This is an old revision of the document!
Table of Contents
Introduction
Etiology
Gliomas are most common malignant tumors found in adults. The onset tends to occur in glial tissues and most commonly in the brain but they could actually occur anywhere in the central nervous system. Gliomas could be astrocytic, oligodendrocytic, or a mix of both. The comparison between the types can be hard to study as they all fall under histologies. Overall, there are scarcely any carcinogenetic causes which can be identified. Exposure to high doses of ionizing radiation is regarded as the only confirmed risk factor.
Epidemiology
The incidence rate of gliomas depends on its histologic type, age at diagnosis, gender, race, and country. However, it is hard to compare and accurately define the incidence rate due to its variation in the different types and its inconsistency of definition resulting in different data collections.
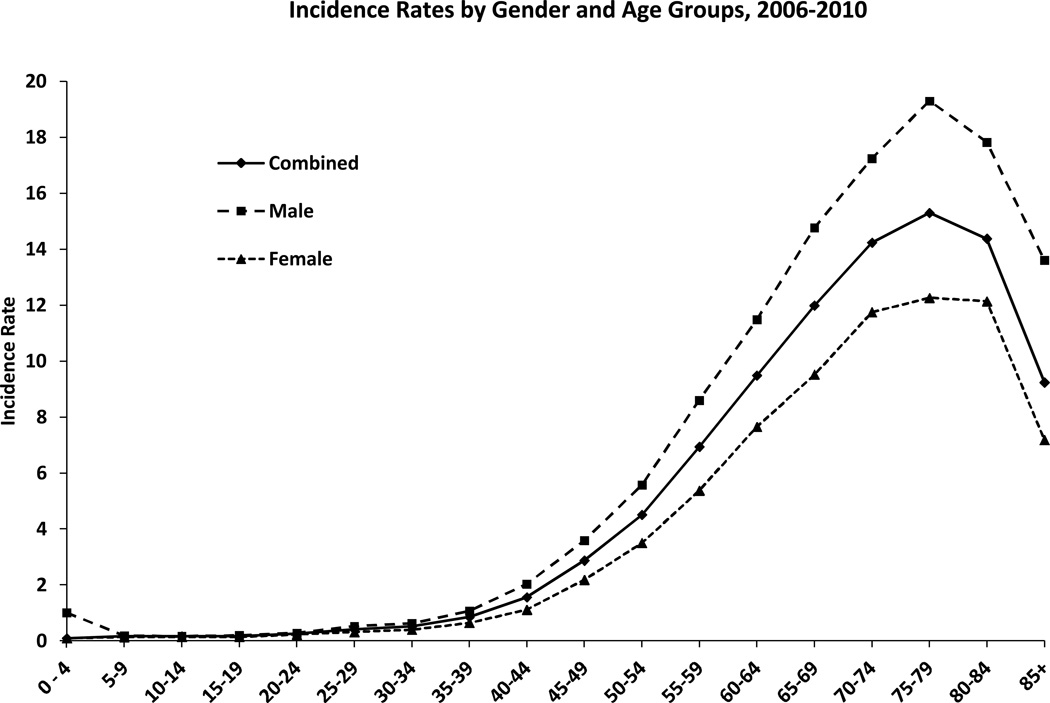
According to a review, the age-adjusted incidence rates range from 4.67 to 5.73 per 100 000 persons (Thakkar et al. 2014). Anaplastic astrocytoma and glioblastoma increase in incidence with age, peaking in the 75–84 age group. Oligodendrogliomas and oligoastrocytomas are most common in the 35–44 age group. Figure 1 provides data on the prevalence of GBM according to age and gender, suggesting that the cancer is more common among men and adults within the 50-70 ranged age group.
Prognostic factors include the extent of tumor resection, the age of diagnosis, and the Karnofsky performance. Glioblastomas have a low survival rate of around 0.05% to 4.7% 5 years past diagnosis. Oligodendrogial types have a relatively higher survival rate in comparison with the astrocytic type. Overall, early surgical resection had a higer chance of surviving compared to waiting and biopsy.
Figure 1 : This figure depicts the age and gender specific incidence rates of glioblastoma at the time of diagnosis
Clinical Presentation
• Headache • Pain • Nausea/ vomiting • Seizures • Cognition • Confusion • Problems with mobility (Sizoo et al. 2010)
Moreover, GBM symptoms may be focal depending on the location of the tumor. For instance, the development of a tumor in the eloquent cortex may interfere with cognition, speech, vision (Young et al. 2015).
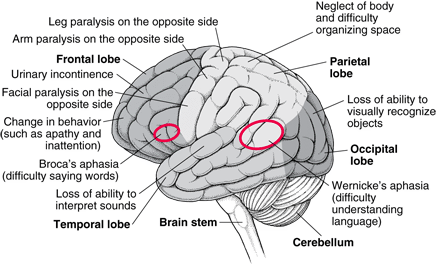 Figure _: Effect glioblastoma has on each area of the brain.
Figure _: Effect glioblastoma has on each area of the brain.
Diagnosis
Given that glioblastomas have an unusually high turnover rate and lead to poor patient prognosis, diagnosis of the disease should be completed immediately. At present, there are several techniques practiced in the accurate diagnosis of all stages of glioblastoma.
Biopsy
The needle biopsy procedure is a low invasive procedure that involves extraction of cancerous tissue for tumor diagnosis. This procedure is ideal for patients presenting tumors in deep regions of the brain, for which a fine needle aspiration biopsy that protects healthy brain tissue by the use of 3-D imaging technology (Young et al. 2015). Following tissue extraction, a pathologist uses microscopic analysis to determine the grade of the tumor and other diagnostic characteristics of the tumor.
Radiographic Techniques
Initially, a computed tomography (CT scan) which looks at the soft tissue of the brain and is accurate in the detection of larger scale tumors of varying stages. However, CT scanning may not possess the resolution to detect small multifocal tumors that may present or transform into glioblastoma multiform. In addition, cerebrospinal fluid (CSF) spread may be difficult to detect with CT scanning (Davis 2016).
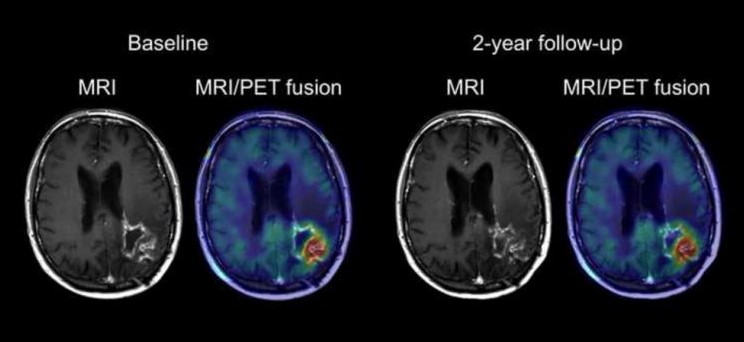
Moreover, an MRI scan applies a gadolinium contrast dye to the glioblastoma cells to identify the presence of an irregularly shaped tumor exhibiting a dense cellular outer ring and an inner ring of necrosis, a key feature indicative of GBM (Bosnyak et al. 2016). Moreover, brain scans may also reveal the presence of vasogenic edema peripheral to the primary tumor location, and as a result, potential hemorrhaging and displacement of the brain’s ventricles containing cerebrospinal fluid (Davis 2016). Figure 2 depicts an MRI scan and an MRI/PET fusion scan to illustrate a high grade glioma with central necrosis, high glucose metabolism and the presence of peripheral edema.
Furthermore, in approximately 13% of cases, GBM is presented as more than 2 lesions (multifocal) with a distant secondary lesion that is discontinuous with the primary lesion (Bosnyak et al. 2016).
Figure 2 : This figure depicts an MRI scan and an MRI/PET fusion scan of a patient brain exhibiting a left parieto-occipital glioblastoma.
Tumor Classification
Grade I : tumors are classified as juvenile pilocytic astrocytomas. Notable characteristics include a benign disposition, slow-growth rate, association with long-term survival and are least likely of the four grades to recur following treatment (Louis et al. 2016).
Grade II : tumors are classified as developed astrocytomas. Notable characteristics include increased hypercellularity, the absence of mitosis and vascularization, a lack of necrosis, and the higher likelihood of recurrence as a higher-grade tumor (Louis et al. 2016).
Grade III : tumors are classified as anapalastic astrocytomas. Notable characteristics include a high rate of hypercellularity and mitosis, a lack of vascularization and necrosis, and an overall high rate of recurrence (Louis et al. 2016).
Grade IV : tumors are classified as glioblastomas. Notable characteristics include a very high rate of hypercellularity and mitosis, the presence of vascularization surrounding the tumor, and the presence of necrosis in the tumor core (Louis et al. 2016).
Brain Physiology
The brain can be divided into two major components; white matter and grey matter. The grey matter consists of the cell bodies, axons and dendrites, it is where all the neuronal connections occur. The white matter consists of the glial cells and the myelinated axons that connect cell bodies to one another (Henson, 1999). Glioblastomas tend to occur in two subsets of the glial cells—astrocytes and oligodendrocytes. Astrocytes maintain the appropriate environment for neuronal signalling and are star-shaped cells that give the brain its shape. Astrocytes are the most common cell type to become tumours. Oligodendrocytes are responsible for forming myelin sheaths around axons. Tumours of oligodendrocytes are less common than astrocytomas. Many tumours contain a mixture of astrocytoma and oligodendroglioma cells. Tumours of other cell types in the brain are less common. For instance, tumours of neurons are very rare in adults (Henson, 1999).
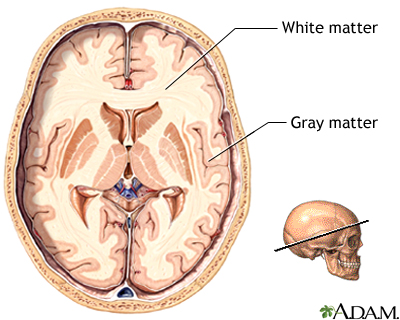 Figure 3: Glioblastomas occur in glial cells, which are located in the white matter of the brain.
Figure 3: Glioblastomas occur in glial cells, which are located in the white matter of the brain.
Pathophysiology of Glioblastomas
Overview of Tumorigenesis
Six general intracellular events which lead to the development of glioblastoma. These steps do not occur in isolation, but the accumulation of these will lead to tumorigenesis. The first event is loss of cell cycle control. Normal cells have many checkpoints during the cell cycle to keep proliferation in control. However, in glioblastoma, genetic defects occur leading to uncontrolled regulation of the cell cycle causing proliferation. The second event that occurs is overexpression of growth factors and their receptors. This overexpression provides growth advantage to tumor cells, increasing their survivability. A third event that occurs in glioblastoma is angiogenesis. Glioblastoma is a vascular tumor, and thus needs angiogenesis to support itself. Another critical feature in glioblastoma is the mutation of stem cells into tumor cells*. Moreover, abnormal apoptosis is a significant feature in tumorigenesis. Genetic mutations, such as p53, disrupt normal apoptotic responses, and allow further progression of the cell cycle. Lastly, genetic instability allows for the accumulation of mutations. Some of these mutations will be advantageous since it will be a more malignant clone (Nakada et al. 2011).
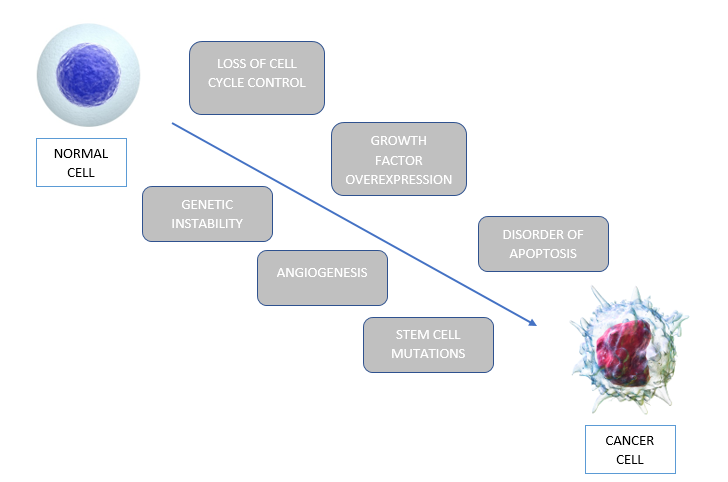 Figure_: The six intracellular events that allow normal cells to be transformed to cancerous cells, through a process called tumorigenesis.
Figure_: The six intracellular events that allow normal cells to be transformed to cancerous cells, through a process called tumorigenesis.
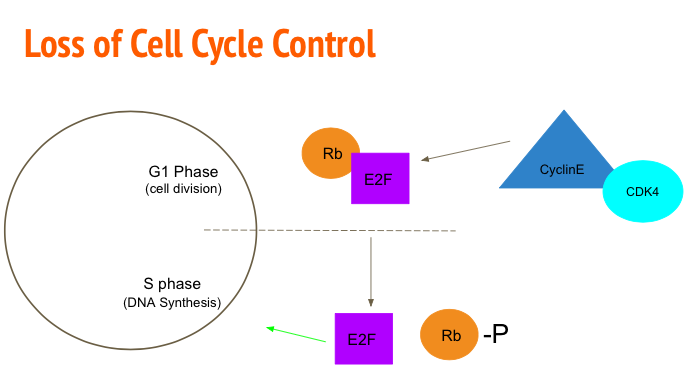
Loss of Cell Cycle Control
In normal conditions, the process of cellular division is highly regulated with numerous checkpoints that either allow the progression of the cell cycle or induce apoptosis in the presence of mutations. Cancerous cells evade these restrictions resulting in uncontrolled cellular proliferation. One mechanism to accomplish this is mutating the Rb/E2F pathway. Rb/E2F mediates the transition of G1 phase to S phase (Alifieris, 2015). Transcription factor E2F is responsible for upregulating genes to promote DNA synthesis. Ordinarily Retinoblastoma protein (Rb) binds and inhibits the activation of E2F (Nevins, 2001). To progress to S phase, Cyclin D-cyclin dependent kinase 2 (CDK-2) and Cyclin E-CDK4 phosphorylate the Rb protein rendering it inactive. The cell is then able to advance to S phase via E2F (Nevins, 2001). Tumour analysis showed mutations in the Rb gene allowed the cell to enter a cancerous state. After this conversion, cancerous cells are also able to upregulate genes coding for Cylin-CDK4 to promote the phosphorylation of Rb and therefore the activation of E2F (Nevins, 2001).
Figure_: Cyclin E and CDK4 phosphorylate Rb rendering it inactive and allowing E2F to up regulate DNA synthesis genes.
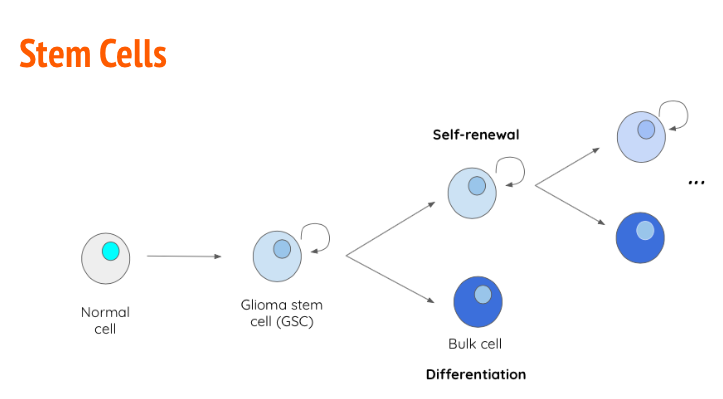
Stem Cells
Stem cells are undifferentiated cells that are able to undergo continuous proliferation to give rise to specialized differentiated cells. During fetal growth, embryonic stem cells are known as pluripotent, meaning they can give rise to any tissue type (Ahmed, 2013). As development progresses post-partum, stem cells lose plasticity and become multipotent, meaning they can only give rise to cells of a particular tissue type (Ahmed, 2013). In many cases, cancer arises due to the uncontrollable proliferation of stem cells. In the case of GBM, glioma stem cells (GBS) have the capability of becoming tumor initiating cells (TICs) (Ahmed, 2011). The primary reason GBM has a high relapse rate is because of the heterogeneity of the GBM tumour. When GSCs divide, they give rise to another GSC with self-renewal properties and a differentiated bulk cell which contributes to the tumour size (Ahmed, 2013). Along with unregulated proliferation, GSCs also have the capability to upregulate transcription factors such as VEGF to promote angiogenesis (Alifieris, 2015). Tt has been determined that GSCs upregulate certain antigens on their cellular membrane to increase tumor-like characteristics that are not observed in regular differentiated cells. An example of this, is antigen CD133+ which shown in vitro to give rise to “tumor spheres” when cultured in optimal conditions (Ahmed, 2013).
Figure_: GSCs give rise to a daughter GSCs with self-renewal properties as well as a differentiation bulk cell with contributes to tumour size.
PI3K pathway
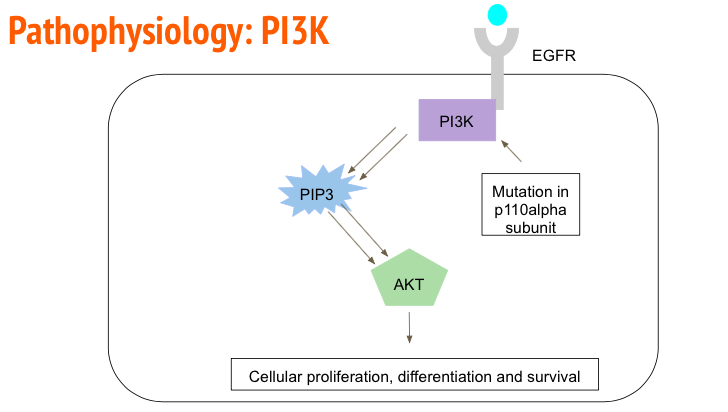
Upregulation of many Receptor Tyrosine Kinase, such as Endothelial Growth Factor Receptor (EGFR), can be observed in a cancerous environment. EGFR activation leads to upregulation of angiogenesis, proliferation, differentiation and cell metastasis (Herbst, 2003). In GMB cell samples it has been shown that the extracellular domain of the EGFR has been deleted leading to ligand independent cell signaling (Herbst, 2003). EGFR activates by auto phosphorylating its tyrosine domain which allows cellular kinases such as Phosphoinositide 3-Kinase (PI3K) (Alifieris, 2015). PI3K then goes on to phosphorylate proteins such as PIP3 and PIP2 via its p110alpha catalytic subunit. Phosphorylation of PIP3 and PIP2 leads to the activation of Akt which then geos on to upregulate cells to promote cellular proliferation, differentiation and survival (Alifieris, 2015). In a cancerous environment, it has been observed that the p110alpha domain of PI3K has been upregulated to accelerated cellular division (Alifieris, 2015).
Figure_: EFGR auto-phosphorylates to recruit PI3K. PI3K phosphorylates PIP3 and PIP2 which then go on to activate Akt to up regulate genes that promote cell survival.
Angiogenesis
Angiogenesis is initiated by angiogenic factor such as VEGF and FGF, which stimulate growth of endothelial cells. These are secreted in high concentrations from endothelial cells near the tumor, in a hypoxic microenvironment. The angiogenic factors bind to cognate receptors found on endothelial cells, leading to endothelial cell proliferation and migration. Then, there is a breakdown of local extracellular matrix, via MMPs, to provide room for new blood vessels to form. Next, angiopoietins (Ang-2) are secreted from the glioma cells to allow stability and maintenance of the new vasculature. Ang will bind to Tie-2 and destabilize vessels. After this breakdown and destabilization, endothelial cells gather into a tubular lumen. Individual sprouts are connected and form a vascular loop to allow for blood flow. Pericytes are then recruited and assemble on the outside off the new vasculature to allow maturation. Once a basement membrane is formed angiogenesis is complete (Nakada et al. 2011).
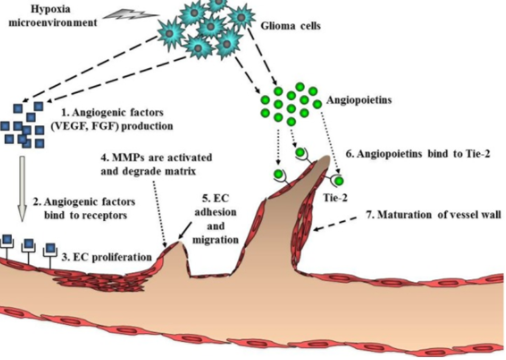 Figure _: Steps for glioblastoma to undergo angiogenesis. Angiogenesis is a necessary process that allows glioma cells to survive and continue to proliferate.
Figure _: Steps for glioblastoma to undergo angiogenesis. Angiogenesis is a necessary process that allows glioma cells to survive and continue to proliferate.
p53 Pathway Mutation
The p53 pathway is often mutated and ultimately leads to tumorigenesis. Normally, p53 acts to detect DNA damage and other cytotoxic stressors. Once detected, the cell cycle is arrested, and apoptosis occurs, thus playing a significant role in tumor suppression. The p53 pathway controls over 2500 genes which are involved in tumorigenesis, and tumor invasion. In secondary glioblastoma, mutated p53 is the first detectable genetic mutation.
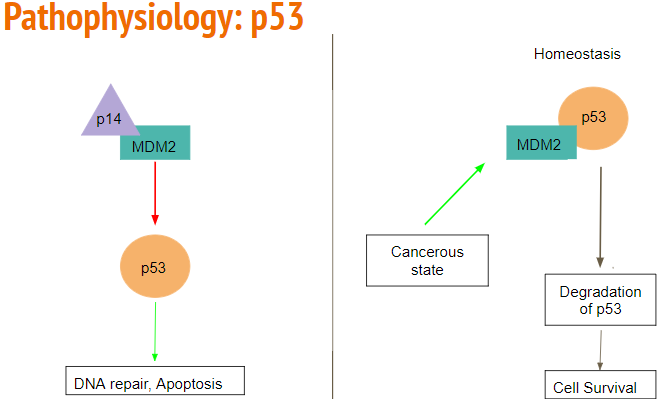
MDM2 acts as a E3 ubiquitin ligase and controls the activity of the p53 pathway. It can inhibit the p53 pathway through direct binding, or by E3 ligase degradation, leading to the continuation of a cell cycle, and ultimately proliferation. MDM2 is overexpressed in 10% of glioblastoma patients, leading to tumorigenesis without direct mutation to the p53 pathway.
ARF is a tumor suppressor protein that is an upstream regulator of the p53 pathway. ARF can directly bind to MDM2 and inactivate the E3 ligase activity, leading to activation of the p53 pathway. However, in glioblastoma, ARF is inactivated or mutated, leading to proliferation by continuation of the cell cycle (Mao et al. 2012).
 Figure_: Rb binds to inhibit E2F. When the cell is ready to move to S phase, Cyclin E-CDK4 phosphorylates Rb rendering it inactive, allowing E2F to up regulate genes responsible for DNA synthesis.
Figure_: Rb binds to inhibit E2F. When the cell is ready to move to S phase, Cyclin E-CDK4 phosphorylates Rb rendering it inactive, allowing E2F to up regulate genes responsible for DNA synthesis.
Genetic Instability
Normal cells have tight regulations during the cell cycle to prevent mutations from occuring. However, when this tight regulation of the cell cycle is lost there will be an accumulation of genomic mutations during a series of cell divisions. These alterations will transform normal cells into precancerous cells. These cells would not be classified or diagnosed as cancer cells, however, these cells have a higher potential to be cancerous. The adition of more mutations that lead to increased growth trasform these cells into cancerous cells, and thus can be diagnsed as cancer. Furthermore, the cancer cells will continue to alter these cancer cells to be more malignant. Thus, genetic instability not only leads to tumorigenesis, but maintains the survivability of these cancer cells (Shen. 2011)
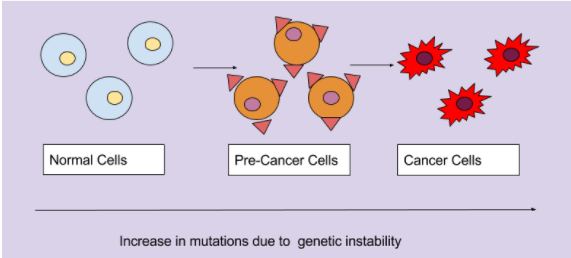
Figure_: The accumulation of mutations due to genetic instability transforms normal cells into pre-cancerous cells, and finally into cancerous cells.
Treatments: Current and Future
Surgical Resection
Due to the aggressive nature of GBM, proactive measures are taken to localize the tumor with respect to the eloquent cortex which composed of the primary motor cortex or speech center. This is completed to determine the proportion of the tumor that can be resected without inducing lethal injury.Namely, maximal surgical resection of the tumor is performed to improve patient survivability and reduce the chances of tumor progression and recurrence. The cancer cannot be cured through surgical resection alone, but must be used in conjunction with neoadjuvant treatment. For grade 1 astrocytomas that present a low rate of mitosis and are classified as benign, surgical resection can be considered curative, however residual adhesions may remain from the removed tumor and can lead to tumor recurrence. Depending on the grade and location of the tumor, surgical resection may be difficult to achieve without impairing neurological function (Young et al. 2015).
A form of surgical resection classified as: awake craniotomy, is implemented for tumors that are localized in Broca’s speech area, Wernicke’s area and the brain stem (regions of the eloquent cortex). Though the patient is sedated, they are awake due to cortical stimulation and are able to respond to surgical probing that can determine areas for safe maximal resection. Moreover, the neurosurgeon is able to map the brain tissue peripheral to the glioma using intraoperative MRI and functional mapping to accurately determine the areas that are outside of the eloquent cortex for safe resection (Young et al. 2015).
Bevacizumab (Avastin®)
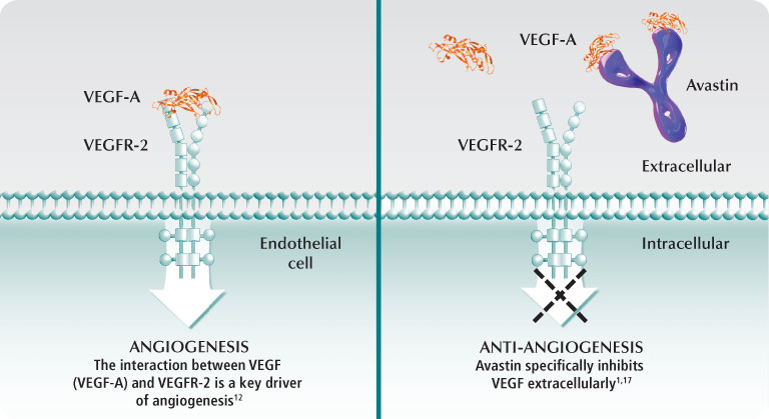
Bevacizumab (Avastin®) is a monoclonal antibody implemented in GBM chemotherapeutic regiments to target vascular endothelial growth factor (VEGF) and was approved by the FDA in 2009 after clinical trials had demonstrated excellent performance. The VEGF protein is needed for angiogenesis, a process that involves the synthesis of new vasculature from pre-existing vessels and occurs during wound healing and when cells are deficient in oxygen levels. The synthesis of new blood vessels among tumor cells mediated by VEGF leads to a greater blood and oxygen supply necessary for the proliferation of GBM cells (Gil-Gil et al. 2013). Avastin works to reduce vascular permeability and edema, enhancing delivery of oxygen to brain cells and reducing necrosis in the tumor core when administered in conjunction with radiation therapy (Davis 2016). According to Gil-Gil et al. 2013, GBM cells generate the proangiogenic factor called VEGF which binds with its tyrosine kinase receptor on the surface of endothelial cells, initiating a signal cascade resulting in angiogenesis. Figure 7 demonstrates that Bevacizumab is easily able to penetrate the selectively permeable blood-brain barrier with a molecular weight of 149 kDa and binds with a high affinity to VEGF, sterically inhibiting the factor from binding to its receptors on endothelial cells and effectively reducing angiogenesis, inhibiting tumor growth, and edema in the brain.
Figure 7 : This figure depicts the mechanism by which Bevacizumab works to inhibit VEGF receptors on the surface of endothelial cells
Radiation Therapy and Temozolomide
Following resection patients undergo various forms of radiation therapy (RT), most commonly RT is externally delivered in which ionizing radiation is targeted at cancer cells left in the brain following resection, damaging DNA and resulting in apoptosis (Davis, 2016). One the most effective forms of RT is brachytherapy, particularly the use of a new device approved in 2002 called GliaSite. This involves the administration of 125I solution which is filled into a closed catheter ballon and inflated to fill the resection cavity (Chan et al., 2005). Following and often times, concurrently with RT, patients are put on a 4-6 week chemotherapeutic regime, they are administered a drug called temozolomide (TMZ) (Davis, 2016). Temozolomide is a small (194 Da) lipophilic molecule that is available as a orally administered chemotherapeutic pill. Because of TMZ’s small size and lipophilic character it readily crosses the blood-brain barrier (Agarwalla & Kirkwood, 2000). Once TMZ is in the body it is rapidly metabolized into MTIC, which is an alkylating agent that adds a methyl group to the O6 position of guanine in DNA resulting in the incorporation of a thymine residue instead of a cytosine across the guanine (Zhang, Stevens & Bradshaw, 2012). This abnormal pair leads DNA mismatch repair activation, which excises the mispaired thymine but leaves the the methylated guanine, this eventually leads to DNA strand breaks and apoptosis. There can also be resistance to TMZ and its alkylating activity, specifically via a protein known as O6-methylguanine methyltransferase (MGMT). MGMT protects cells from carcinogens and consequently, it can also protect and resist chemotherapeutic agents like TMZ. MGMT is able to transfer the O6-alkyl group on guanine to its active site (cysteine 145), effectively repairing DNA and therefore evading apoptosis (Zhang, Stevens & Bradshaw, 2012).
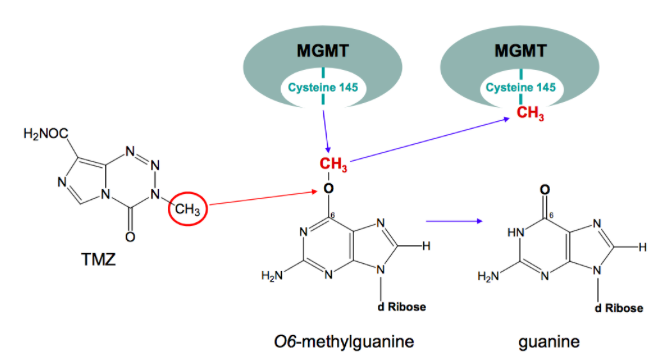 Figure 8: Repair of O6-methylguanine by MGMT
Figure 8: Repair of O6-methylguanine by MGMT
Immune Checkpoints
Novel progression of sequencing methods have led to the identification of specific molecular signatures of GBM. There are several biomarkers which are used for identification. The types include, diagnostic, prognostic, and predictive. Predictive biomarkers help in the treatment allowing the use of patient-specific biology. This uproots anti-GBM therapies to be used as molecular inhibitors that will target the growth factor receptors. Part of the GBM's growth is correlated to its ability to escape the individual's immune system surveillance. GBM dampens the immune response via expression of immunosuppressive cytokines such as prostaglandin E2 and transforming growth factor beta. It also increases the activation of T-cells. It affects two major checkpoint proteins, cytotoxic T-lyphocyte antigen 4 (CTLA-4) and the programmed cell death protein 1 (PD-1). CTLA-4 will increase T-cell activity while also competing with CD28 for binding with B7 ligands. PD-1 regulates immunity at several phases of the immune response. High expression of PD-L1 mRNA and CTLA-4 in GBM suggests a correlation between the immune checkpoint proteins and the severity of
Conclusion
References
Agarwala, S. A., & Kirkwood, J. M. (2000). Temozolomide, a Novel Alkylating Agent with Activity in the Central Nervous System, May Improve the Treatment of Advanced Metastatic Melanoma. The Oncologist, 5, 144-151. doi: 10.1634/theoncologist.5-2-144
Avastin: Important Safety Information. (n.d.). Retrieved September 28, 2017, from https://www.avastin-hcp.com/about-avastin/proposed-moa.html
Bosnyak, E., Kamson D. O., Robinette, N. L., Barger, G. R., Mittal, S., & Juhasz, C. (2016). Tryptophan PET predicts spatial and temporal patterns of post-treatment glioblastoma progression detected by contrast-enhanced MRI. J Neurooncol, 126(2), 317-325. doi: 10.1007/s11060-015-1970-3
Chan, T. A., Weingart, J. D., Parisi, M., Hughes, M. A., Olivi, A. Borzillar S., Alahakone D., Detoria, N. A., Wheram, M. D., & Kleinber, L. (2005). Treatment of recurrent glioblastoma multiform with GliaSite Brachytherapy. Int J Radiat Oncol Biol Phys. 65(4), 1133-1139. doi: 10.1016/j.ijrobp.2004.12.032
Davis, M. E. (2016). Glioblastoma: Overview of Disease and Treatment. Clinical Journal of Oncology Nursing, 20(5), S2–S8. http://doi.org/10.1188/16.CJON.S1.2-8
Gil-Gil, M. J., Mesia, C., Rey, M., & Bruna, J. (2013). Bevacizumab for the Treatment of Glioblastoma. Clinical Medicine Insights. Oncology, 7, 123–135. http://doi.org/10.4137/CMO.S8503
Henson, J. W. (1999). Glioblastoma multiform and anaplastic gliomas: A patient guide. MGH Brain Tumor Center. Retrieved from https://brain.mgh.harvard.edu/patientguide.htm
Louis, D. N., Perry, A., Reifenberger, G., Von Deimling, A., Figarella-Branger, D., Cavenee, W. K., Ohgaki, H., Wiestler, O. D., Kleihues, P., Ellison, D. W. (2016). The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica, 131(6), 803-820. https://doi.org/10.1007/s00401-016-1545-1
Mao, H., LeBrun, D. G., Yang, J., Zhu, V. F., & Li, M. (2012). Deregulated Signaling Pathways in Glioblastoma Multiforme: Molecular Mechanisms and Therapeutic Targets. Cancer Investigation, 30(1), 48–56. http://doi.org/10.3109/07357907.2011.630050
Nakada, M., Kita, D., Watanabe, T., Hayashi, Y., Teng, L., Pyko, I. V., & Hamada, J.-I. (2011). Aberrant Signaling Pathways in Glioma. Cancers, 3(3), 3242–3278. http://doi.org/10.3390/cancers3033242
Sizoo, E. M., Braam, L., Postma, T. J., Pasman, H. R. W., Heimans, J. J., Klein, M., … Taphoorn, M. J. B. (2010). Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro-Oncology, 12(11), 1162–1166. http://doi.org/10.1093/neuonc/nop045
Thakkar, J. P., Dolecek, T. A., Horbinski, C., Ostrom, Q. T., Lightner, D. D., Barnholtz-Sloan, J. S., & Villano, J. L. (2014). Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 23(10), 1985–1996. http://doi.org/10.1158/1055-9965.EPI-14-0275
Young, R. M., Jamshidi, A., Davis, G., & Sherman, J. H. (2015). Current trends in the surgical management and treatment of adult glioblastoma. Annals of Translational Medicine, 3(9), 121. http://doi.org/10.3978/j.issn.2305-5839.2015.05.10
Williamson, J. (n.d.). What Is a Fat Burner? Retrieved from http://www.healthguidance.org/entry/14407/1/What-Is-a-Fat-Burner.html
Zhang, J., Stevens, F. G., & Bradshaw, T. D. (2012). Temozolomide: Mechanisms of Action, Repair and Resistance. Curr Mol Pharmacol., 5, 102-114.