This is an old revision of the document!
Table of Contents
Introduction
Epilepsy is a chronic condition that affects the central nervous system and is characterized by a disruption in the normal functioning of neuronal signaling and activity. Epilepsy is characterized by the presence of unprovoked or spontaneously recurring epileptic seizures ranging in severity and occur within a short span of time. There is often no immediately identifiable trigger for epileptic seizures and two or more seizures occurring within a span of 24 hours is considered an isolated event. Seizures that occur during the neonatal period of life, and acute symptomatic seizures linked to substance abuse are not considered epileptic. (Banerjee, Filippi, & Hauser, 2009) (Scharfman, 2007). The spectrum of epileptic seizures range from a lapse in concentration to unconsciousness with regards to severity. In addition, the World Health Organization defines epilepsy as a prevalent and major health concern world wide, and statistics show that over 50 million individuals world-wide present the disorder (World Health Organization, 2015).
Epidemiology
The World Health Organization has characterized epilepsy as a major cause of morbidity and mortality, affecting an estimated 50 million people world-wide at any given time. Several published epidemiological studies have published prevalence and incidence rates that vary due to study methodologies, population structure (age, gender, geographic location). Moreover, given that some areas in the world stigmatize the condition due to cultural values, rates may be underestimated (Banerjee et al., 2009).
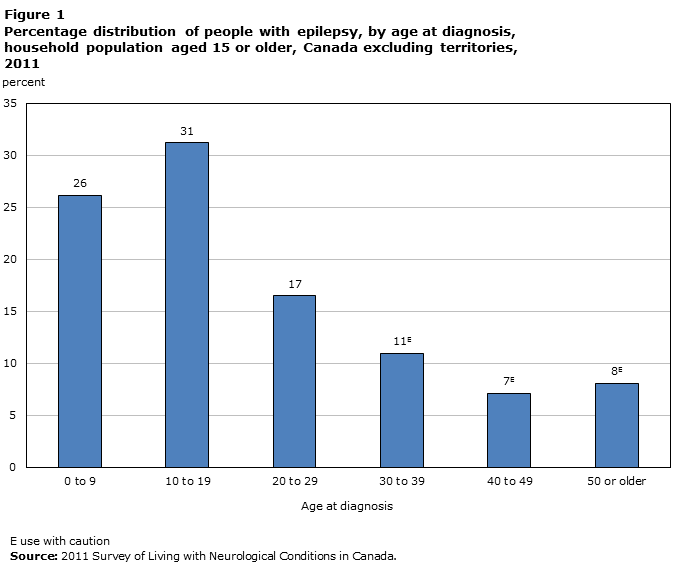
Moreover, epilepsy is diagnosed in a variety of age groups and typically is associated with a 65-85% chance of long-term remission. In North America, the prevalence of epilepsy ranges from 5.0-11.5 cases per 1000 and according to the Center of Disease Control, the estimated median incidence rate is 9.1 cases per 1000 each year. (Banerjee et al., 2009). Specifically, Canadian statistics summarize that from the time period of 2010-2012, an estimated 139 200 Canadians were diagnosed with epilepsy and males were more likely than females to have epilepsy, with a prevalence ratio of 57.1 per 1000 for men compared to 31.6 per 1000 cases for women. In addition, approximately 26% of epileptic cases had been diagnosed before age 10, 75% were diagnosed before age 30, and 15% were diagnosed in their 40s or later (Figure 1). For the studied Canadian population, on average, diagnosed individuals lived with the condition for 24 years, though this period extended to over 60 years. Furthermore, in approximately 64% of the cases studied, epilepsy was the only neurological condition observed and there were no co-morbidities (Gilmour et al., 2016)
Figure 1: Percentage of individuals diagnosed with epilepsy by age during 2011 in all Canadian Provinces, with exception of the Canadian Territories
Etiology
Essentially, recurrent epileptic seizures may range in severity from benign episodes to life-threatening or disabling instances. In general, epileptic seizures are caused by an interference in the regular neuronal patterning, resulting in abnormal sensations and behaviours, including convulsions, twitching, muscle spasticity, and a loss of consciousness. Moreover, epilepsy may be attributable to an abnormality in brain circuitry, nerve signaling imbalances, deviant neurotransmitter concentrations, and abnormalities of gated ion channels integral to neuronal signaling. (PUBMED HEALTH) (Bromfield et al., 2006)
The causes of epileptic seizures can be attributable to several inducing factors, namely:
- 1. Idopathic cause (spontaneous/ unknown cause): assumed to have genetic basis, however, no gross anatomical or pathological abnormality evident. General onset is during childhood, and seen in in over 50% of all cases of epilepsies.
- 2. Symptomatic cause: identified brain insult is the cause, tumor, infection, head injury, lack of oxygen (at birth), stroke. Seizures may occur due to developmental disorders or malformations or which progress due to changes in response to an insult or injury
- 3. Cryptogenic: obscure or uncertain cause (more than half of cases)
(Shorvon, 2011)
Several risk factors have been suggested causative agents for epilepsy, namely in disrupting the normal neuronal signaling patterns of the CNS. Some of these risk factors include:
- Age: most common during early childhood and after the age of 60, however can occur at any age
- Family history of epilepsy
- Head injury
- Stroke
- Brain infections: bacterial/viral meningitis, cerebral malaria, etc.
Epileptic Seizure Classifications and Effects
According to the International League Against Epilepsy (ILAE) 2017, epileptic seizures are classified based on three key features (Fisher, 2017):
- Where the seizure begins in the brain
- Level of awareness during the seizure
- Other features of seizures
Where Seizures Begin
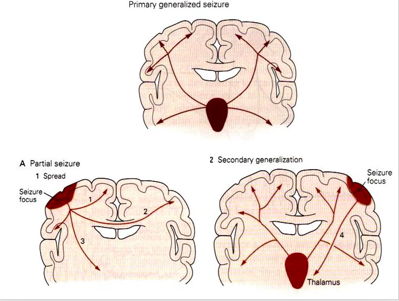
- Focal seizure: starts in an area or network of cells on one side of the brain
- Generalized seizure: involves network on both sides of the brain
- Unknown onset: onset of the seizure is not known
- Focal to bilateral seizure: starts on one side or part of the brain and spreads to both sides
Figure: visual representation of partial, primary and secondary generalized seizures.
Describing Awareness
- Focal aware: awareness remains intact, even if the person is unable to talk or respond during the seizure
- Focal impaired awareness: awareness is impaired at the time of the seizure, even if the person has a vague idea of what happened
- Awareness unknown: sometimes it is not possible to know, i.e. if a person lives alone or has seizures at night
- Generalized seizures: these types of seizures affect a person’s awareness in some way, there is no need for a specific term to describe the affect on awareness
Other Features
- Focal motor seizure: means some type of movement occurs during the seizure: twitching, jerking, stiffening, licking lips, rubbing hands etc.
- Focal non-motor seizure: other symptoms occur first, i.e. changes in sensation, emotions, thinking or experiences
- Generalized motor seizure: experience stiffening (tonic) and jerking (clonic)
- Generalized non-motor seizure: brief changes in awareness, staring, and can experience autonomic/repeated movements like lip smacking
(Fisher, 2017)
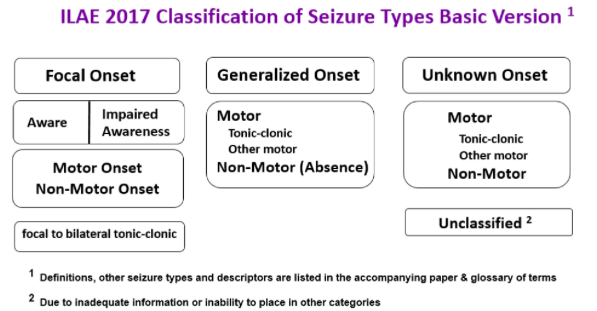 Figure _: Revised seizure classification system developed by the International League Against Epilepsy
Figure _: Revised seizure classification system developed by the International League Against Epilepsy
Moreover, epileptic seizures vary in severity and duration, usually lasting seconds or mintues but have the potential to continue beyond normal time frames. Regardless, notable symptoms associated with epileptic seizures include:
- Loss of consciousness
- Fatigue upon recovery
- Uncontrolled movements and muscle spasticity
- Irregular heartbeat
- Difficulty respiring
(Bromfield et al., 2006)
EEG patterns and behaviour
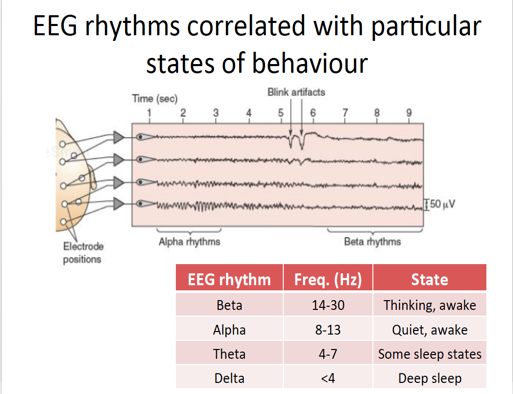
Electroencephalogram (EEGs) is a diagnostic test conducted to detect any irregularities in neuronal activity in the brain. There 5 main types of EEG rhythms, each associated with different states of consciousness (refer to Figure_). Beta rhythms occur during thinking and wakeful periods and are characterized by low amplitudes and high frequencies. This pattern is due to the complex firing of neurons needed for thinking. There are many inputs being processed simultaneously and these signals can be excitatory and inhibitory (Bear, 2007). However, when they fired asynchronously the waves destructively interfere and cancel each other out, leading to low amplitudes. Delta and Theta rhythms fire during deep sleep periods and are characterized by high amplitudes and low frequencies. When there is no real active processes occurring, firing is controlled by the thalamus which is regarded as the rhythmic pace maker in the brain. These neurons fire synchronously and constructively interfere with each other leading to the superimposition of brain waves (Bear, 2007).
Figure _: EEG rhythms and associated behaviours. Beta waves occur during thinking periods and are characterized by low amplitudes. Delta waves occur during sleeping periods and are characterized by larder amplitudes.
Diagnosis
Whether or not an individual has epilepsy depends on the symptoms before, during, and after a seizure episode occurs. In addition, a physician will likely not be present when the episode occurs, and therefore, a number of diagnostic questions are asked and several tests are conducted to gather data on the seizure and reach a difinitive diagnosis. Several diagnostic tests include:
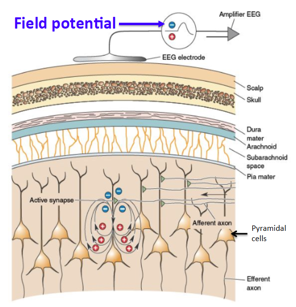
Electroencephalogram (EEG): This is the primary tool for recording electrical activity of the brain and involves the attachment of small metal disk electrodes to the scalp, enabling changes in voltage to be recorded. Physicians look for abnormal electrical activity, including synchronous firing and hyperexcitable neurons. The EEG records fluctuations in the extracellular environment, therefore if a neuron is depolarized, the EEG will record a negatively charged environment. This is due to the influx of positively charged ions leaving the extracellular environment and entering the neuron. In order to produce a strong signal on the EEG, there needs to be thousands of underlying neurons firing in a synchronous manner. The EEG signal measures field potentials, and not an excitatory postsynaptic potential (EPSP) as it is the recording of many neurons and not a single neuron firing (Bear, 2007). The EEG can be done while the patient is conscious or unconscious. Furthermore, the physician may also rear the patient on video to assess the patient’s muscular reactions during a seizure, subsequently demanding overnight examination of the patient. Moreover, a normal EEG reading does not rule out the presence of epilepsy.
Figure_: EEG recording field potential in pyramidal cells.
Computerized Tomography (CT) Scan and/or Magnetic Resonance Imaging (MRI): CT scans use X-rays to create cross sectional images of the brain, effectively revealing distinct structures of the brain. Furthermore, an MRI reveals a more detailed image of the brain and soft tissue, however both of these imaging techniques allow the doctor to determine potential causative agents of epileptic seizures, including tumours, internal hemorrhaging, and cysts.
Blood Test: A blood test and subsequent genetic analysis may identify any genetic conditions or infections, like viral meningitis for instance, that may be causative agents for epileptic seizures.
Neuropsychological tests: The doctor can test speech, cognitive, and memory skills to assess what areas of the brain have been affected by epileptic seizures.
(Epilepsy Support Centre, 2015)
Normal Synaptic Function and Neuronal Firing
Action Potentials
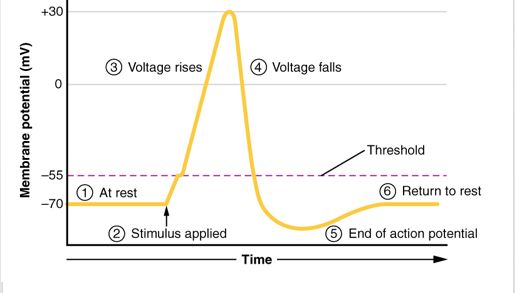
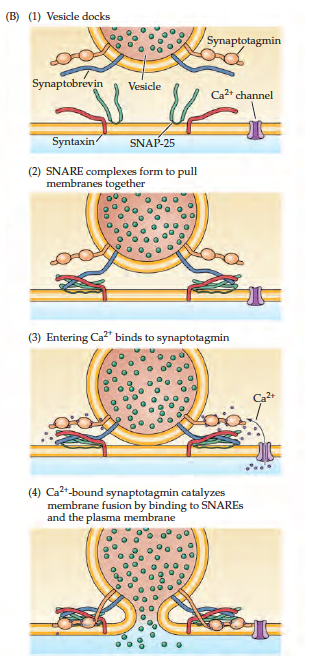
Neurons communicate with each other through the release of neurotransmitters into the synaptic cleft to elicit an excitatory or inhibitory effect. The release of these neurotransmitters is regulated by fluctuations in the axon membrane potential. There are two main channels that are located on axon membrane; one being sensitive to Na+ ions and the other K+ ions. These channels are referred to as voltage gated because their activation is dependent on membrane potential (Purves, 2012). In the presence of a stimulus, the resting membrane potential (-70mV) begins to depolarize through the activation of Na+ voltage gated channels which allows the influx of Na+ ions. Once the membrane potential has crossed the threshold of excitation (-50mV) an action potential is guaranteed (Purves, 2012). The axon continues to depolarize until it peaks (+50mV), at this point the inactivation gate on the Na+ channel closes preventing an Na+ influx. Since voltage gated K+ channels activate in response to depolarization the channels open causing an efflux of K+ ions and a decrease of membrane potential (Purves, 2012). Voltage gated K+ channels are less sensitive to polarization so often times the channels stay open even after resting membrane potential has been reached, which leads to hyperpolarization. The K+ channels eventually close and the cell is able to return to its resting membrane potential. The action potential moves throughout the axon until it reaches the presynaptic terminal. The depolarization causes voltage gated Ca2+ channels to open leading to an influx of Ca2+ ions. The influx of Ca2+ ions leads to further depolarization and the fusing of neurotransmitter vesicles with the presynaptic membrane (Purves, 2012). Attached to the vesicle membrane are SNARE complexes made up of Synaptobrevin protein and Synaptotagmin. The presynaptic membranes also have a SNARE complex made up of Syntaxin and SNAP-25. These bind to the Synaptobrevin from the vesicle. The influx of Ca2+ ions bind onto the vesicle Synaptotagmin subunit which allows the fusing of the vesicle with the membrane and finally the release of neurotransmitters into the synaptic cleft (Purves, 2012).
Figure _: The steps of action potential
Figure _: Mechanisms of vesicle fusion with axonal membrane leading to the release of neurotransmitters
Excitatory Neurotransmission
The brain’s primary excitatory neurotransmitter is glutamate. The influx of Ca2+ following depolarization of the terminal axon prompts vesicles to release glutamate into the synaptic cleft where it can bind either NMDA (N-methyl-D-aspartate) or non-NMDA (kainate and AMPA) receptors (Stafstrom, 1998). The binding of glutamate to non-NMDA receptors causes the influx of Na+ into the post synaptic neuron via the rector’s pore. This brings the membrane potential inside the postsynaptic neuron closer to firing threshold and is called an excitatory postsynaptic potential (EPSP). Non-NMDA receptors mediate a fast-EPSP, which is often followed by an action potential once firing threshold is reached. To activate a NMDA receptor, glutamate must bind to the NMDA receptor, a glycine co-agonist must bind to the NMDA receptor complex and a Mg2+ ion that blocks the channel pore of the NMDA receptor complex must be expelled (Stafstrom, 1998). At resting membrane potential the Mg2+ ion blocks the pore and prevents the influx of ions into the postsynaptic neuron. Once the postsynaptic neuron has been depolarized by a non-NMDA mediated fast-EPSP the Mg2+ ion is relieved, permitting the flow of Na+ and Ca2+ into the postsynaptic neuron, this results in a NMDA-mediated prolonged-EPSP. During the longer NMDA-mediated depolarization several action potential may fire (Stafstrom, 1998).
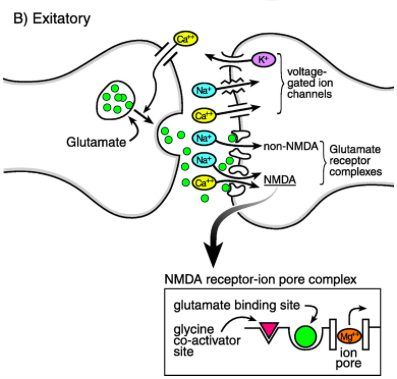 Figure_: Glutamate is released from the presynaptic neuron into the synaptic cleft and binds to either a NMDA or non-NMDA receptor on the postsynaptic neuron. This allows positive ions to move into the postsynaptic neuron and causes either a prolonged-EPSP or a fast-EPSP.
Figure_: Glutamate is released from the presynaptic neuron into the synaptic cleft and binds to either a NMDA or non-NMDA receptor on the postsynaptic neuron. This allows positive ions to move into the postsynaptic neuron and causes either a prolonged-EPSP or a fast-EPSP.
Inhibitory Neurotransmission
The brain’s primary inhibitory neurotransmitter is gamma-amino-butyric acid (GABA). Glucose is the primary precursor of GABA, but it can also be produced from pyruvate and glutamine. Tricarboxylic acid cycle enzymes convert glucose to glutamate which is then converted to GABA via glutamic acid decarboxylase (GAD). GABA is eventually degraded into succinate by GABA transaminase and is free to enter the Krebs cycle (Purves, 2012). The influx of Ca2+ following the depolarization of the terminal axon causes vesicles to release GABA into the synaptic cleft. Once GABA is in the synaptic cleft it can bind to three receptors: GABA-A, GABA-C, GABA-B. The former two are ionotropic receptors and therefore, the activation of these two receptors leads to an influx of Cl-, which inhibits postsynaptic neurons. Receptor GABA-B is a G-coupled protein, therefore when GABA binds to the receptor it activates the G-subunit of the receptor. Stimulation of the G-protein subunit leads to the recruitment of secondary messengers to activate downstream ion channels. In the case of GABA-B, downstream K+ channels are activated so K+ flows out of the neuron and Ca2+ channels are blocked so they cannot flow into the neuron, resulting in hyperpolarization of the postsynaptic neuron (Treiman, 2001). Essentially, the binding of GABA to any of its respective receptors results in a greater negative charge inside the postsynaptic neuron, this change in membrane potential is called an inhibitory postsynaptic potential (IPSP), which reduces the firing frequency of a neuron by keeping the membrane potential away from firing threshold (Stafstrom, 1998).
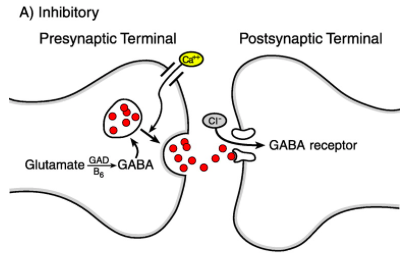 Figure_: GABA is released from the presynaptic neuron into the synaptic cleft and binds onto a GABA receptor on the postsynaptic neuron, this permits the influx of Cl- ions into the postsynaptic neuron and leads to an IPSP.
Figure_: GABA is released from the presynaptic neuron into the synaptic cleft and binds onto a GABA receptor on the postsynaptic neuron, this permits the influx of Cl- ions into the postsynaptic neuron and leads to an IPSP.
Pathophysiology of Epilepsy
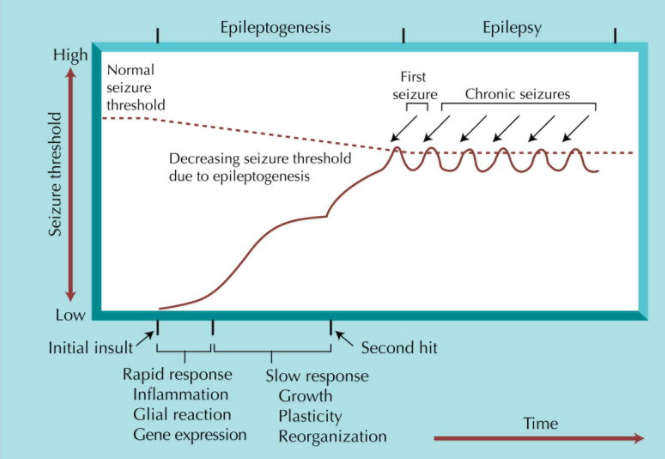
Epileptogenesis is the process in which a normally functioning brain changes toward a brain that produces an abnormal amount of electrical activity known resulting in seizures. Epilepsy occurs due to any biological feature that results in the brain’s production of unprovoked seizure. These can include anatomical, molecular, or circuit level alterations. For example, neuron death, or abnormality in ion channels or neurotransmitter receptors.
The process of epileptogenesis occurs in three steps. The first one being a brain injury. The second step, categorized by a latent period in which the brain changes to an epileptic brain due to the injury. The third and final phase is known as chronic, characterised by epilepsy (Goldberg et al. 2013).
During the second phase, there is both rapid and slower progressive changes. Rapid changes contain neuronal excitation and calcium influx, which triggers events that such as second messenger and immediate early gene responses, modifications to pre-existing proteins, and protein synthesis. Immediately, there is cell death and proliferation as well as inflammatory, glia, and vascular responses. Slower responses include growth such as; axon outgrowth, synaptogenesis, angiogenesis, leading to synaptic reorganization. Over a period of time, there is a growing increase in excitability, and thus the risk of a seizure increases. Therefore, genes, development, and the responses to the initial insult are likely to act together to result in a state of chronic seizures (Scharfman 2007)
 Abnormal synchronization of neurons in the brain results in an epileptic seizure. These seizure present various symptoms, which depend on the origin of the seizure and the connections in the brain. Seizure develop from either increased excitation or decreased inhibition (Moshé et al. 2015)
Abnormal synchronization of neurons in the brain results in an epileptic seizure. These seizure present various symptoms, which depend on the origin of the seizure and the connections in the brain. Seizure develop from either increased excitation or decreased inhibition (Moshé et al. 2015)
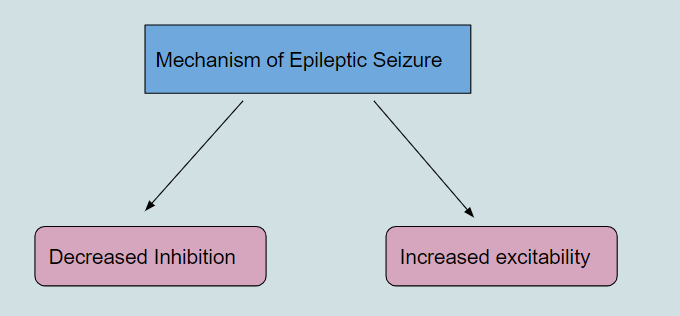
Increased Excitability
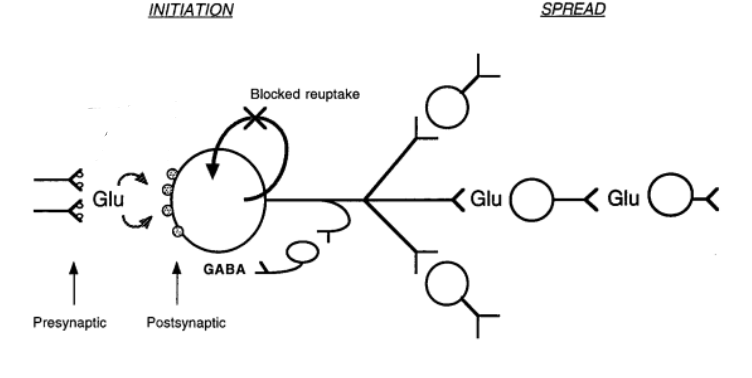
There are elevated levels of glutamate in the brain of patients with epilepsy, which is known as glutamate-induced excitotoxicity. Glutamate is normally degraded by an enzyme called glutamine synthetases (GS). However, GS has been shown to be deficient in patients with acquired epilepsy, also known as TLE. The GS deficiency causes high glutamate levels in astrocytes and in extracellular areas such as the synaptic cleft. This leads to prolonged effects of glutamate. Furthermore, GTL-1 is a major glutamate uptake molecule found in the brain, and its downregulation causes increased glutamate levels, leading to more excitability (Cho 2013). Glutamate itself and glutamate receptors, such as NMDA, cause high excitability as characterized in epilepsy. The cell death and tissue damage which occur in the initial stage of acquired epilepsy leads to the high concentrations of glutamate leakage into the extracellular space. Furthermore, tissue reorganization, occurring in epileptogenesis impairs high efficiency uptake of glutamate from the synaptic cleft. The decreased uptake of glutamate, allows for prolonged excitation of postsynaptic neurons (Brandford 1995).
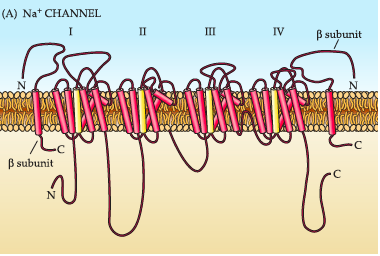
As mentioned before, voltage gated Na+ channels play a vital role in axon depolarization and therefore it can be assumed that dysregulation in their function can lead to epileptic symptoms. The voltage gated Na+ channels are composed of four homologous subunits (I-IV) and each subunit is composed of 6 transmembrane segments (Escayg, 2010). Subunits III and IV are connected by an intracellular loop which forms the inactivation gate. The channels are also connected to accessory beta units; 1, 2, 3, 4 which help modulate voltage dependence. Voltage gated Na+ channels are encoded by nine genes, SCN1A to SCN11A which gives rise to nine distinct channel isoforms. These isoforms differ in their location, ranging from CNS to embryonic heart muscle and also their threshold for activation. Mutations in these SNCA genes lead to increased frequency of depolarization of neurons and can even lead to excitotoxicity. For example, alterations in SCN1A and SCN2A lead to disorders such as genetic epilepsy with febrile seizures plus (Escayg, 2010).
Figure_: Visual representation of voltage gated Na+ channels, composed of four subunits which are each made up of 6 transmembrane proteins to form the channel and inactivation gate.
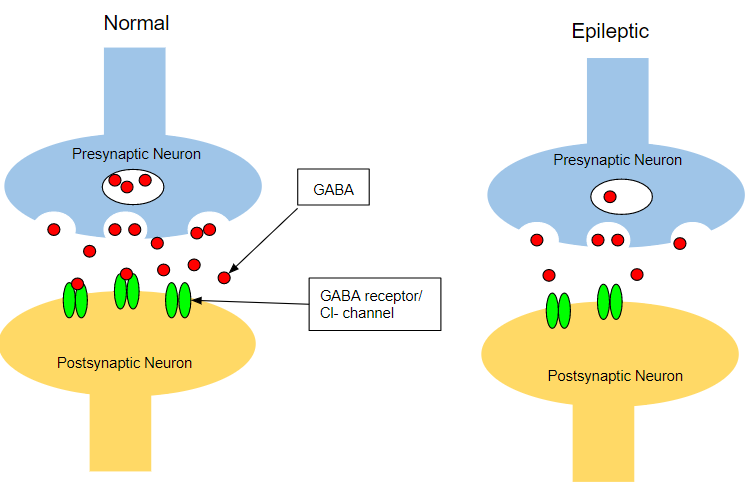
Decreased Inhibition
Deviations in normal GABA synthesis and release has been shown to produce epileptic symptoms. There has been observed abnormalities in GABAergic function found in both genetic and acquired epilepsy. Decreased GABA-mediated inhibition has been observed in epilepsy. There is defective GABA synthesis in humans with genetic epilepsy, which is characterized by seizures in infants (Treiman, 2012). GABA-receptor densities have been shown to be decreased in people with epilepsy. Furthermore, it was also shown that GABA release in epileptic patients were reduced when compared to non-epileptic people. In a particular study, it was shown that there are two main mechanisms in which GABA can be released into the synaptic cleft (Treiman, 2012). The first being vesicular fusion mediated by Ca2+ influx (discussed earlier). The second is non-vesicular secretion which is regulated via Na+ influx. When comparing an epileptic hippocampus to a non-epileptic hippocampus, it was observed that non-vesicular release of GABA in the epileptic hippocampus was not as robust as the control, resulting in a decreased amount of GABA in the synaptic cleft and by extension a decrease in inhibitory effects on postsynaptic neurons (Treiman, 2012).
Treatments
Antiepileptic Drugs (AEDs)
Antiepileptic drugs decrease the membrane excitability via interaction with neurotransmitter receptors or ion channels such as sodium, calcium, or potassium. For example, AEDs that were developed before 1980 acted on sodium channels, GABA receptors (inhibitory), or calcium channels. Drugs such as benzodiazepines and barbiturates enhance GABA receptors to inhibit HFS and activation of neurons. Some drugs (CBZ) mechanism of action is theorized to inhibit the HFS that is sustained via sodium action potentials (Macdonald et al, 1995).
Gabapentin is one of the four clinically FDA approved drugs in the United States that has proven to have relatively significant effects. The mechanism of action of gabapentin is not fully understood, but its distinct profile of anticonvulsant activity in animal seizure models and its lack of activity at many drug binding sites associated with other antiepileptic drugs indicate that its mechanism of action is novel. Gabapentin has no effect on the GABA receptors uptake but instead, it will interact with a high affinity binding site in the brain membranes: the auxiliary subunit of voltage-sensitive Ca2+ channels. Investigation in rat cortical neurones reported inhibitory effects of GBP on high voltage-activated calcium channels and a specific interaction (Graeme et al, 2006). Gabapentin crosses several lipid membrane barriers via L amino acid transporters. The L amino transport system is responsible for the absorption of the drug from the small intestine and expressed at the blood brain barrier. Once transported, it will increase GABA synthesis as indicated by NMR spectroscopy. The increased non-synaptic GABA responses and the reduced release of several mono-amine neurotransmitters are the main results of clinical effects for the treatment of epilepsy.
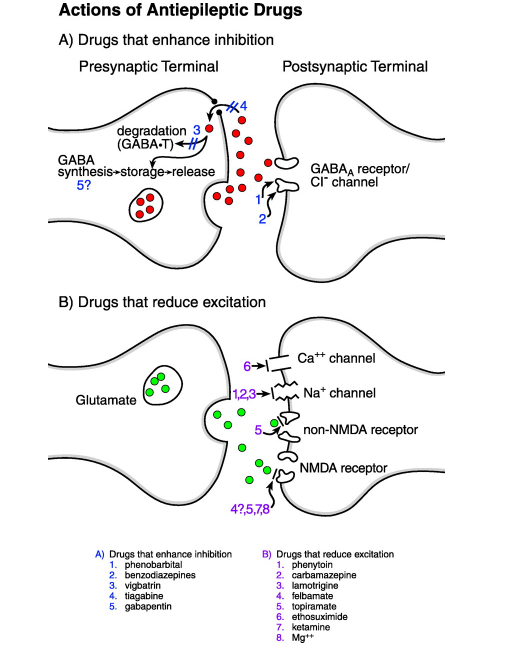
Antiepileptic Drugs (AEDs) Mechanism
Antiepileptic drugs (AEDs) can target either the inhibitory system or excitatory system. Both Phenobarbital (PHB) and Benzodiazepines (BZD) can improve inhibition by allowing Cl- to flow into the GABA receptors by either increase duration of chlorine channel opening (BZD) or increase the frequency of opening (PHB). Vigbatrin can act to decrease GABA degradation by inhibiting GABA degradatory enzymes. Furthermore, Tiagabine (TGB), with also increase inhibitory effects of GABA by preventing reuptake into the presynaptic neuron (Stafstrom 1998).
AEDs also will affect excitation. Phenytoin, carbamazepine, and Lamotrigine all block voltage gated sodium channels, resulting in a reduction of continuation of electric signal. Furthermore, Ethosuximide will block calcium channels to prevent the post synaptic neuron from firing. Felbamate, ketamine, and Mg++ will block NMDA receptors (which bind to glutamate) to prevent an excitatory signal, while topiramate can block both NMDA and non- NMDA receptors to produce the same effect (Stafstrom 1998).
 Vagus Nerve Stimulation (VNS)
Vagus Nerve Stimulation (VNS)
It is a novel therapy that has become available for epilepsy. This is used for patients with partial onset with or without secondary generalization after the age of 12. It is basically a system compromised to battery generators which send intermittent electrical stimuli which is controlled by software and an interrogating wand. The generator is implanted in the left upper chest which connects to the left cervical vagus nerve using two semi-circular helical electrodes. It takes an approximate of 2 hours to complete under general anesthesia. There are some effects that are present during VNS such as vice alteration and tingling sensation in the throat. The intensity of the effects starts to decrease over several weeks. This is one of the less invasive methods for anti-epilepsy. This is however not a replacement for resection surgery which is known to produce seizure-free effects in patients with a chance of 70-90%.
(Uthman, 2000)
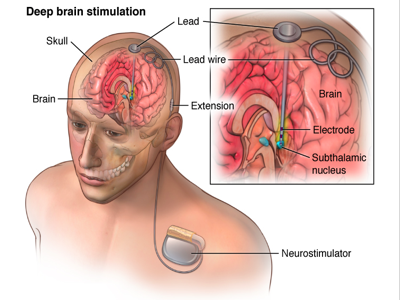
Deep Brain Stimulation (DBS)
Brain stimulation uses intraoperative stimulation to map epileptogenic focus in relative to specific regions of the brain. Its purpose is to stimulate areas of the brain that play a role in the inhibition of seizure activity and its spreading. The caudate plays such a role, discovered after various research experiments on different parts of the brain including locus coeruleus (brainstem) and the corpus callosum. Other successful regions for DBS include the hippocampus, sub thalamic nucleus, posterior hypothalamus and the anterior nucleus of thalamus.
The mechanism behind DBS remains limited, but there are some proposed mechanisms supported by limited data. DBS occurs at the level of individual neurons, synapses, and neuronal networks. The stimulation depends on the response of the neuron which is based on its orientation, membrane resistance, and conductance. They primarily rely on stimulation parameters which either results in excitation or inhibition in the proximal region of stimulation. DBS blocks epileptiform patterns via shifts of potassium from inside neurons to the extracellular space, causing a temporary refractory period for neuronal firing. The sub thalamic nucleus and ventral intermediate nucleus act as inhibitory lesions. However, both excitation and inhibition depend on the stimulation of neuronal networks. DBS is slowly reaching clinical utility as more research is being done (Dostrovsky, 2002).
There are four general hypotheses behind the mechanism of DBS: Depolarization blockade, Synaptic inhibition, Synaptic depression, and Stimulation-induced disruption of pathological network activity. It is theorized that they show suppression of somatic activity but high-frequency axonal output. Despite producing HFS (high frequency stimulation) which would be theorized to increase epileptic effects, one possibility is that neurons activated by the stimulus train are unable to sustain the HFS on efferent targets because of a depletion in neurotransmitters. Therefore, a more explanatory theory is that there exists two volumes of activation induced by stimulation-induced disruption of pathological network activity (STN).The smaller volume 1 represents the activation of projection neurons. The larger volume 2 represents the activation of afferent inputs. The difference of these two volumes represents a volume of projection neurons, subthreshold for direct activation by the applied field, suppressed by the stimulation induced trans-synaptic inputs. However, most projection neurons within volume 2 will exhibit suppression of somatic firing independent of their efferent output. Despite DBS being able to override pathological activity patterns of epilepsy, the patterns induced are not considered normal (Cameron, 2004).
Figure_:
Brain Surgery
The goal of surgery is to find the epileptogenic focus which is not eloquent cortex and resect it without causing any neurological deficits. The most common focus point in adults is the temporal lobe (mainly the hippocampus). There are many ways to discover the area of epileptogenic focus: Video and scalp EEG, fMRI, MEG (magnetoencephalography), or seizure semiology. Surgery can be divided into either palliative or curative procedures. Curative procedures include lesional resection, lobectomy, and multiple subpial transections. For the treatment of temporal love epilepsy a gamma knife radiosurgery is used.
The main goal of curative surgery is to allow the patient to fully alleviate epileptic causes and no need of AEDs. An early surgery is favourable as the patients will not experience all the problems associated with epilepsy including cognitive, behavioural, and psychological. This may help the patient in their social interactive skills. Also, subsequent seizures result in neurological damage over time.
Palliative procedures only very rarely result in cessation of seizures. Palliative surgeries are used to prevent the occurrence of life threatening epilepsy types such as drop attacks or reduce the frequency and severity of the seisures. Examples include hemispheric surgery and disconnection procedures including corpus colostomy.
Surgery is either used to define and resect an area of epileptogenic focus or disrupt the spread of seizure activity which in turn reduces the likelihood of seizures. Electrodes are used to record from the surface of the brain. One of the most common procedures is for medial temporal lobe epilepsy where the hippocampus is the main target for surgery.
(Bromfield, 2006) (Kawai, 2015)
References
Banerjee, P. N., Filippi, D., & Hauser, W. A. (2009). The descriptive epidemiology of epilepsy-a review. Epilepsy Research, 85(1), 31–45. http://doi.org/10.1016/j.eplepsyres.2009.03.003
Bhalla, D., Godet, B., Druet-Cabanac, M., Preux, P.M. (2011). Etiologies of epilepsy: a comprehensive review. Expert Review of Neurotherapeutics. 11(6):861-76 https://journals-scholarsportal-info.libaccess.lib.mcmaster.ca/pdf/14737175/v11i0006/861_eoeacr.xml
BRADFORD, H.F. (1995). Glutamate, GABA and epilepsy. Prog. Neurobiol., 47, 477–511.
Bromfield EB, Cavazos JE, Sirven JI, editors. An Introduction to Epilepsy [Internet]. West Hartford (CT): American Epilepsy Society; 2006. Chapter 1, Basic Mechanisms Underlying Seizures and Epilepsy.Available from: https://www.ncbi.nlm.nih.gov/books/NBK2510/
Cho, C.-H. (2013). New mechanism for glutamate hypothesis in epilepsy. Frontiers in Cellular Neuroscience, 7, 127. http://doi.org/10.3389/fncel.2013.00127
Dostrovsky, J. O. and Lozano, A. M. (2002), Mechanisms of deep brain stimulation. Mov. Disord., 17: S63–S68. doi:10.1002/mds.10143
Epilepsy Support Centre. (2015). Diagnosing Epilepsy. Available at: http://epilepsysupport.ca/seizure-education/about/diagnosing
Fisher, R.S. (2017). The New Classification of Seizures by the International League Against Epilepsy 2017. Current Neurology and Neuroscience Reports, 17(48). https://doi.org/10.1007/s11910-017-0758-6
Gilmour, H., Ramage-Morin, P., & Wong, S.L. (2016). Epilepsy in Canada: Prevalence and impact. Statistics Canada. http://www.statcan.gc.ca/pub/82-003-x/2016009/article/14654-eng.htm
Goldberg, E. M., & Coulter, D. A. (2013). Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nature Reviews. Neuroscience, 14(5), 337–349. http://doi.org/10.1038/nrn3482
Kawai, K. (2015). Epilepsy Surgery: Current Status and Ongoing Challenges. Neurologia Medico-Chirurgica, 55(5), 357–366. http://doi.org/10.2176/nmc.ra.2014-0414
Macdonald, R. L. and Kelly, K. M. (1995), Antiepileptic Drug Mechanisms of Action. Epilepsia, 36: S2–S12. doi:10.1111/j.1528-1157.1995.tb05996.x
McIntyre, C.C., Savasta, M., Kerkerian-Le Goff, L., Vitek, J.L. (2004). Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clinical Neurophysiology. 115(6); 1239-1248.https://doi.org/10.1016/j.clinph.2003.12.024.
Moshé SL, Perucca E, Ryvlin P, and Tomson T. (2015). Epilepsy: new advances. Lancet 385:884–898.
Scharfman, H. E. (2007). The Neurobiology of Epilepsy. Current Neurology and Neuroscience Reports, 7(4), 348–354.
Shorvon SD. The etiologic classification of epilepsy. Epilepsia 2011; 52(6): 1052-7. Banerjee, P. N., Filippi, D., & Hauser, W. A. (2009). The descriptive epidemiology of epilepsy-a review. Epilepsy Research, 85(1), 31–45. http://doi.org/10.1016/j.eplepsyres.2009.03.003
Sills, G.J.(2006). The mechanisms of action of gabapentin and pregabalin. Current Opinions in Pharmacology.6(1);108-126. https://doi.org/10.1016/j.coph.2005.11.003. (http://www.sciencedirect.com/science/article/pii/S1471489205001906)
Stafstrom, C.E. (1998). Back to Basics: The Pathophysiology of Epileptic Seizures: A Primer For Pediatricians. Pediatrics in Review, 19(10).
Treiman DM.(2001). GABAergic mechanisms in epilepsy. Epilepsia;42:Suppl 3:8-12
World Health Organization. Epilepsy. Fact Sheet No. 999. Geneva: World Health Organization, 2015. Available at: http://www.who.int/mediacentre/factsheets/fs999/en/index.html
Uthman, B. M. (n.d.). (2000). Vagus nerve stimulation for seizures. Retrieved October 27, 2017, from https://www.ncbi.nlm.nih.gov/pubmed/11036181