Table of Contents
What is Cloning?
Clones are referred to as organisms that are exact genetic copies of each other. To say the least, every single piece of their DNA sequence is identical (“Cloning LiteratureWatch '', 2000). Cloning can occur both naturally and artificially. We observe many examples of natural cloning around us, for example bacteria reproducing asexually through the process of binary fission is an example of cloning (Noh & Neumann, 2001). Our cells undergo cloning everyday through mitosis. And lastly, another really cool example of cloning are identical twins. All these events have inspired scientists to experiment cloning through artificial means. Science has gone from cloning single cells to entire organisms and advancements continue to happen today (Noh & Neumann, 2001). The three types of artificial cloning that we will be discussing today include reproductive cloning, therapeutic cloning and gene cloning (Noh & Neumann, 2001).
History
The idea of cloning began as early as 5000 B.C. when humans discovered that the quality of corn crops could be improved by planting seeds from the best plants (Cloning’s Historical Timeline, n.d.). However, the first artificial inducement of natural cloning didn’t occur until 1902, when Hans Spemann, a German scientist, divided a salamander embryo in two to show how early embryo cells could retain all genetic information needed to create a new organism (Cloning’s Historical Timeline, n.d.). In 1938, he proposed a “fantastical experiment” of transferring a cell’s nucleus into an egg without a nucleus - this would eventually be the basic method used in cloning (Cloning’s Historical Timeline, n.d.). Forty years later, in 1978, David Rorvick released a book called In His Image: The Cloning of a Man; this ignited sparks around the world regarding the debate on cloning ethics (Cloning’s Historical Timeline, n.d.).
Although most people associate the first cloned mammal with Dolly the sheep, many mammals were cloned before her. For example, in 1984, Steen Willadsen, a Danish scientist, created a genetic copy of a lamb from early sheep embryo cells through a process known as twinning (Cloning’s Historical Timeline, n.d.). Twinning is a process that leads to the production of more than one offspring at birth (“Twinning”, n.d.). Another example is in 1986, First, Prather, and Eyestone cloned a cow from embryo cells (Cloning’s Historical Timeline, n.d.). Following, in 1995, Ian Wilmut replicated their experiment using differentiated cells from sheeps (Cloning’s Historical Timeline, n.d.). However, he put the embryo cells into an inactive state before transferring their nuclei to sheep eggs, as a result, the eggs developed into normal lambs. Finally, in 1996, the most famous cloned animal, Dolly - the sheep, was born into the world (Cline, 2019). She was the first animal to be cloned from adult cells, however, her birth was not announced until 1997 (Cline, 2019). It was also in 1997, when the scientist who produced Dolly announced Polly, a lamb created with a human gene in every cell of its body (Cloning’s Historical Timeline, n.d.).
All the mammals cloned previously had been female, but in 1999, researchers from the University of Hawaii produced the first male cloned mouse - Fibro (Cloning’s Historical Timeline, n.d.). Then, in 2001, the first human clone embryo was produced (Cloning’s Historical Timeline, n.d.). This was for the purpose of embryonic stem cell harvesting and not for cloning, however, the stem cells stopped dividing before they could be harvested. Twelve years later, in 2013, human stem cells were successfully created.
Artificial Cloning
Reproductive Cloning of organisms
The two methods of artificially creating clones include Embryonic Splitting and Somatic Cell Nuclear Transfer (SCNT).
Embryonic splitting
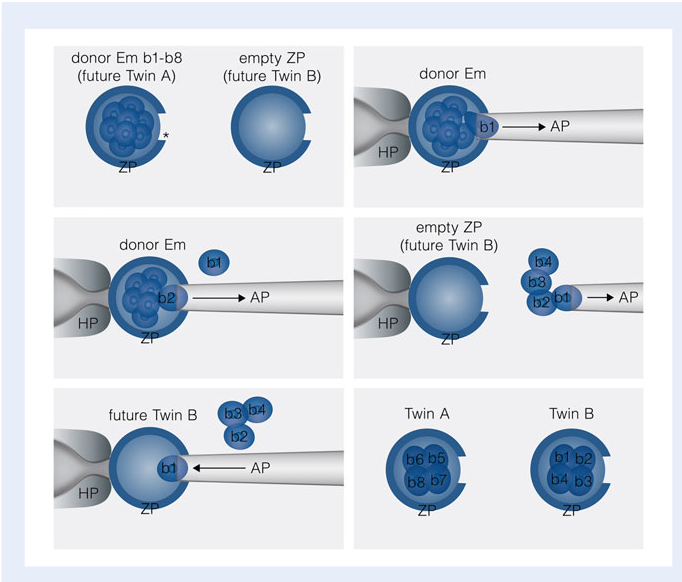
This technique is inspired by the formation of identical twins in organisms. This was one of the initial approaches towards creating clones artificially (Keefer, 2015). Embryonic splitting can be further broken down into two different procedures called the blastomere splitting for cleavage stage, or bisection if the fertilized egg has reached the blastocyst or morula stage. Embryonic splitting is a simpler method of creating clones, however it results in only a limited number of clones. The limitation is due fact that the initial stages of division following fertilization involve cell division without any significant overall cell growth. Thus, too much splitting can lead to a loss of mass necessary to give rise to a viable organism (Keefer, 2015).
Blastomere splitting involves the separation of blastomeres from an early embryo to form two or more embryos. The process involves removal of one or more blastomeres from a cleavage stage embryo followed by insertion into an empty Zona Pellucida (the protective membrane of an oocyte). Prior to insertion through the Zona Pellucida, the donor blastomere is treated with acidified tyrode’s solution to make an opening in the donor cell Zona Pellucida. Then using an aspirating pipette the blastomere is removed and then inserted into a separate empty Zona Pellucida (Noli, Ogilvie, Khalaf and Ilic, 2017).
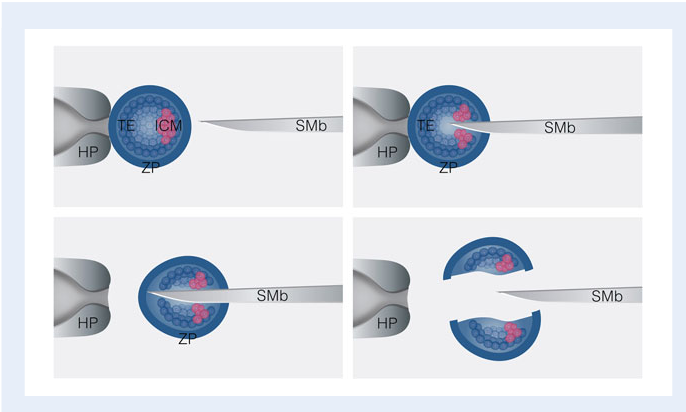
Another method of performing embryonic splitting is by performing a bisection on post-compacted embryo. An embryo in the post-compacted stage is differentiated into an inner cellular mass (ICM) and the trophoblast which eventually becomes the placenta. . Bisection involves precisely cutting the embryo and thus the ICM in half using a surgical microblade (Noli et al, 2017).
Somatic Cell Nuclear Transfer (SCNT)
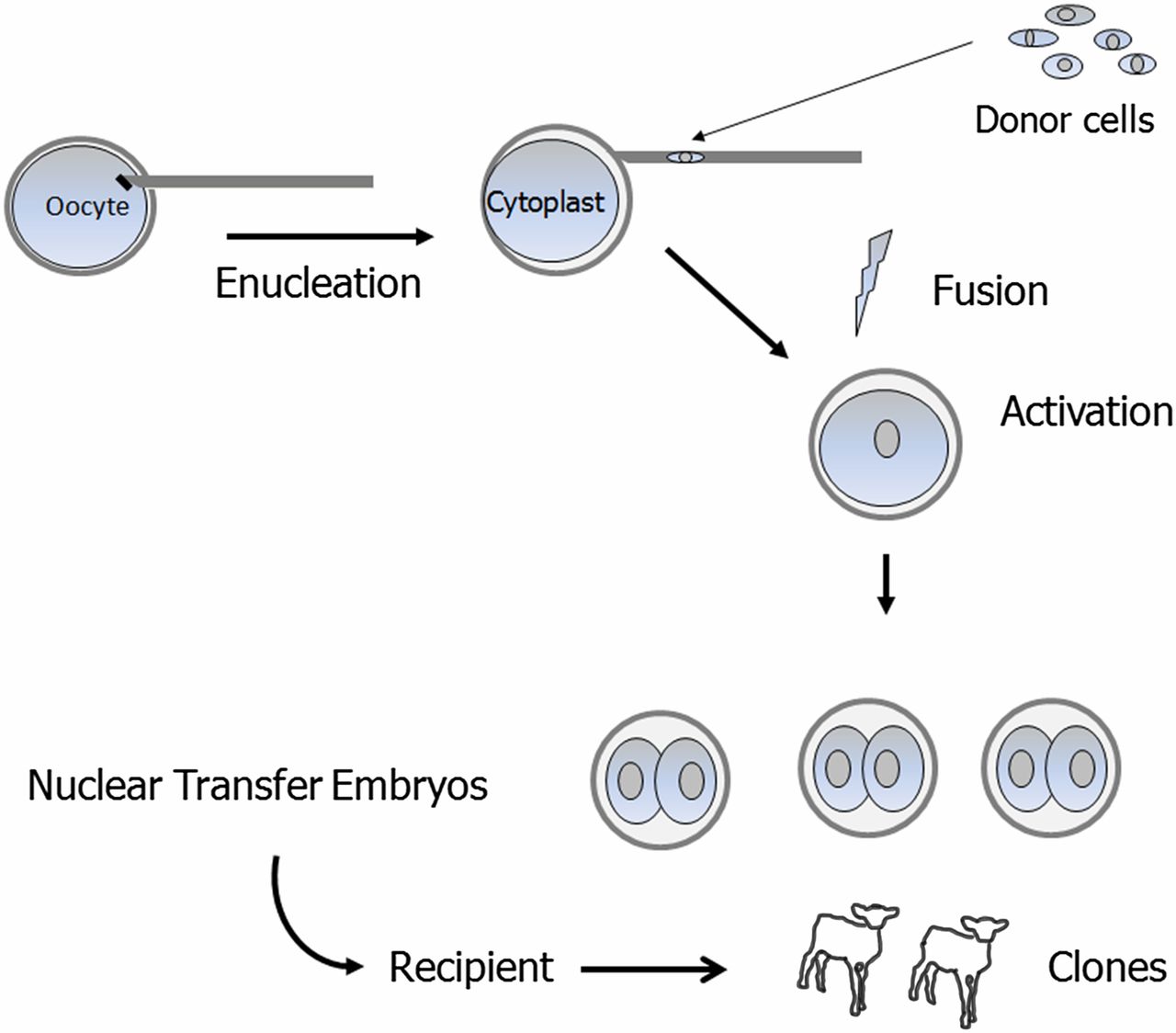
Compared to embryonic splitting, theoretically this method should be capable of producing multiple clones as it does not involve any loss of mass, however further improvements are required to reach a significant success rate. The first stage of SCNT involves preparation of the somatic cell, typically a skin fibroblast as it is easy to access and is less invasive and painful (Wilmut, Schnieke, McWhir, Kind and Campbell, 1997). To retrieve the cells, a tiny amount of skin is obtained and placed in a trypsin enzyme buffer solution that allows the cell to detach from its surrounding extracellular matrix. The cell is then placed in a serum medium for three weeks to obtain a single layer containing only fibroblasts. Once the skin fibroblasts are cultured, the nucleus or karyoplast is isolated to be later inserted in an egg without a nucleus.The second stage involves the preparation of an oocyte. To do this, Human Chorionic Gonadotropin Hormone (hCG) is used to induce ovulation (French et al, 2008). The ovulated egg is extracted and the egg’s DNA is fluorescently labelled to assist with efficient removal (Heindryckx, De Sutter, Gerris, Dhont and Van der Elst, 2007). Once the nucleus is removed from the oocyte, it’s referred to as a cytoplast. The karyoplast obtained from the skin fibroblasts is transferred into the cytoplast. Electrical pulses are then used to induce fusion between the karyoplast and the cytoplast which results in an activated oocyte. The oocyte contains factors or chemicals that allow it to reprogram the genetic information in the somatic nucleus to induce pluripotency. The oocyte is then implanted in a surrogate mother who carries the pregnancy until delivery (Niemann & Lucas‐Hahn, 2012).
Human Cloning
Several claims have been made in the past, but all without evidence. Human cloning is scientifically a more complicated and sensitive process, with many ethical concerns attached to it. It is also currently banned in many countries (“Cloning Fact Sheet”, 2017). Scientifically speaking, it is quite challenging to ensure that the human clones created would lead a normal, healthy life. Clones of other organisms created in the past have faced a lot of health challenges pre-natally and post-natally and many die at a young age. Thus, similar concerns regarding human cloning pose an ethical challenge.
Current advancements and Lifespan
Over 20 species of mammals have been cloned thus far including pigs, goats, rabbits, sheep, cattle and even two rhesus monkeys (Greshke, 2018). The lifespan of these species varies and many are afflicted with abnormalities leading to either abortion or an early death after birth (Burgstaller & Brem, 2017). These abnormalities include increased birth size, organ defects, premature aging and issues in the immune system. Despite these anomalies, certain clones of goats, and mice have been able to survive the maximum lifespan for their species. Some argue that the abnormalities observed could be due to factors like telomere aging and accumulated DNA damage in the donor DNA, however, it has also been observed that oocyte reprogramming is capable of restoring telomere length and other abnormalities (Burgstaller & Brem, 2017). Hence, it could be speculated that the probability of cloning a healthy organism depends on the ability of these occyte factors to fully reprogram the genetic information. Despite these anomalies, certain clones of goats, and mice have been able to survive the maximum lifespan for their species. Currently, the longest living cloned organism is a goat in China called Yangyang cloned in 2000 and according to recent news is still alive (Burgstaller & Brem, 2017).
Transgenic organisms can also be cloned via somatic cell nuclear transfer. Organisms containing foreign DNA, that makes their traits desirable can be cloned using the SCNT method. The Scottish researchers responsible for cloning Dolly the sheep, also genetically modified and cloned other sheep to produce a human clotting factor protein, bio-thrombin in its milk in the hopes of providing treatment for individuals lacking the protein. Other experiments have been performed with transgenic clones containing genes for industrial proteins as well as human polyclonal antibodies (“Cloning Fact Sheet”, 2017).
Furthermore, cloned animals have also been used for clinical trials, allowing scientists to observe uniform responses for a given genetic makeup since all model organisms are genetically identical. In 2008, FDA approved meat and milk from cloned animals as safe to consume, which allows scientists to freely clone organisms with the most desirable traits, however, due to the cost associated with cloning it will still take some time for these products to become available to the public (“Cloning Fact Sheet”, 2017).
Other Types of Artificial Cloning
Reproductive cloning, therapeutic cloning and gene cloning are three types of artificial cloning. Reproductive cloning produces an animal that is genetically identical to the donor animal (Cloning Fact Sheet, 2017). This is done through somatic cell nuclear transfer (SCNT). Reproductive cloning involves implantation of a cloned embryo into a uterus (Rugnetta, 2019). This allows the embryo to develop into a fetus. This type of cloning experiment was performed for over 40 years through embryo splitting (Rugnetta, 2019). In the 1990s, reproductive cloning saw major changes. This was after the birth of “Dolly the sheep”. Dolly was generated through SCNT (Rugnetta, 2019). Reproductive cloning using SCNT is considered to be very harmful because fetuses of embryos cloned through this process are usually born with birth defects. Also, fetuses born this way rarely survive gestation (Rugnetta, 2019).
In therapeutic cloning, embryo is created in a similar way to reproductive cloning but the difference is that cloned cells are not implanted into the uterus (Cloning Fact Sheet, 2017). Therapeutic cloning is used to extract stem cells from the embryo (Cloning Fact Sheet, 2017). This type of cloning allows the cultivation of stem cells that are genetically identical to the patient. Stem cells can differentiate into different cell types (Rugnetta, 2019). Then, these differentiated cells can be transplanted into the patient. This transplantation helps to replace damaged cells without the risk of rejection (Rugnetta, 2019). These cells are able to treat diseases such as Parkinson, Alzheimer and stroke. Stems cells can also be used to test drugs (Rugnetta, 2019). The generation of stem cells from cloned embryo primates has been very difficult. In 2007, stem cells derived from cloned macaque embryo differentiated into heart cells and neurons (Rugnetta, 2019). This experiment started with 304 eggs but only two lines of stem cells were developed. One of these stem cells had an abnormal Y chromosome (Rugnetta, 2019). Progress in research on therapeutic cloning in humans has been slow compared to reproductive cloning in animals because of ethical reasons and technical challenges (Rugnetta, 2019).
Gene cloning that is also known as DNA cloning is a completely different process from reproductive and therapeutic cloning. This type of cloning produces identical copies of a particular piece of DNA (Cloning Fact Sheet, 2017). In this process, the DNA fragment of interest is first inserted into a plasmid. This produces a molecule of recombinant DNA (Cloning Fact Sheet, 2017). Then, the recombinant plasmid is introduced into bacteria. Bacteria that are carrying the plasmid grow and as they reproduce, they replicate the plasmid and pass it to their offspring (DNA cloning, 2007).
Cloning extinct and endangered species
Although much research is still being conducted in regards to cloning extinct and endangered species, many attempts have been done in the past by researchers (White, 2000). One of the first exposures was in 2003 when the first extinct-animal clone was created (White, 2000). How was it exactly created? A sample of frozen skin was extracted to clone a bucardo, also known as Pyrenean ibex (White, 2000). This was a subspecies of the spanish ibex that went extinct originally in 2000 (White, 2000). Even though it was one of the successful attempts, the bucardo died shortly after birth (White, 2000). However, upon examination, the process was tedious to say the least. The scientists first cloned the embryos by inserting the bucardo's DNA into domestic goat eggs removed from their original genetic material (“Cloning LiteratureWatch”, 2000). Initially, researchers implanted about 208 embryos, of which only 7 goats became pregnant, and 1 survived (“Cloning LiteratureWatch”, 2000). Through further investigation, researchers noted that the ibex eventually died from respiratory failure due to significant lung abnormalities right after its birth (“Cloning LiteratureWatch”, 2000).
Other researchers soon followed and cloned two primates using the same technique that gave rise to Dolly the sheep (Wilmut, 1999). Being the first time in history in which primates have been cloned in an effective and appropriate manner, this outlined a major achievement in cloning history (Wilmut, 1999). After years-long experimentation, the chinese researchers named the two female macaques Zhong Zhong and Hua Hua, are genetically identical and were born after a years-long effort (Wilmut, 1999). This discovery along with other milestones gave hope and for replicating the same technique in endangered species (Noh & Neumann, 2001).
One of the first instances where scientists tried to clone an endangered species involved a species known as Bos gaurus, an asian ox with a declining population (Noh & Neumann, 2001). Genetic engineers retrieved gaur somatic cells, used an embryo of a domestic cow as well as had another cow for surrogate purposes (Seidel, 2004). This was one of the first cloning procedures that occurred using the SCNT approach (Seidel, 2004). Like the ibex as mentioned previously, the gaur clone, named Noah, also died after two days due to dysentery (Seidel, 2004). When examining previous research, it would be safe to say that a mixed success overall for cloning extinct species has occurred. When it comes to cloning domestic animals, the chances are better but not by much (Wilmut, 2001). Researchers are often found using interspecies SCNT when cloning domestic animals (Wilmut, 2001). But, only a few reported cases have been successful in producing offspring as most tend to not thrive in the current environment or die relatively early due to other bodily issues (Saadeldin, 2015). Currently, there is a major debate that considers the pros and cons surrounding the ethics of cloning endangered species. One side argues that cloning will provide a great opportunity to create desirable traits, additional research benefits, and restore lost species as well as help to eradicate problematic diseases widely occurring across the world (Saadeldin, 2015). However, cloning endangered species has also proved to be expensive and ineffective even though science has progressed greatly (Saadeldin, 2015). Not only that but it also is a factor in the reduction of genetic diversity (Wilmut & Dominko, 2000).
Ethical issues
This cloning debate involves scientists, legislators, religious leaders, philosophers and international Organizations, not always harmoniously (Maclin, 2005).
Science, technology and innovation are at a constant growth for the field of cloning. However for decades now questions about the ethics behind human cloning continue to grow. Questions such as: “How do genes, and different forms of genetic intervention (gene therapy, genetic enhancement, genetic diagnosis of preimplantation embryos, and so on) affect the identities of the people who already exist and those we bring into existence? How do these interventions benefit or harm the people we bring into existence in the near future and those who will exist in the distant future? Are we morally obligated to prevent the existence of people who would be severely diseased and disabled? Do the claims of people who will exist in the distant future have less moral weight than the claims of those who will exist in the near future? Would genetic enhancement of cognitive, physical, and emotional capacities to raise them above the normal level of functioning for people be morally objectionable, and if so, on what grounds? How could we ensure equal and fair access to both therapeutic and enhancement genetic technologies for all people?” (Glannon, 2019).
The birth of Dolly the sheep, on July 5th 1996 using the cloning technique referred to as somatic cell nuclear transfer raised a lot of concerns by the public, which were amplified largely by fictional content (Shaprio, 1997). Immediately, after the publication of the cloned sheep’s report, President Clinton placed a ban on research and funding for cloning humans using the somatic cell nuclear transfer. Additionally, President Clinton appointed the National Bioethics Advisory Commission (NBAC) to report on the ethical and legal issues regarding cloning humans within 90 days.
Dolly, at the age of 6.5 years, was put down in 2003 due to her development of progressive lung disease and premature arthritis. Similarly so were other cloned animals, these high failure rates (more than 90%) and high morbidity rates suggests that this is inapplicable to humans (Maclin, 2005).
As news of successfully cloned animals increased, it began to catch the attention of the general public, however, scientists are far from controlling the results. Success rates were continuing to remain low, and successful clones were being born with a wide range of abnormalities and defects (“Human Cloning: ethical issues”, 2005). If we were to do this to humans, is it moral?
One of the biggest concern comes from the basic fact that cloning would allow humans to procreate in an asexual manner which would give us the capacity for complete control over the genetic profile of our children. Upon further investigation fears about harm to the children's psychology and personality were revealed (Shapiro, 1997). Majority of these fears arise from opponents and their concerns that the technology is not yet safe enough and could be abused (AAAS Scientific Responsibility, Human Rights and Law Program, 2002). In addition to being harmful to the child itself, some expressed a concern for its negative effects on important social values. Social concerns such as how these cloned individuals will be able to integrate themselves within their societies and families (McGee, 2000). As this somatic cell nuclear transfer cloning would open doors to a form of eugenics or tempting some to manipulate others as if they were objects instead of people with emotions and lives (Sapiro, 1997). Religion also played a role in the ethics of human cloning. Many religious individuals are against the technology because it is taking ‘God’s Place’ and because this will be leading to destroying a human life (in this case an embryo) (Sullivan, 2003). However, there are a few religious groups who support therapeutic cloning because of its potential life-saving benefits (Bainbridge, 2003).
The First Human Cloned Embryo
Jose B. Cibelli, Robert P.Lanza and Michael D.West, with Carol Ezzel.
This study was performed to hopefully, in the future be able to offer therapeutic cloning or cell therapy arising from parthenogenesis to sick patients. The first human embryos produced using the technique of nuclear transplantation. This study helped demonstrate that the goal of therapeutic cloning is within reach. Therapeutic cloning which seeks, for example, uses genetic material from patients’ own cells to generate pancreatic islets to treat diabetes or nerve cells to repair damaged spinal cords . Note this is different from reproductive cloning: implantation of a cloned embryo into a women’s uterus leading to birth of a cloned baby
Steps:
- Consulted ethics advisory board, independent ethicists, lawyers, fertility specialist and counselors
- Recruited women willing to contribute eggs to be used in the cloning procedure and also collect cells from individuals to be cloned (the donors)
- Women where between the ages of 24 and 32 who have at least one child
- Potential egg contributors to go through psychological and physical tests, including screening for infectious diseases, to ensure that women were healthy and that contributing eggs would have no effects on them
- The timing of collecting eggs was important, they depended on the menstrual cycles of women and injected them with hormone injections for several days, therefore, they ovulate 10 or so eggs at once instead of the normal one or two.
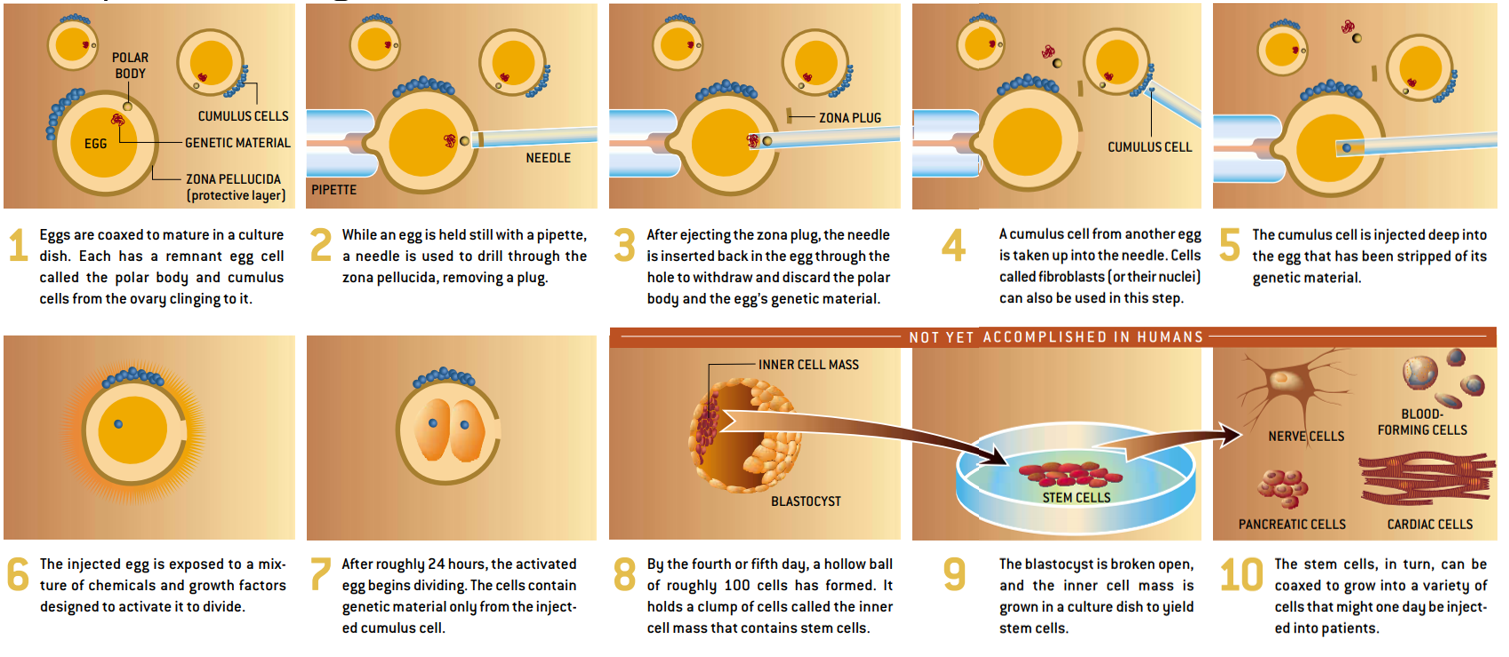
- Began nuclear transfer technique, in which they used an extremely fine needle to suck the genetic material from a mature egg
- Following this, they inject the nucleus of the donor cell (sometimes a whole cell) into the enucleated egg and incubate it under special conditions that prompt it to divide and grow
- It took 71 eggs from seven volunteers before they could create their first cloned early embryo.
- Of the eight eggs we injected with cumulus cells, two divided to form early embryos of four cells—and one progressed to at least six cells—before growth stopped.
Link to powerpoint presentation: https://1drv.ms/p/s!AkW_XMx1tFYxumquXn_Knr16kTFC?e=fGa3br
References
AAAS - AAAS Scientific Responsibility, Human Rights and Law Program. (2002, February 14). Retrieved February 12, 2020, from https://web.archive.org/web/20120911093343/ http://www.aaas.org/spp/sfrl/docs/cloningstatement.shtml
Ayala, F. J. (2015). Cloning humans? Biological, ethical, and social considerations. Proceedings of the National Academy of Sciences, 112(29), 8879–8886. Doi: 10.1073/pnas.1501798112
Bainbridge, W. (2003). Religious Opposition to Cloning. Journal of Evolution and Technology, 13. Retrieved from https://www.jetpress.org/volume13/bainbridge.html
Burgstaller, J. P., & Brem, G. (2017). Aging of cloned animals: A mini-review. Gerontology, 63(5), 417-425.
Cline, A. (2019, March 23). The Science of Cloning Over Time. Retrieved February 7, 2020, from https://www.learnreligions.com/timeline-of-cloning-history-4070856
Cloning Fact Sheet. (2017, March 21). Retrieved from https://www.genome.gov/about- genomics/fact-sheets/Cloning-Fact-Sheet
Cloning’s Historical Timeline. (n.d.). [PDF file]. Retrieved February 7, 2020, from https://www.nsta.org/images/news/legacy/scope/0603/cloningtimeline.pdf
Cibelli, J. B., Kiessling, A. A., Cunniff, K., Richards, C., Lanza, R. P., & West, M. D. (2001). Rapid communication: somatic cell nuclear transfer in humans: pronuclear and early embryonic development. e-biomed: the Journal of Regenerative Medicine, 2(5), 25-31.
DNA cloning. (2007, November 28). Retrieved from https://www.sciencelearn.org.nz/resources/2031-dna-cloning
Dominko, T., Mitalipova, M., Haley, B., Beyhan, Z., Memili, E., McKusick, B., & First, N. L. (1999). Bovine oocyte cytoplasm supports development of embryos produced by nuclear transfer of somatic cell nuclei from various mammalian species. Biology of reproduction, 60(6), 1496-1502.
French, A. J., Adams, C. A., Anderson, L. S., Kitchen, J. R., Hughes, M. R., & Wood, S. H. (2008). Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem cells, 26(2), 485-493.
GLANNON, W. A. L. T. E. R. (2019). Genes And Future People: philosophical issues in human genetics. Retrieved from https://content.taylorfrancis.com/books/download? dac=C2017-0-71243-0&isbn=9780429968785&format=googlePreviewPdf
Greshko, M. (2018, January 24). Monkey Clones created in the lab. Now What? Retrieved from https://www.nationalgeographic.com/news/2018/01/monkey-clones-dolly-sheep-china-m edicine-science/
Heindryckx, B., De Sutter, P., Gerris, J., Dhont, M., & Van der Elst, J. (2007). Embryo development after successful somatic cell nuclear transfer to in vitro matured human germinal vesicle oocytes. Human Reproduction, 22(7), 1982-1990.
Human cloning: ethical issues. (2005). Retrieved from https://unesdoc.unesco.org/ark:/48223/ pf0000135928
Keefer, C. L. (2015). Artificial cloning of domestic animals. Proceedings of the National Academy of Sciences, 112(29), 8874-8878.
Macklin, R. (2005). Yet Another Guideline? The Unesco Draft Declaration. Developing World Bioethics, 5(3), 244–250. doi: 10.1111/j.1471-8847.2005.00122.x
McGee, G. (2000). The perfect baby: parenthood in the new world of cloning and genetics. Lanham, Mar.: Rowman & Littlefield.
Niemann, H., & Lucas‐Hahn, A. (2012). Somatic cell nuclear transfer cloning: practical applications and current legislation. Reproduction in Domestic Animals, 47, 2-10.
Noli, L., Ogilvie, C., Khalaf, Y., & Ilic, D. (2017). Potential of human twin embryos generated by embryo splitting in assisted reproduction and research. Human reproduction update, 23(2), 156-165.
Rugnetta, M. (2019, December 12). Reproductive cloning. Retrieved from https://www.britannica.com/science/cloning/Reproductive-cloning
Shapiro, H. T. (1997). Ethical and Policy Issues of Human Cloning. Science, 277(5323), 195–196. doi: 10.1126/science.277.5323.195
Sullivan, B. (2003, November 26). Beyond Dolly: Human Cloning on Religions reveal little consensus on clonin. NBC News. Retrieved from http://www.nbcnews.com/id/3076930/#.XkRmYehKg2x
Twinning. (n.d.). Retrieved February 7, 2020, from https://www.britannica.com/science/twinning-biology
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J., & Campbell, K. H. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature, 385(6619), 810-813.