactase production as a result of an illness, surgery, or injury that has affected the small intestine. Lactase is located on the tip of the intestinal villi causing it to be more susceptible to intestinal issues that lead to cell damage than other disaccharidases, which are usually located deeper (Vesa et al., 2000). This form of the condition, can vary in severity and is sometimes transient, depending on the nature of the primary disorder (Rusynyk & Still, 2001). Treating the underlying disorder affecting the intestines can restore lactase levels. There are numerous potential causes for secondary lactose intolerance, and some include severe gastroenteritis, cystic fibrosis, and even chemotherapy (Vesa et al., 2000).
Congenital lactose intolerance is very rare (Rusynyk & Still, 2001). In this form, babies are born with the inability to produce any lactase (Rusynyk & Still, 2001). As a result, the consumption of any amount of lactose is intolerable and even dangerous as their diarrhea can quickly lead to dehydration (Rusynyk & Still, 2001). This is a genetic, autosomal recessive condition, meaning that both the mother and father must pass on the same gene variant for their child to be affected (Vesa et al., 2000). This form of the disorder is generally apparent within a few days after birth. For these individuals, they must be fed a lactose-free diet (Rusynyk & Still, 2001).
TREATMENTS & LIFESTYLE
Lactose intolerance can be treated with simple dietary changes. The following are some of the strategies that can be used to reduce the amount of milk or daily products in his or her diet.
Lactase Supplements
When dietary restriction of lactose consumption is not a suitable option, lactase supplements are available (Mattar et al., 2012). Lactase enzyme produced industrially by fungi of the genus Aspergillus, are suitable supplements to the lactase produced in the small intestines of humans (Mattar et al., 2012). This enzyme in the form of beta-galactosidase tablets, is available over the counter in several countries. The beta-galactosidase tablets must be taken at the time of lactose consumption in order to effectively aid in the digestion of lactose (Mattar et al., 2012). In addition, most available forms of this tablet are pH sensitive and are only active in acidic environments such as that of the stomach (Mattar et al., 2012). It is important to not that excessive acidity can denature the enzyme so consumption while fasting is not recommended (Mattar et al., 2012).
Dietary Management
Dietary management of lactose intake involves restricting the daily intake of lactose in order to reduce the onset of symptoms caused by lactose malabsorption (Shaukat et al., 2010). This restriction applies to all products containing the disaccharide lactose, which includes any dairy or dairy based products (Vesa et al., 2000). Lactose intolerance is caused by lactose malabsorption, which stems from lactose deficiency. Individuals with primary and secondary hypolactasia will express reduced concentrations of lactase enzyme in the small intestine (Mattar et al., 2012). These individuals can tolerate a varyingly small portion of lactose consumption, which can be determined via dietary management (Mattar et al., 2012). Dietary management involves restricting lactose digestion until symptoms of lactose intolerance are no longer present, which does not necessarily mean a complete shutdown of lactose consumption (Shaukat et al., 2010). Thus, these individuals are able to consume lactose within their varying limits. On the other hand, individuals with primary congenital alactasia lack the expression of lactase enzyme production, and as such, cannot tolerate any lactose consumption (Mattar et al., 2012).
Adaptation
Frequent consumption of lactose products can lead to adaptation by colonic bacteria (Mattar et al., 2012). Small amounts of lactose consumption in regular intervals throughout the day can promote a favorable microbiome for lactose digestion (Mattar et al., 2012). In essence, this can reduce lactose intolerance, allowing individuals with primary or secondary hypolactasia to consume higher doses of lactose over time (Mattar et al., 2012). Furthermore, the ingestion of live yogurt cultures containing lactobacilli can improve lactose consumption (Mattar et al., 2012). Lactobacilli can aid in the digestion of lactose, thus reducing lactose malabsorption (Mattar et al., 2012).
Lactose Substitutes
Individuals with primary or secondary hypolactasia can tolerate varying concentrations of lactose consumption, which is often considerably lower than lactose tolerant individuals (Mattar et al., 2012). Thus, dietary modifications for these individuals often involve the consumption of lactose-reduced or lactose-free substitutes (Mattar et al., 2012). Milk has many lactose-free substitutes that are available in wide distribution across the world. These substitutes include soy milk, almond milk, coconut milk, rice milk, hazelnut milk, hemp milk, and many other variants (Shaukat et al., 2010).
Milk provides a good source of nutrients essential for bone mineralization and growth. This is especially important during peak growth periods. Some nutrients found in milk include: protein, calcium, vitamin D, vitamin A, vitamin B12, riboflavin and phosphorus.
There are many products currently on the market that are lactose-free. This is a good option for those that don't want to give up their favorite milk products. There are more options on the way. The lactose-free food market is predicted to grow 11.10 percent between 2017 and 2021.Individuals avoiding foods containing milk may lose source essential nutrients from their diet, and therefore foods must be chosen with care to replace these lost nutrients. In some cases, cross-reactivity may when the proteins in one food are similar to the proteins in another. When that happens, the body's immune system sees them as the same. There is a high degree of cross-reactivity between cow's milk and the milk from other mammals such as goat and sheep. In studies, the risk of allergy (resulting in symptoms) to goat's milk or sheep's milk in a person with cow’s milk allergy is about 90%. The risk is much lower, about 5%, for allergy to mare's milk which is less cross-reactive with cow's milk.
Foods from the meat and alternative food category can provide the proteins necessary to meet daily requirements However, to replace calcium the individual may need to consume a lots of non-dairy foods containing calcium which may be more than they are cable of consuming. Furthermore, calcium fortified juices can provide additional calcium but are not a good source of other nutrients.
Many milk alternatives are also based on legumes, seeds and grains. These include rice, hemp, soy, sunflower, oat, and coconut milks. Those not allergic to nuts may also consider nut milks (the most common of which is almond milk). Most of these milks can be substituted one-for-one in recipes. Many are available in original or other flavors. They are also available in sweetened and unsweetened varieties. Generally, the unsweetened and unflavored versions work best as substitutes in recipes. The following are some examples of milk alternatives based on legumes, seeds and grains.
Soy milk Fortified rice milk Fortified grain or seed milks (oat milk, flax milk) Fortified nut milks (almond or cashew milk) Fortified coconut milk
Any of these milk substitutes can be used, if tolerated. Make sure they are a good source of calcium and additional nutrients. Review the nutrition information on the package to check the amount of protein, which should be 8 grams per 8 ounce serving. Soy or rice milk can be used for children who are eating a variety of other foods. The soy or rice milk must be calcium fortified or contain 30 percent calcium.
When cooking, you can substitute non-dairy margarine or oils (in equal amounts) for butter. Soy-based, coconut-based, and pea-based yogurt, sour cream, and cream cheese products are available at many grocery stores. Milk-free ice creams and other products are also available. Be sure to check the ingredient statements to make sure that they contain no milk ingredients How to Read a Label for Milk
Always read the entire ingredient label to look for the names of milk. Milk ingredients may be within the list of the ingredients. Or milk could be listed in a “Contains: Milk” statement beneath the list of ingredients. Advisory statements such as “may contain milk” or “made in a facility with milk” are voluntary. Advisory statements are not required by any labeling law. It is recommend for the individual to consult their doctors if they consume products with these labels.
REFERENCES
Arola, H. (1994). Diagnosis of Hypolactasia and Lactose Malabsorption. Scandinavian Journal of Gastroenterology, 29(sup202), 26–35. doi:10.3109/0036552940909174 Fang, L., Ahn, J. K., Wodziak, D., & Sibley, E. (2012). The human lactase persistence-associated SNP− 13910* T enables in vivo functional persistence of lactase promoter–reporter transgene expression. Human genetics, 131(7), 1153-1159. doi: 10.1007/s00439-012-1140-z
Itan, Y., Jones, B. L., Ingram, C. J. E., Swallow, D. M., & Thomas, M. G. (2010). A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evolutionary Biology, 10, 36. doi: /10.1186/1471-2148-10-36
Itan, Y., Powell, A., Beaumont, M. A., Burger, J., & Thomas, M. G. (2009). The origins of lactase persistence in Europe. PLoS computational biology, 5(8), e1000491. doi: 10.1371/journal.pcbi.1000491
Lactose Intolerance: Background, Pathophysiology, Etiology. (2017). Retrieved from https://emedicine.medscape.com/article/187249-overview
Louvard, D., Kedinger, M., & Hauri, H. P. (1992). The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annual review of cell biology, 8(1), 157-195.
Mattar, R., de Campos Mazo, D. F., & Carrilho, F. J. (2012). Lactose intolerance: diagnosis, genetic, and clinical factors. Clinical and experimental gastroenterology, 5, 113-121. doi: 10.2147/CEG.S32368
Misselwitz, B., Pohl, D., Frühauf, H., Fried, M., Vavricka, S. R., & Fox, M. (2013). Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European gastroenterology journal, 1(3), 151-159. doi: 10.1177/205064061384463
Patel, Y. T., & Minocha, A. (2000). Lactose intolerance: Diagnosis and management. Comprehensive Therapy, 26(4), 246–250. doi:10.1007/s12019-000-0025-6
Prevalence of lactose intolerance. (2013). Retrieved from https://www.food-intolerance-network.com/food-intolerances/lactose-intolerance/ethnic-distribution-and-prevalence.html
Ridefelt, P., & Håkansson, L. D. (2005). Lactose intolerance: Lactose tolerance test versus genotyping. Scandinavian Journal of Gastroenterology, 40(7), 822–826. doi: 10.1080/0036552051001576
Rusynyk, R. A., & Still, C. D. (2001). Lactose intolerance. Journal of the American Osteopathic Association, 101(4 supplement 1), 10S.
Simoons, F. J. (1969). Primary adult lactose intolerance and the milking habit: A problem in biological and cultural interrelations. The American Journal of Digestive Diseases, 14(12), 819–836. doi: 10.1007/BF02233204
Simren, M., & Stotzer, P. O. (2006). Use and abuse of hydrogen breath tests. Gut, 55(3), 297-303. doi:10.1136/gut.2005.075127
Shaukat, A., Levitt, M. D., Taylor, B. C., MacDonald, R., Shamliyan, T. A., Kane, R. L., & Wilt, T. J. (2010). Systematic review: effective management strategies for lactose intolerance. Annals of internal medicine, 152(12), 797-803. doi: 10.7326/0003-4819-152-12-201006150-00241
Solomons, N. W., García-Ibañez, R., & Viteri, F. E. (1980). Hydrogen breath test of lactose absorption in adults: the application of physiological doses and whole cow’s milk sources. The American Journal of Clinical Nutrition, 33(3), 545–554.doi:10.1093/ajcn/33.3.545
Srinivasan, R., & Minocha, A. (1998). When to suspect lactose intolerance: symptomatic, ethnic, and laboratory clues. Postgraduate medicine, 104(3), 109-123. doi: 10.3810/pgm.1998.09.577
Swagerty, D. L., Walling, A. D., & Klein, R. M. (2002). Lactose intolerance. American family physician, 65(9), 1845-1860.
Swallow, D. M. (2003). Genetics of lactase persistence and lactose intolerance. Annual review of genetics, 37(1), 197-219.
Vesa, T. H., Marteau, P., & Korpela, R. (2000). Lactose intolerance. Journal of the American College of Nutrition, 19(sup2), 165S-175S. doi: 10.1080/07315724.2000.10718086
Villako, K., & Maaroos, H. (1994). Clinical Picture of Hypolactasia and Lactose Intolerance. Scandinavian Journal of Gastroenterology, 29(sup202), 36–54. doi:10.3109/00365529409091743